Содержание
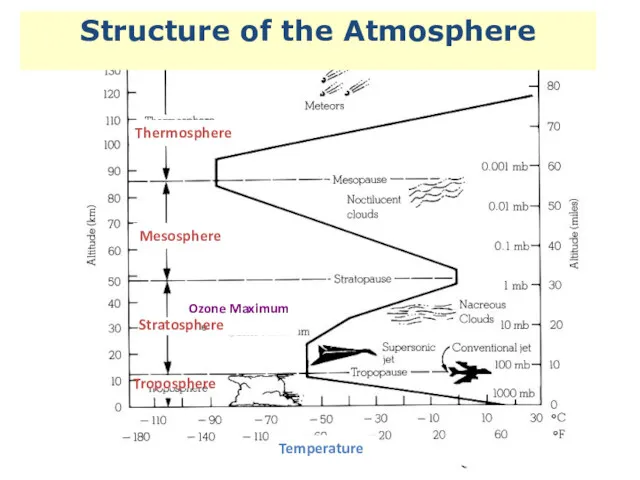
- 2. Structure of the Atmosphere Thermosphere Mesosphere Ozone Maximum Stratosphere Troposphere Temperature
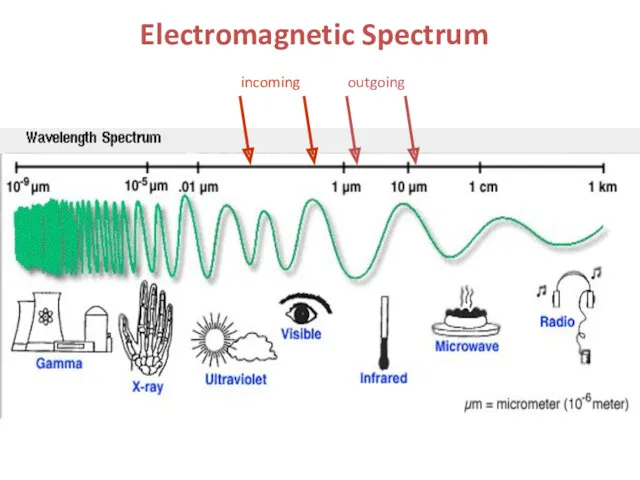
- 3. Electromagnetic Spectrum incoming outgoing
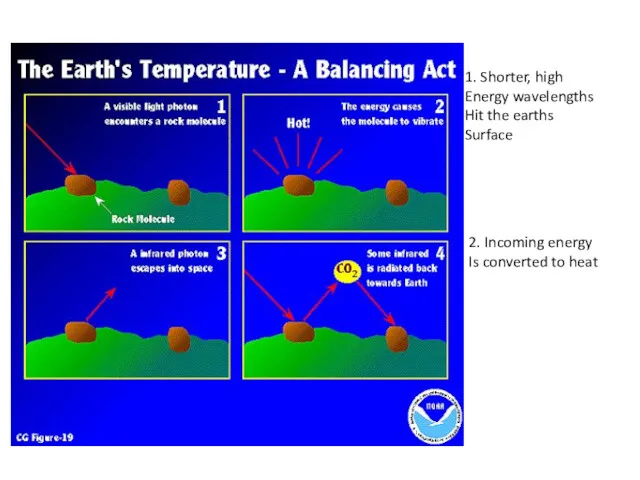
- 4. 1. Shorter, high Energy wavelengths Hit the earths Surface 2. Incoming energy Is converted to heat
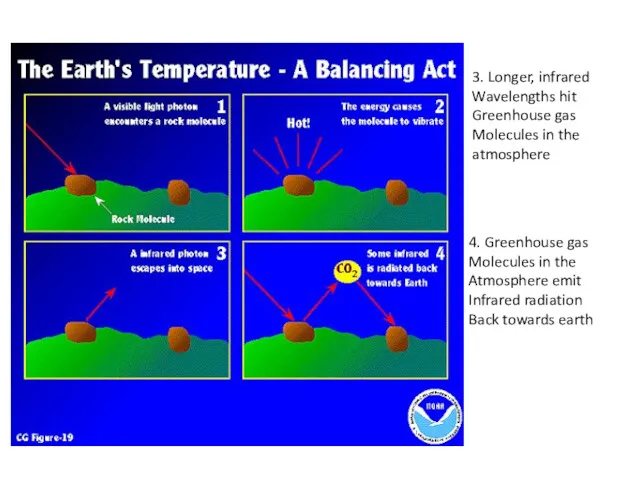
- 5. 3. Longer, infrared Wavelengths hit Greenhouse gas Molecules in the atmosphere 4. Greenhouse gas Molecules in
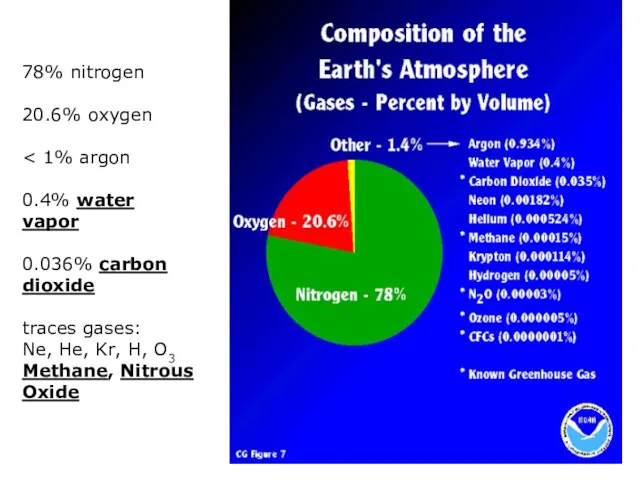
