Содержание
- 2. Overview: Carbon: The Backbone of Life Living organisms consist mostly of carbon-based compounds Carbon is unparalleled
- 3. Figure 4.1
- 4. Concept 4.1: Organic chemistry is the study of carbon compounds Organic chemistry is the study of
- 5. Vitalism, the idea that organic compounds arise only in organisms, was disproved when chemists synthesized these
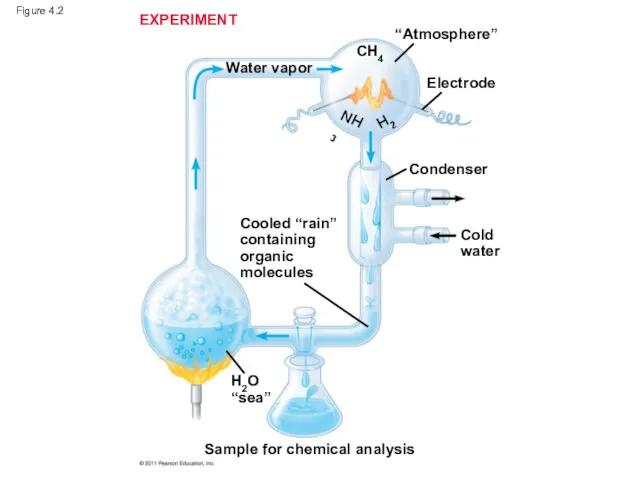
- 6. Organic Molecules and the Origin of Life on Earth Stanley Miller’s classic experiment demonstrated the abiotic
- 7. Figure 4.2 EXPERIMENT “Atmosphere” Electrode Condenser CH4 H2 NH3 Water vapor Cooled “rain” containing organic molecules
