Слайд 2
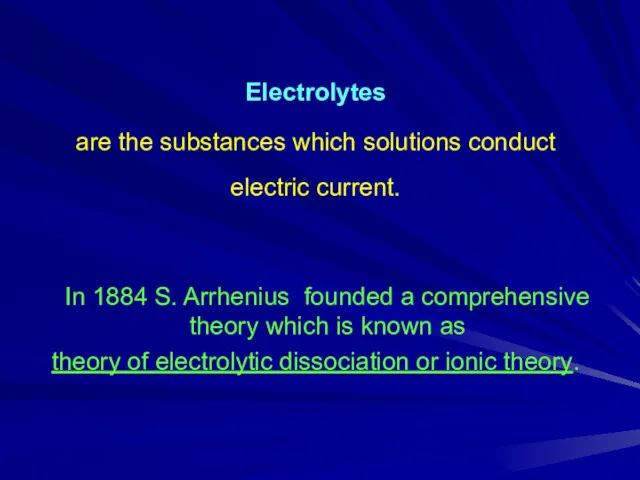
Electrolytes
are the substances which solutions conduct electric current.
In 1884
S. Arrhenius founded a comprehensive theory which is known as
theory of electrolytic dissociation or ionic theory.
Слайд 3
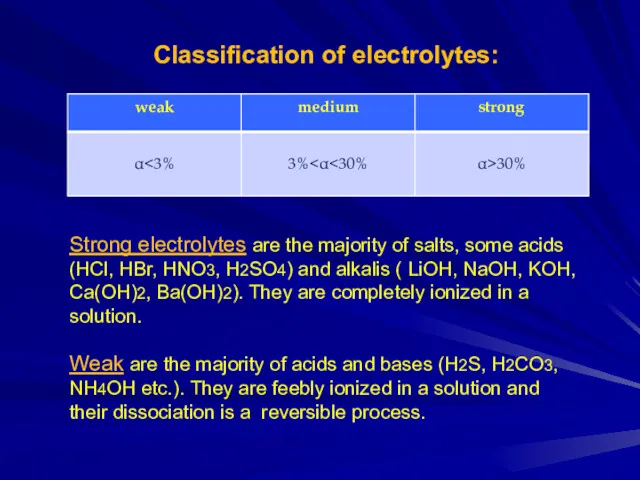
Classification of electrolytes:
Strong electrolytes are the majority of salts, some acids
(HCl, HBr, HNO3, H2SO4) and alkalis ( LiOH, NaOH, KOH, Ca(OH)2, Ba(OH)2). They are completely ionized in a solution.
Weak are the majority of acids and bases (H2S, H2CO3, NH4OH etc.). They are feebly ionized in a solution and their dissociation is a reversible process.
Слайд 4
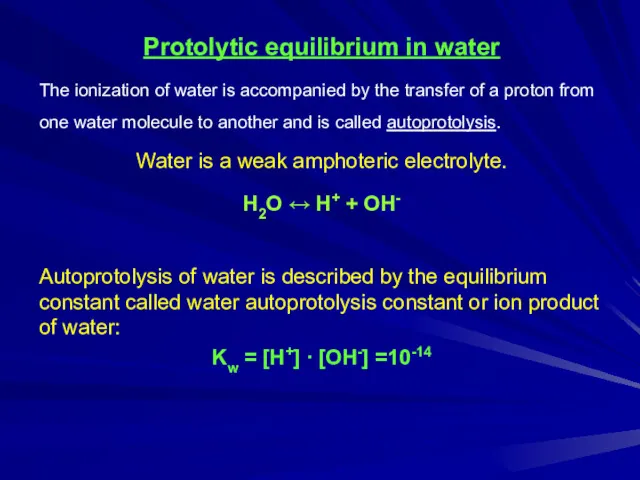
Protolytic equilibrium in water
The ionization of water is accompanied by the
transfer of a proton from one water molecule to another and is called autoprotolysis.
Water is a weak amphoteric electrolyte.
H2О ↔ H+ + OH-
Autoprotolysis of water is described by the equilibrium constant called water autoprotolysis constant or ion product of water:
Kw = [H+] ∙ [OH-] =10-14
Слайд 5