- 6. 78% nitrogen 20.6% oxygen 0.4% water vapor 0.036% carbon dioxide traces gases: Ne, He, Kr, H,
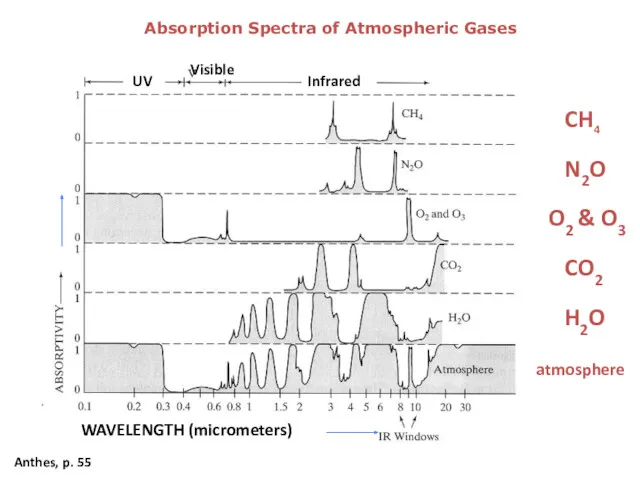
- 7. Absorption Spectra of Atmospheric Gases Anthes, p. 55 CH4 CO2 N2O H2O O2 & O3 atmosphere
- 8. Greenhouse gases absorb infrared radiation and prevent it from escaping to space. Carbon dioxide, methane, and
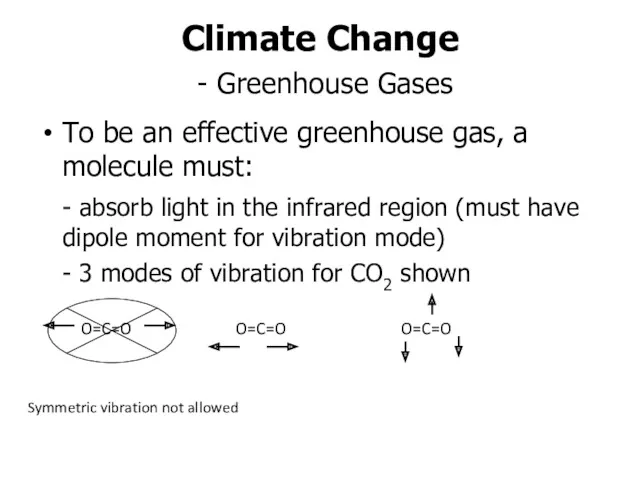
- 9. Climate Change - Greenhouse Gases To be an effective greenhouse gas, a molecule must: - absorb
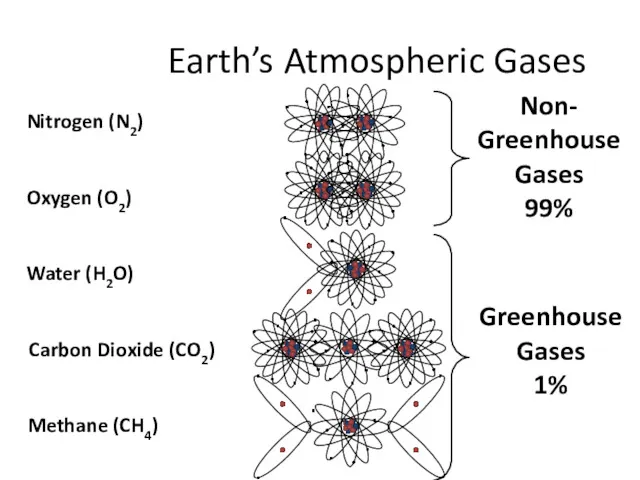
- 10. Earth’s Atmospheric Gases Non- Greenhouse Gases 99% Greenhouse Gases 1%