- 8. Concept 4.2: Carbon atoms can form diverse molecules by bonding to four other atoms Electron configuration
- 9. The Formation of Bonds with Carbon With four valence electrons, carbon can form four covalent bonds
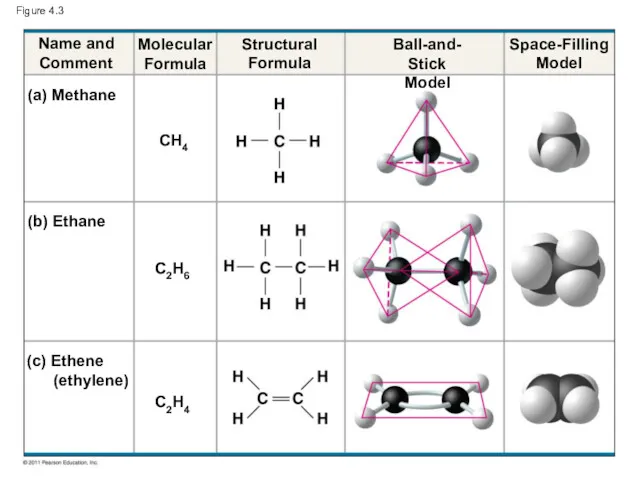
- 10. Figure 4.3 Name and Comment Molecular Formula (a) Methane (b) Ethane CH4 Ball-and- Stick Model Space-Filling
- 11. The electron configuration of carbon gives it covalent compatibility with many different elements The valences of
- 12. Figure 4.4 Hydrogen (valence = 1) Oxygen (valence = 2) Nitrogen (valence = 3) Carbon (valence
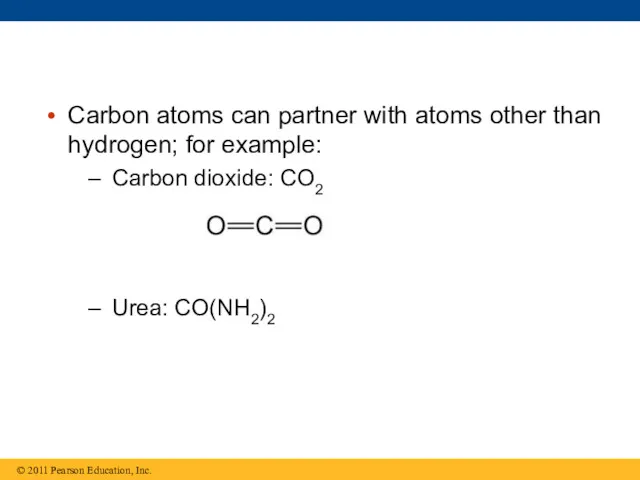
- 13. Carbon atoms can partner with atoms other than hydrogen; for example: Carbon dioxide: CO2 Urea: CO(NH2)2
