Содержание
- 2. Why are you late?
- 3. Occurrence and classification. The genus Brucella includes three medically relevant species—B. abortus, B. melitensis, and B.
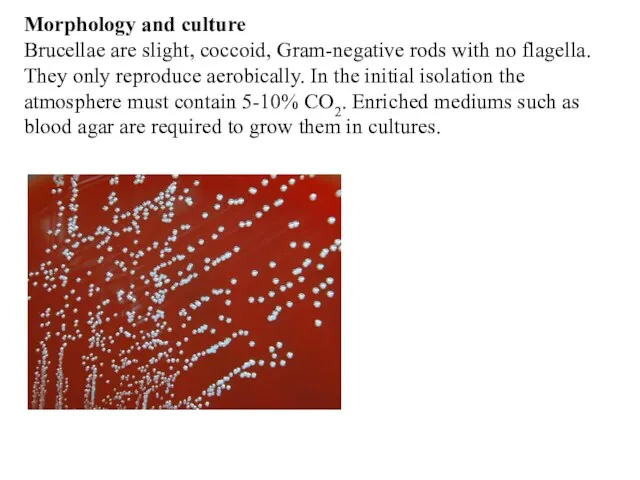
- 4. Morphology and culture Brucellae are slight, coccoid, Gram-negative rods with no flagella. They only reproduce aerobically.
- 5. Pathogenesis and clinical picture Human brucellosis infections result from direct contact with diseased animals or indirectly
- 6. Diagnosis This is best achieved by isolating the pathogen from blood or biopsies in cultures, which
- 7. Epidemiology and prevention Brucellosis is a zoonosis that affects animals all over the world. Infections with
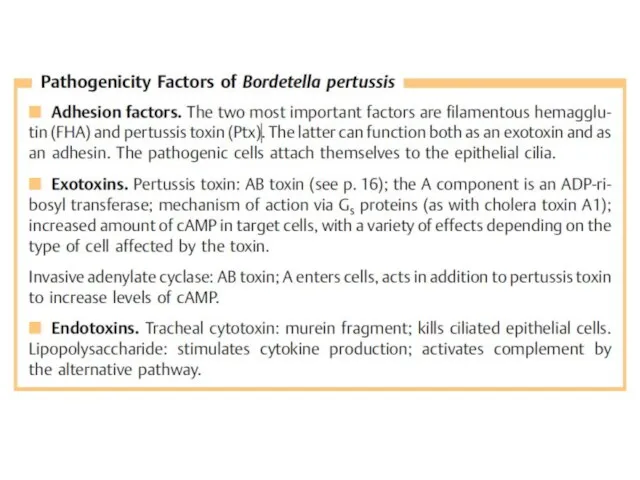
- 8. The genus Bordetella, among others, includes the species B. pertussis, B. parapertussis, and B. bronchiseptica. Of
- 10. Diagnosis The pathogen can only be isolated and identified during the catarrhal and early paroxysmal phases.
- 11. Therapy. Antibiotic treatment can only be expected to be effective during the catarrhal and early paroxysmal
- 12. Treponema (Syphilis, Yaws, Pinta) Morphology and culture. These organisms are slender bacteria, 0.2 μm wide and
- 13. Left untreated, the disease manifests in several stages: Stage I (primary syphilis). Hard, indolent (painless) lesion,
- 14. Stage III (tertiary or late syphilis). Late gummatous syphilis: manifestations in skin, mucosa, and various organs.
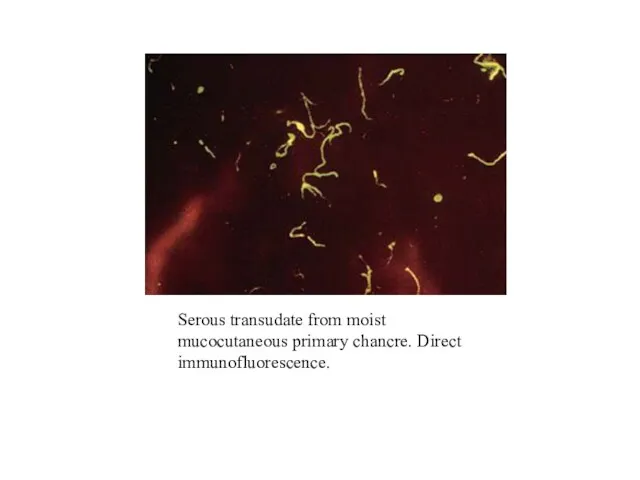
- 15. Serous transudate from moist mucocutaneous primary chancre. Direct immunofluorescence.