Hydrogen ion exponent
In pure liquid water (neutral medium) concentrations of hydrogen
and hydroxyl ions are the same:
[H+] = [OH-] = 10-7
рН = – lg[H+] = 7
рОН = –lg[ОH-] = 7
pKw = pH + pOH = 14
рН is hydrogen ion exponent – a value that is equal to negative decimal logarithm of hydrogen ions concentration (mole/litre).
Слайд 6
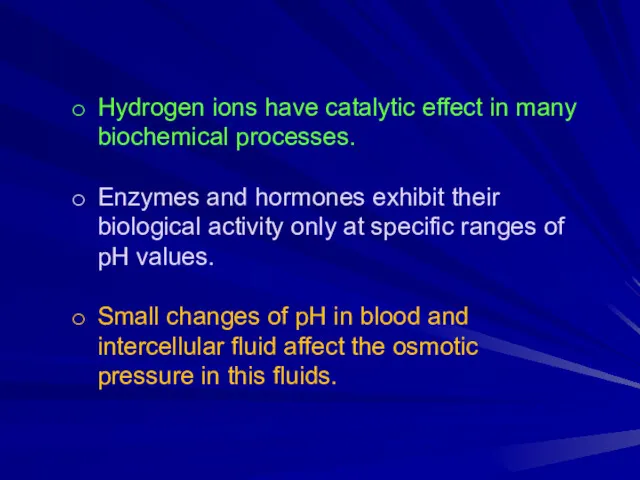
Hydrogen ions have catalytic effect in many biochemical processes.
Enzymes and hormones
exhibit their biological activity only at specific ranges of pH values.
Small changes of pH in blood and intercellular fluid affect the osmotic pressure in this fluids.
Слайд 7

BUFFER SOLUTIONS
are the solutions which pH values do not practically change
when moderate amounts of either a strong acid or a strong base are added and also as a result of dilution.
Buffer solutions consist of weak acids and their salts (conjugate bases) or of weak bases and their salts (conjugate acids).
Слайд 8
Buffer systems of blood
are the most important among the buffer systems
of all biological fluids.
Hydrocarbonate and phosphate buffers are present in blood plasma and in erythrocytes.
Proteins buffer system is in plasma.
Hemoglobin buffer system is in erythrocytes.







 Продолговатый мозг и мост
Продолговатый мозг и мост Влияние стресса на продуктивность сельскохозяйственных животных
Влияние стресса на продуктивность сельскохозяйственных животных Размножение и оплодотворение у растений. Двойное оплодотворение у цветковых растений
Размножение и оплодотворение у растений. Двойное оплодотворение у цветковых растений Общая остеология
Общая остеология Цитология. Биохимия клетки
Цитология. Биохимия клетки Одноклеточные и многоклеточные организмы. Ткани и органы
Одноклеточные и многоклеточные организмы. Ткани и органы Деление покрытосеменных растений на классы и семейства
Деление покрытосеменных растений на классы и семейства Мужская половая система
Мужская половая система Кровеносная система человека: артерии
Кровеносная система человека: артерии Витамины. Действие на организм
Витамины. Действие на организм Немембранные и двумембранные органоиды
Немембранные и двумембранные органоиды Презентация к уроку биологии 6 кл.
Презентация к уроку биологии 6 кл. В гости к лесным зверям (фотографии)
В гости к лесным зверям (фотографии) Золотая осень
Золотая осень Методологическая роль лабораторных и практических работ по биологии в рамках ФГОС.
Методологическая роль лабораторных и практических работ по биологии в рамках ФГОС. Исследование растений в XVI-XVIII века. (Лекция 6)
Исследование растений в XVI-XVIII века. (Лекция 6) Пластическая анатомия
Пластическая анатомия Общие принципы организации тканей. Эпителиальные ткани
Общие принципы организации тканей. Эпителиальные ткани Голонасінні. Ялина європейська
Голонасінні. Ялина європейська Технология кормления рыбы
Технология кормления рыбы Становление классической биологии
Становление классической биологии Витамины. Определение, классификация
Витамины. Определение, классификация Борьба за существование и ее формы
Борьба за существование и ее формы Движущие силы эволюции
Движущие силы эволюции Репродуктивная система человека
Репродуктивная система человека Лимбическая система
Лимбическая система Одноклеточные животные
Одноклеточные животные Влажность воздуха
Влажность воздуха