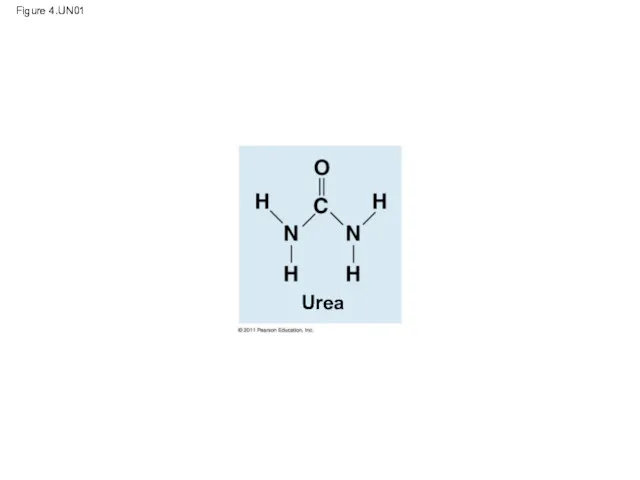
- 14. Figure 4.UN01 Urea
- 15. Molecular Diversity Arising from Carbon Skeleton Variation Carbon chains form the skeletons of most organic molecules
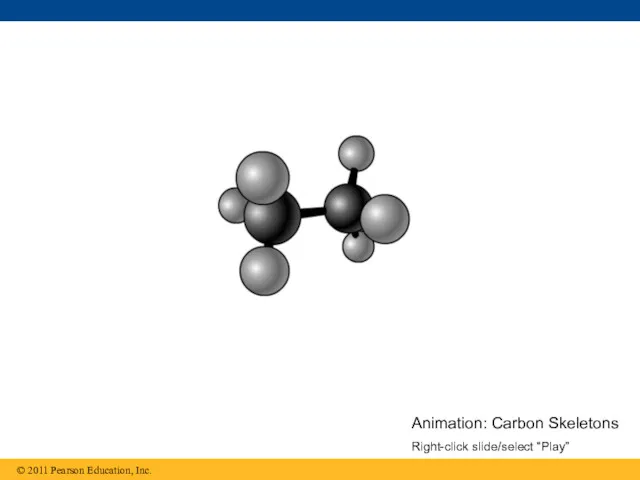
- 16. Animation: Carbon Skeletons Right-click slide/select “Play”
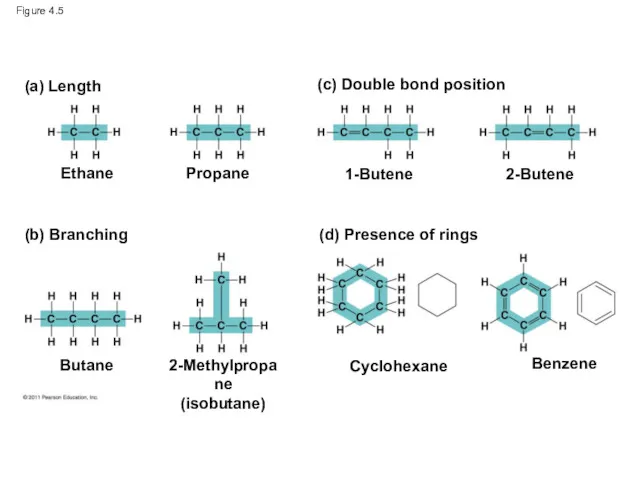
- 17. Figure 4.5 (a) Length Ethane 1-Butene (c) Double bond position 2-Butene Propane (b) Branching (d) Presence
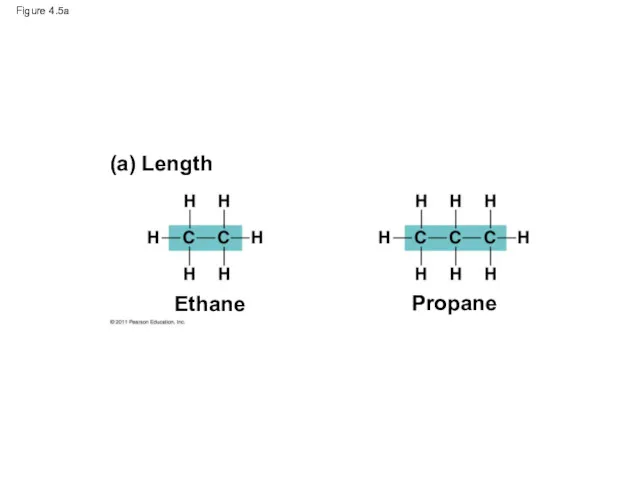
- 18. Figure 4.5a (a) Length Ethane Propane
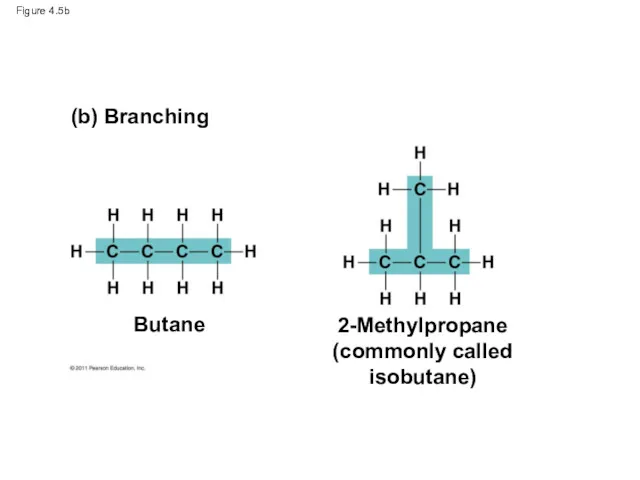
- 19. Figure 4.5b (b) Branching Butane 2-Methylpropane (commonly called isobutane)
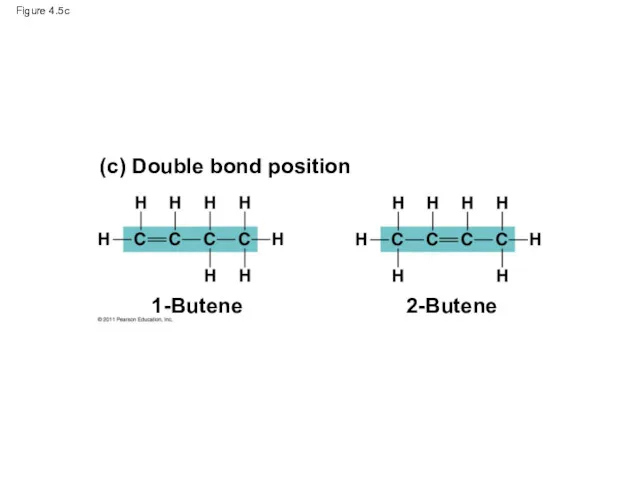