- 16. Therapy. Penicillin G is the antibiotic agent of choice. Dosage and duration of therapy depend on
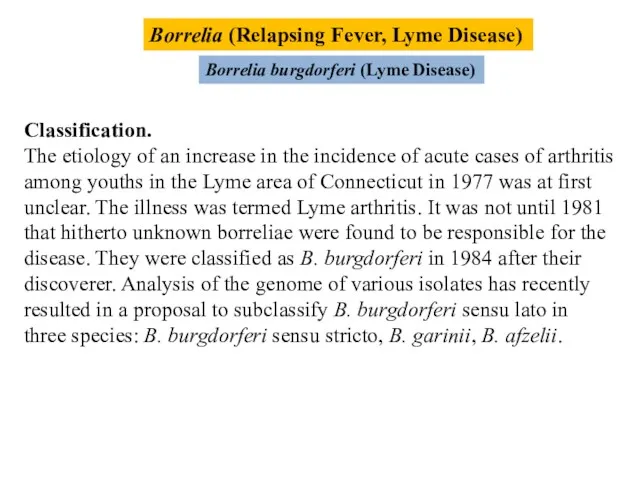
- 17. Borrelia (Relapsing Fever, Lyme Disease) Borrelia burgdorferi (Lyme Disease) Classification. The etiology of an increase in
- 18. Morphology and culture These are thin, flexible, helically wound, highly motile spirochetes. They can be rendered
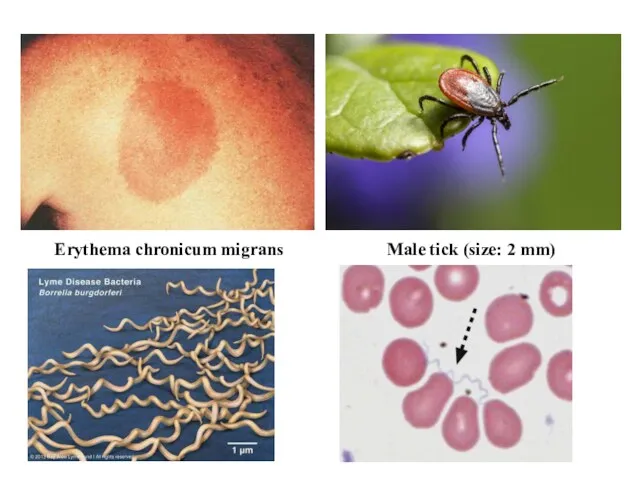
- 19. Erythema chronicum migrans Male tick (size: 2 mm)
- 20. Diagnosis Direct detection and identification of the pathogen by means of microscopy and culturing techniques is
- 21. Epidemiology and prevention Lyme disease occurs throughout the northern hemisphere. There are some endemic foci where
- 22. Leptospira (Leptospirosis, Weil Disease) Classification. Leptospirae belong to the family Leptospiraceae. The genus Leptospira comprises two
- 23. Serogroup icterohemorrhagiae. Culture preparation. Dark field microscopy.
- 24. Pathogenesis and clinical picture. Leptospirae invade the human organism through microinjuries in the skin or the
- 25. Diagnosis Detection and identification of leptospirae are accomplished by growing the organisms in cultures. Blood, cerebrospinal
- 26. Therapy. The agent of choice is penicillin G. Epidemiology and prevention. Leptospiroses are typical zoonotic infections.
- 27. Rickettsia, Coxiella, Orientia, and Ehrlichia (Typhus, Spotted Fever, Q Fever, Ehrlichioses) The genera of the Rickettsiaceae
- 28. Several species of Ehrlichiaceae cause ehrlichiosis in animals and humans. The method of choice for laboratory
- 29. The bacteria of this group belong to the families Rickettsiaceae (Rickettsia and Orientia), Coxelliaceae (Coxiella), and
- 30. Pathogenesis and clinical pictures. With the exception of C. burnetii, the organisms are transmitted by arthropods.
- 31. Diagnosis Direct detection and identification of these organisms in cell cultures, embryonated hen eggs, or experimental
- 32. Epidemiology and prevention The epidemic form of typhus, and earlier scourge of eastern Europe and Russia
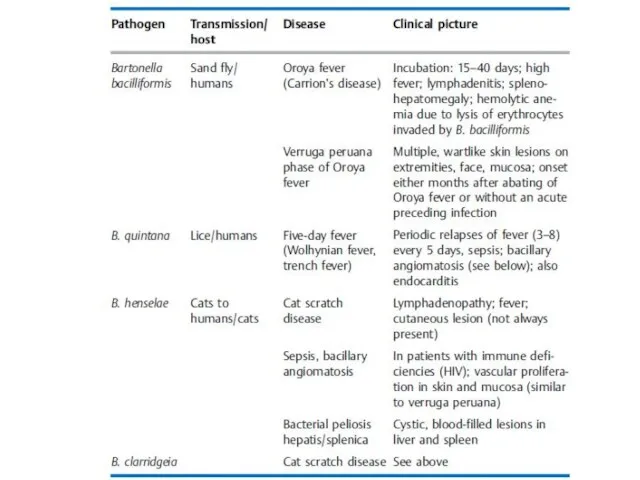
- 33. Bartonella Classification. The genus Bartonella includes, among others, the species B. bacilliformis, B. quintana, B. henselae,
- 35. Diagnosis. Special staining techniques are used to render bartonellae visible under the microscope in tissue specimens.