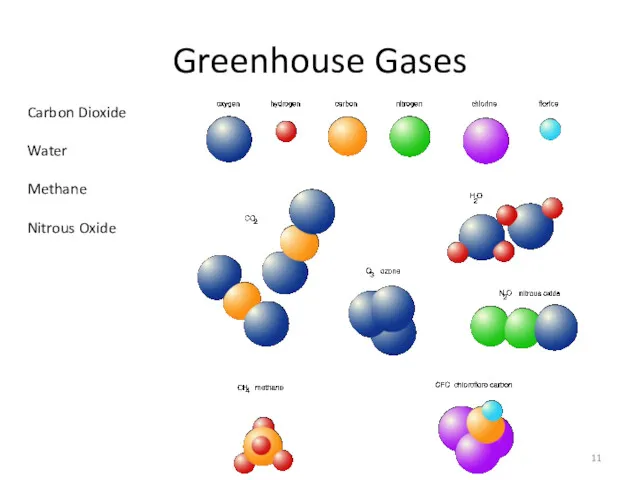
- 11. Greenhouse Gases Carbon Dioxide Water Methane Nitrous Oxide
- 12. Greenhouse Gases Molecules must absorb light in the right regions - roughly 7 to 25 μm
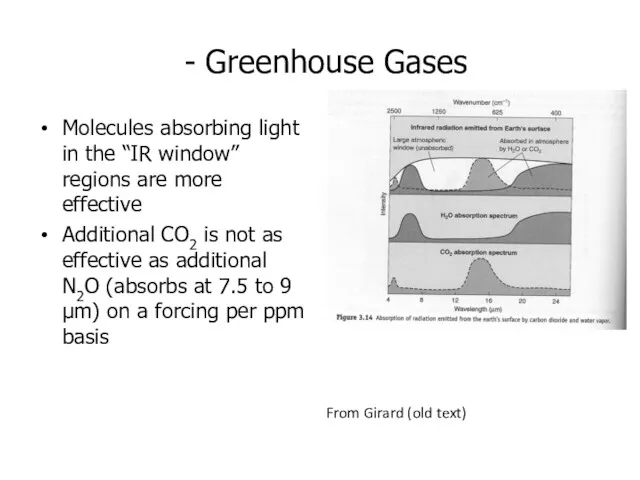
- 13. - Greenhouse Gases Molecules absorbing light in the “IR window” regions are more effective Additional CO2
- 14. Selected Greenhouse Gases Carbon Dioxide (CO2) Source: Fossil fuel burning, deforestation Anthropogenic increase: 30% Average atmospheric
- 15. Greenhouse Effect & Global Warming The “greenhouse effect” & global warming are not the same thing.