- 20. Figure 4.5c 1-Butene (c) Double bond position 2-Butene
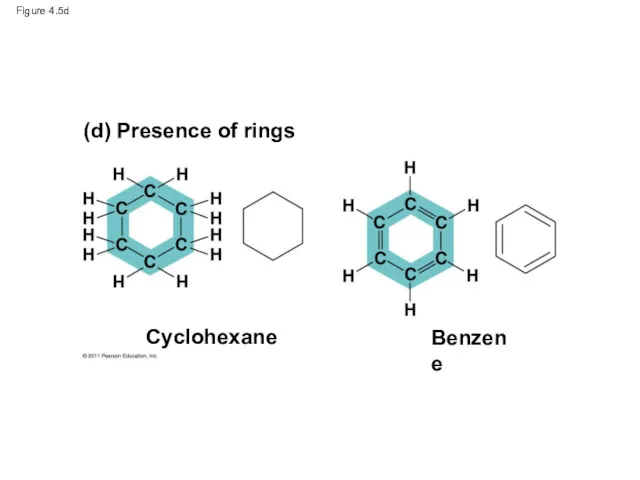
- 21. Figure 4.5d (d) Presence of rings Cyclohexane Benzene
- 22. Hydrocarbons Hydrocarbons are organic molecules consisting of only carbon and hydrogen Many organic molecules, such as
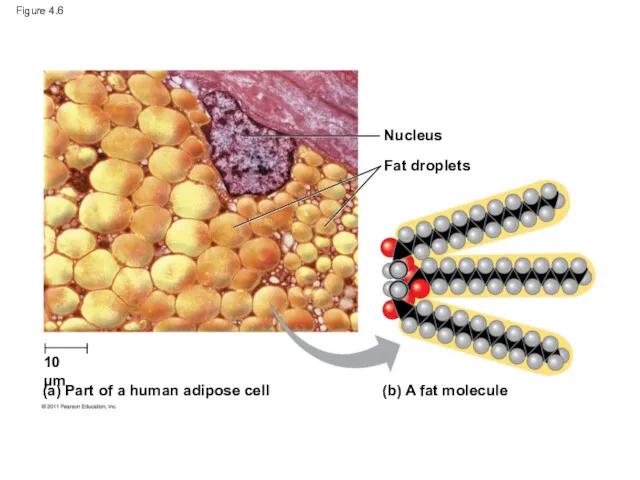
- 23. Figure 4.6 Nucleus Fat droplets (b) A fat molecule (a) Part of a human adipose cell
- 24. Figure 4.6a Nucleus Fat droplets 10 μm
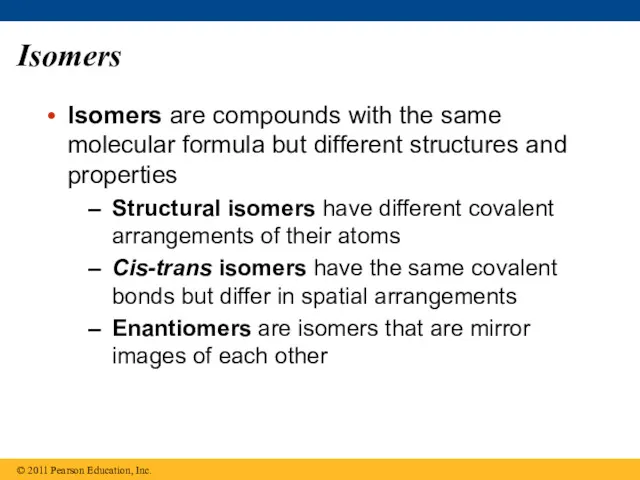
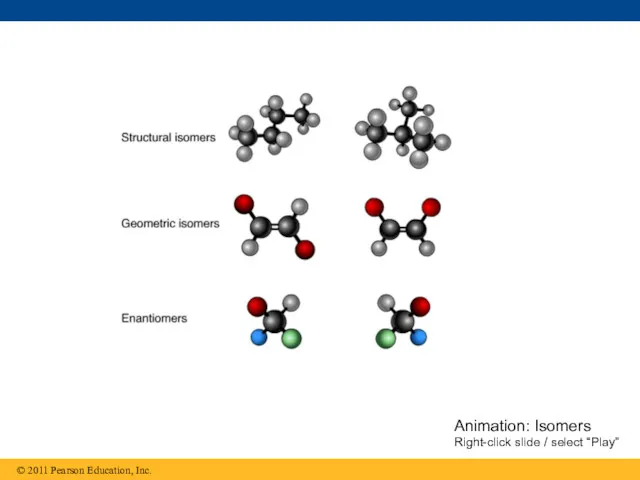
- 25. Isomers Isomers are compounds with the same molecular formula but different structures and properties Structural isomers
- 26. Animation: Isomers Right-click slide / select “Play”