- 36. Chlamydia Chlamydiae are obligate cell parasites. They go through two stages in their reproductive cycle: the
- 37. The bacteria in the taxonomic family Chlamydiaceae are small (0.3–1 μm) obligate cell parasites with a
- 38. Two morphologically and functionally distinct forms are known Elementary bodies. The round to oval, optically dense
- 39. Chlamydia psittaci (Ornithosis, Psittacosis) Pathogenesis and clinical picture The natural hosts of C. psittaci are birds.
- 40. Diagnosis. The pathogen can be grown from sputum in special cell cultures. Direct detection in the
- 41. Chlamydia trachomatis (Trachoma, Lymphogranuloma venereum) C. trachomatis is a pathogen that infects only humans. Trachoma is
- 42. Lymphogranuloma venereum. This venereal disease (syn. Lymphogranuloma inguinale, lymphopathia venerea (Favre-Durand-Nicolas disease) not to be confused
- 43. Chlamydia pneumoniae This new chlamydial species (formerly TWAR chlamydiae) causes infections of the respiratory organs in
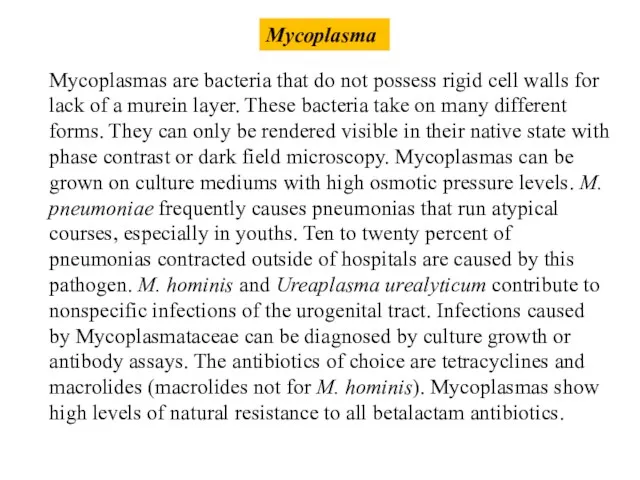
- 44. Mycoplasma Mycoplasmas are bacteria that do not possess rigid cell walls for lack of a murein
- 48. Скачать презентацию



















 Поняття мікроеволюції
Поняття мікроеволюції Дене бітімінің (конституция) жіктеу
Дене бітімінің (конституция) жіктеу Классификация овощных культур. Занятие 4
Классификация овощных культур. Занятие 4 Диво-дивное. Любопытные факты о животных
Диво-дивное. Любопытные факты о животных Эволюция растений
Эволюция растений Декоративные растения: отделы папоротниковидные и сосновые. Лекция 04
Декоративные растения: отделы папоротниковидные и сосновые. Лекция 04 Sunflower seeds
Sunflower seeds Клеткалық биотехнология
Клеткалық биотехнология Эндокринная система человека
Эндокринная система человека Медико-биологические основы БЖД
Медико-биологические основы БЖД Устрицы. Хозяйственное значение
Устрицы. Хозяйственное значение Влияние человека на растительный и животный мир
Влияние человека на растительный и животный мир Суринам ұн жемірі
Суринам ұн жемірі Тренинг по борьбе с курением: Скажем сигарете - НЕТ! (Презентация)
Тренинг по борьбе с курением: Скажем сигарете - НЕТ! (Презентация) Годівля робочих коней
Годівля робочих коней Вирусология. Құрылысын, көбеюін, биохимиясын
Вирусология. Құрылысын, көбеюін, биохимиясын Нейрон и нейроглия. Их виды
Нейрон и нейроглия. Их виды Сердце, ветви дуги аорты. Сосуды головы и шеи
Сердце, ветви дуги аорты. Сосуды головы и шеи Тип Кишечнополостные
Тип Кишечнополостные Биологиялық үрдістер термодинамикасы
Биологиялық үрдістер термодинамикасы Газообмен в легких и тканях
Газообмен в легких и тканях NGS - Секвенирование нового поколения
NGS - Секвенирование нового поколения Своя игра
Своя игра Технология возделывания гречихи
Технология возделывания гречихи Биоинженерия. Поведение брома при атаке его молекулами хрома
Биоинженерия. Поведение брома при атаке его молекулами хрома Систематическое положение
Систематическое положение презентация к уроку биологии общая характеристика царства растений 7 кл
презентация к уроку биологии общая характеристика царства растений 7 кл Биотехнология. Введение в курс
Биотехнология. Введение в курс