Содержание
- 2. FREE ENERGY AND EQUILIBRIA ΔG ΔG > 0 reverse process is spontaneous ΔG = 0 no
- 3. CHEMICAL POTENTIAL The chemical potential can be used to give quantitative meaning to Le Châtelier’s principle.
- 4. DIRECTIONALITY OF A CHEMICAL REACTION Consider a closed system of four components (A, B, C, and
- 5. EXAMPLE H2O (l) H2O (g) 100 °C This is at constant T and P. Its reversible.
- 6. CHEMICAL POTENTIAL AND PARTIAL PRESSURE We found last time that: (T = constant) Defining the standard
- 7. ΔG OF MIXING Consider the isobaric, isothermal mixing of two gases: Gas A at P Atm
- 8. EQUILIBRIUM CONSTANT The Haber process of nitrogen fixation: N2 + 3H2 2NH3 Rewrite letting unitless pressure
- 9. EQUILIBRIUM CONSTANT At any temperature and pressure there exists and equilibrium state for this reaction. A
- 10. EQUILIBRIUM CONSTANT Define equilibrium constant (at constant T and P): More generally: aA + bB cD
- 11. CHEMICAL POTENTIAL EXAMPLE Reactants Products Reactants Products Δμ° Δμ° μ° Chemical potential is a measure of
- 12. EQUILIBRIUM EXAMPLE Oxidation of CO: 2 CO (g) + O2 (g) 2 CO2 (g) The free
- 13. EQUILIBRIUM EXAMPLE Reactants (CO,O2) Products (CO2) Δμ°, ΔG° The conversion of CO and O2 to CO2
- 14. ΔH0 FOR SOME NONCOVALENT INTERACTIONS Na+(g) + Cl-(g) NaCl(s) Ionic -785 NaCl(s) Na+(aq) + Cl-(aq) Ionic
- 15. PROTEIN UNFOLDING Proteins have a native state. (Really, they tend to have a tight cluster of
- 16. PROTEIN UNFOLDING Let’s consider denaturation with heat. We can determine a great deal about the nature
- 17. PROTEIN UNFOLDING In differential scanning calorimetry you have two samples: Your material of interest Control You
- 18. PROTEIN UNFOLDING Data for glyceraldehyde-3-phosphate dehydrogenase. pH8.0 pH6.0 Bacillus stearothermophilus E. coli Rabbit Is the protein
- 20. Скачать презентацию
 Оксиды азота
Оксиды азота Физико-химия поверхностных явлений
Физико-химия поверхностных явлений Материаловедение. Придание металлам и сплавам заданных свойств
Материаловедение. Придание металлам и сплавам заданных свойств Титриметрический анализ. Сущность титриметрического анализа
Титриметрический анализ. Сущность титриметрического анализа Окислительно-восстановительные реакции в аналитической химии
Окислительно-восстановительные реакции в аналитической химии Кислородные соединения азота. Азотная кислота
Кислородные соединения азота. Азотная кислота Тотығу-тотықсыздану титрлеу әдісі
Тотығу-тотықсыздану титрлеу әдісі Открытие Д.И. Менделеевым периодического закона. Периодическая система химических элементов
Открытие Д.И. Менделеевым периодического закона. Периодическая система химических элементов Кислородсодержащие органические соединения. 9 класс
Кислородсодержащие органические соединения. 9 класс Основной государственный экзамен Химия 2021. Задание 5
Основной государственный экзамен Химия 2021. Задание 5 ГИА-9 Химия. А4
ГИА-9 Химия. А4 Мыло. Мылящие вещества в природе
Мыло. Мылящие вещества в природе Сучасні матеріали. Пластмаса
Сучасні матеріали. Пластмаса Классификация химических реакций. 8 класс
Классификация химических реакций. 8 класс Mineralogy. Chemical composition and properties of minerals
Mineralogy. Chemical composition and properties of minerals Общие способы получения металлов
Общие способы получения металлов Відносна молекулярна маса речовини, її обчислення за хімічною формулою
Відносна молекулярна маса речовини, її обчислення за хімічною формулою Химическая связь
Химическая связь Алкины. Общая характеристика, гомологический ряд, номенклатура, изомерия. Методы синтеза алкенов. Лекция №4
Алкины. Общая характеристика, гомологический ряд, номенклатура, изомерия. Методы синтеза алкенов. Лекция №4 Предельные углеводороды
Предельные углеводороды Геохимия рудных месторождений
Геохимия рудных месторождений Количество вещества. 8 класс
Количество вещества. 8 класс №2 Практикалық жұмыс. Химиялық реакция жылдамдығына әртүрлі факторлардың әсерін зерттеу
№2 Практикалық жұмыс. Химиялық реакция жылдамдығына әртүрлі факторлардың әсерін зерттеу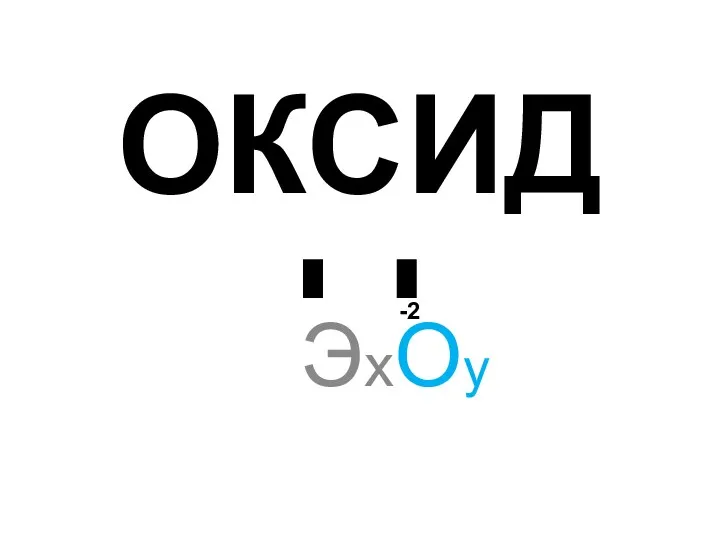 Химические свойства оксидов
Химические свойства оксидов Механизм реакции в органической химии
Механизм реакции в органической химии Природный и синтетический каучуки. Резина
Природный и синтетический каучуки. Резина Масс-Спектроскопия
Масс-Спектроскопия