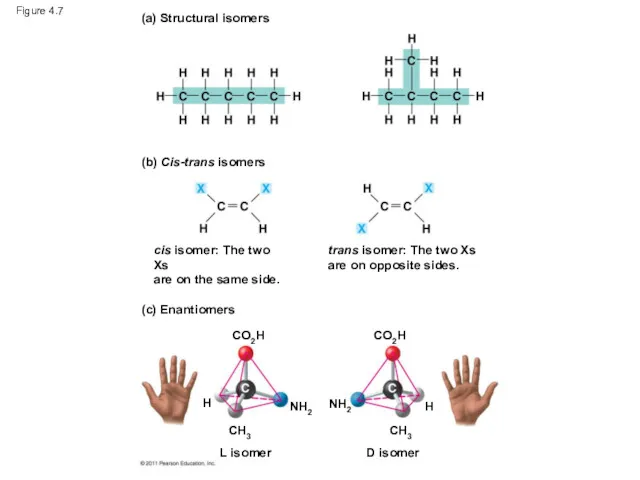
- 27. Figure 4.7 (a) Structural isomers (b) Cis-trans isomers (c) Enantiomers cis isomer: The two Xs are
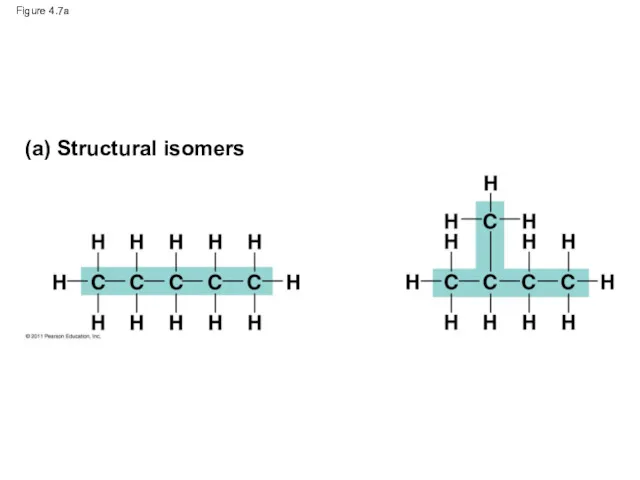
- 28. Figure 4.7a (a) Structural isomers
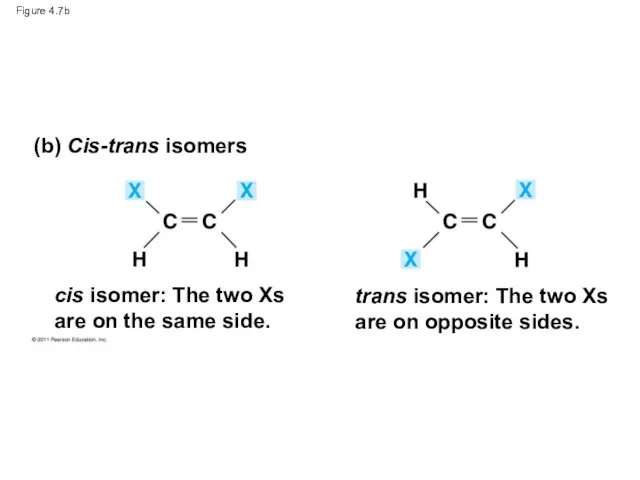
- 29. Figure 4.7b (b) Cis-trans isomers cis isomer: The two Xs are on the same side. trans
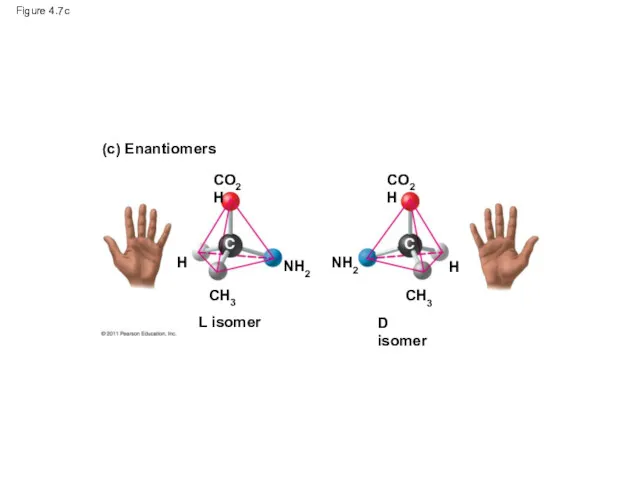
- 30. Figure 4.7c (c) Enantiomers CO2H CO2H CH3 H NH2 L isomer NH2 CH3 H D isomer
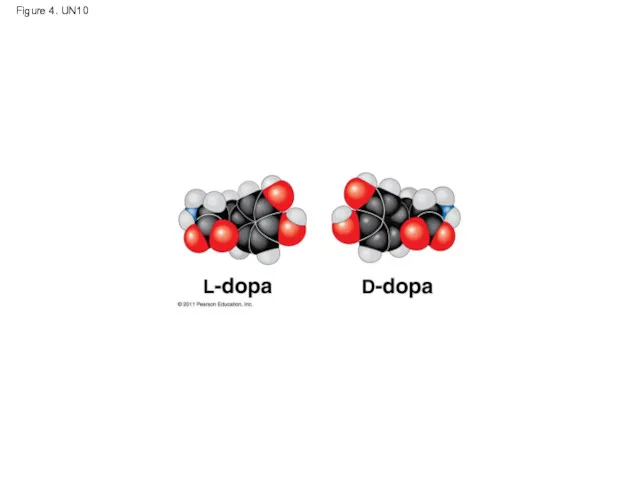
- 31. Enantiomers are important in the pharmaceutical industry Two enantiomers of a drug may have different effects
- 32. Animation: L-Dopa Right-click slide / select “Play”