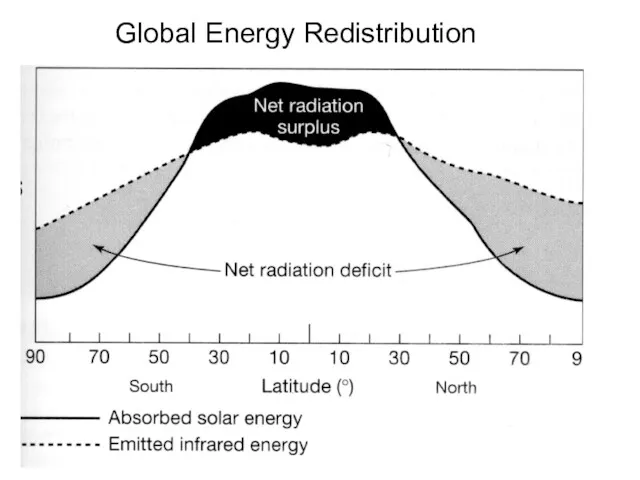
- 16. Global Energy Redistribution
- 17. Radiation is not evenly distributed over the Surface of the earth. The northern latitudes have an
- 18. The climate engine II Since earth does rotate, air packets do not follow longitude lines (Coriolis
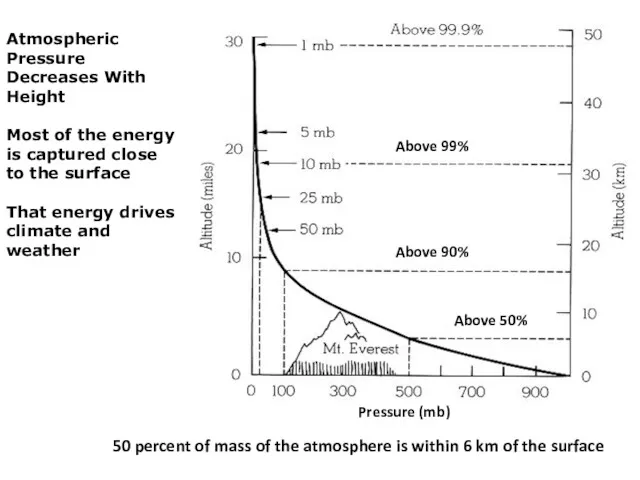
- 19. Atmospheric Pressure Decreases With Height Most of the energy is captured close to the surface That
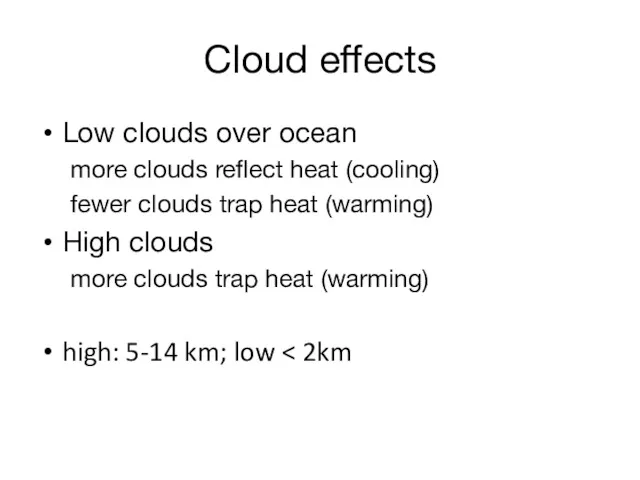
- 20. Cloud effects Low clouds over ocean more clouds reflect heat (cooling) fewer clouds trap heat (warming)
- 21. Fig. 19-10, p. 513
- 22. - Greenhouse Gases H2O as a greenhouse gas - the molecule responsible for the most greenhouse
- 23. The sun plays a key role in the earth’s temperature Researchers think that atmospheric warming is
- 24. Water vapor is one of the most important elements of the climate system. A greenhouse gas,
- 26. Скачать презентацию










 Game. Choose the right option
Game. Choose the right option Food. Has got, have got
Food. Has got, have got Watch the animals and choose
Watch the animals and choose Страдательный залог
Страдательный залог Middle English
Middle English Body Parts Taboo Game
Body Parts Taboo Game Past Tenses
Past Tenses Spelling bee contest
Spelling bee contest TV Quiz. Academy Stars 1. Unit 7
TV Quiz. Academy Stars 1. Unit 7 Individual work upon the lexical topic “Bronchitis”
Individual work upon the lexical topic “Bronchitis” Family review
Family review Colours
Colours Kazakh and English sayings
Kazakh and English sayings Colours game
Colours game Создание словаря аббревиатур на английском языке для использования при СМС сообщениях
Создание словаря аббревиатур на английском языке для использования при СМС сообщениях Lesson 9. Future Perfect Continuous
Lesson 9. Future Perfect Continuous Great Britain
Great Britain International organisations and the united nations
International organisations and the united nations Newspaper Articles Features
Newspaper Articles Features Game. Choose the right option
Game. Choose the right option There are 9 different ways to express future tense in English
There are 9 different ways to express future tense in English How do aircraft jet engines work
How do aircraft jet engines work English Grammar Land
English Grammar Land English-speaking countries
English-speaking countries State exam. Speaking
State exam. Speaking Mumo. Creative presentation template
Mumo. Creative presentation template Money and banking
Money and banking New technologies
New technologies