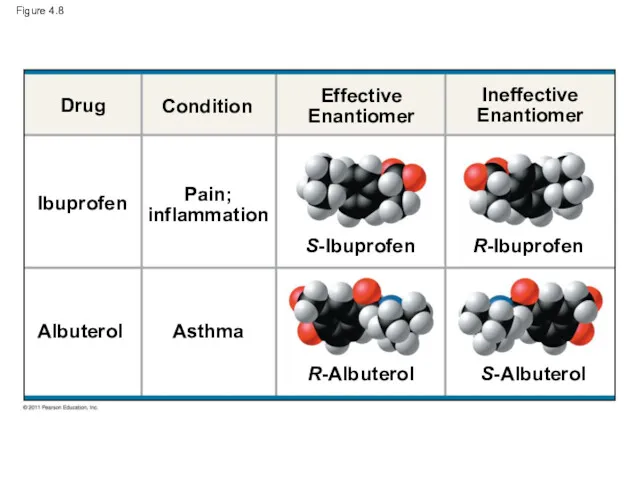
- 33. Figure 4.8 Drug Ibuprofen Albuterol Condition Effective Enantiomer Ineffective Enantiomer Pain; inflammation Asthma S-Ibuprofen R-Ibuprofen R-Albuterol
- 34. Concept 4.3: A few chemical groups are key to the functioning of biological molecules Distinctive properties
- 35. The Chemical Groups Most Important in the Processes of Life Functional groups are the components of
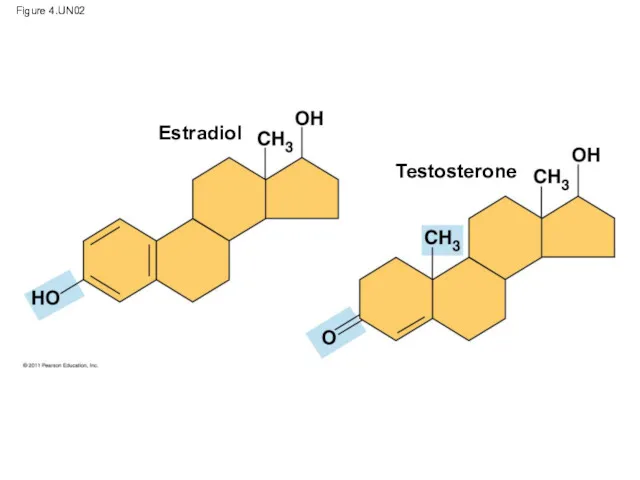
- 36. Figure 4.UN02 Estradiol Testosterone
- 37. The seven functional groups that are most important in the chemistry of life: Hydroxyl group Carbonyl
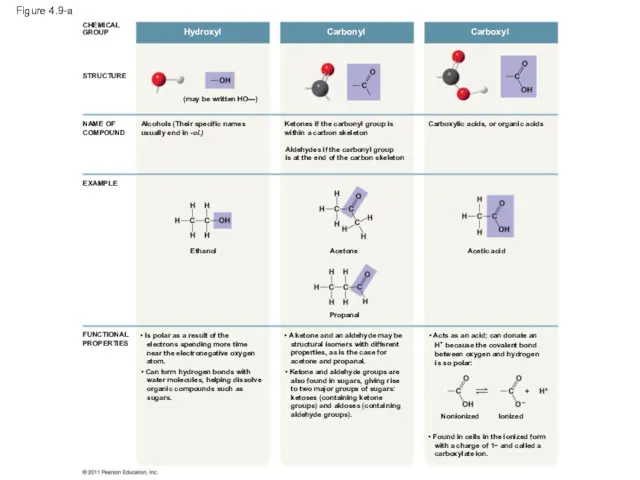
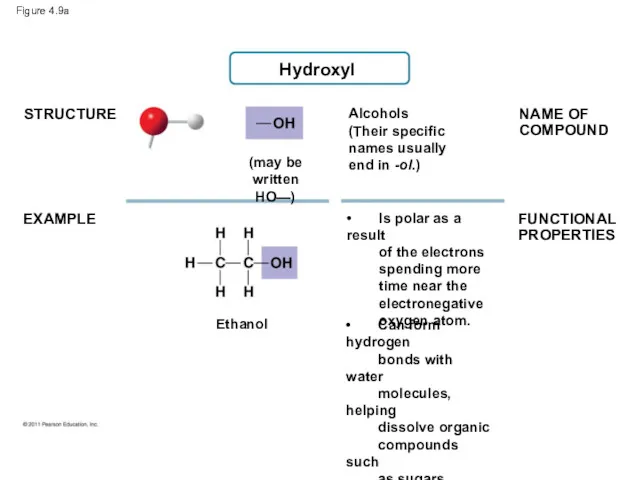
- 38. Figure 4.9-a STRUCTURE CHEMICAL GROUP Hydroxyl NAME OF COMPOUND EXAMPLE Ethanol Alcohols (Their specific names usually
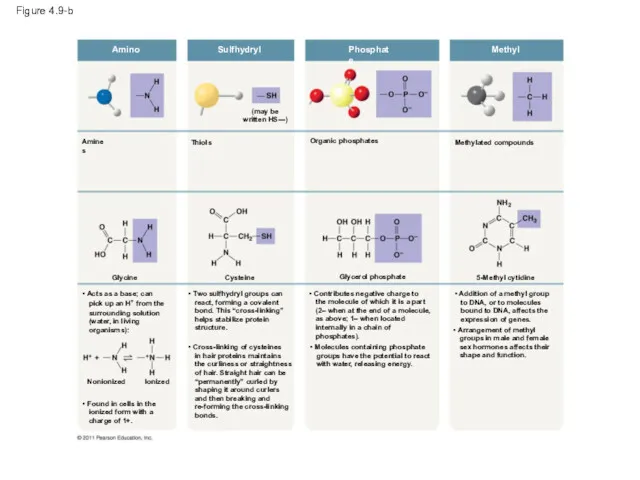
- 39. Figure 4.9-b Amino Sulfhydryl Phosphate Methyl Methylated compounds Organic phosphates (may be written HS—) Thiols Amines
- 40. Figure 4.9a STRUCTURE EXAMPLE Alcohols (Their specific names usually end in -ol.) NAME OF COMPOUND FUNCTIONAL
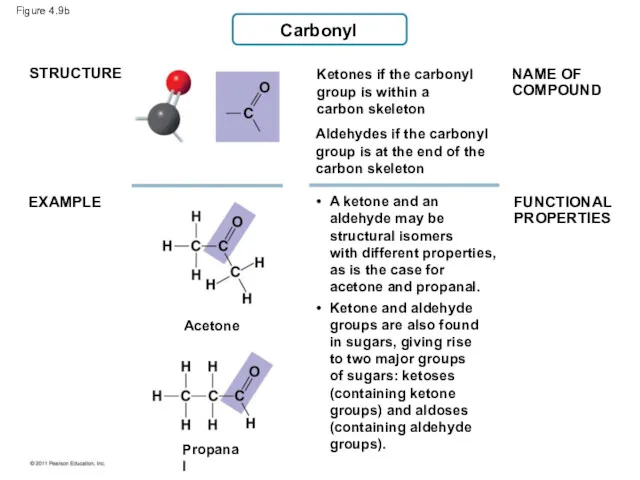
- 41. Figure 4.9b Carbonyl STRUCTURE EXAMPLE Ketones if the carbonyl group is within a carbon skeleton NAME
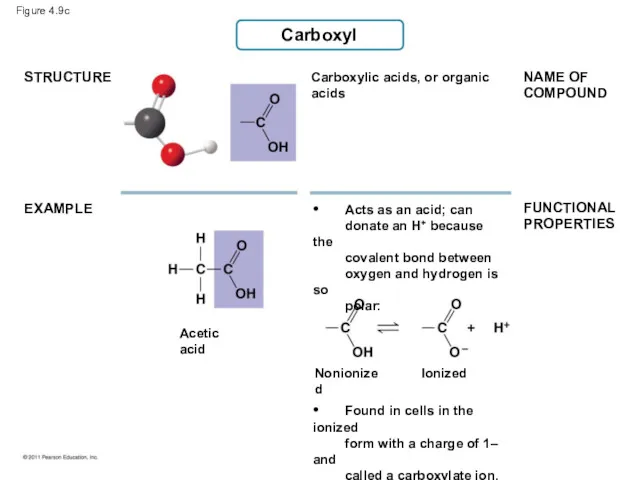
- 42. Carboxyl STRUCTURE EXAMPLE Carboxylic acids, or organic acids NAME OF COMPOUND FUNCTIONAL PROPERTIES Acetic acid •
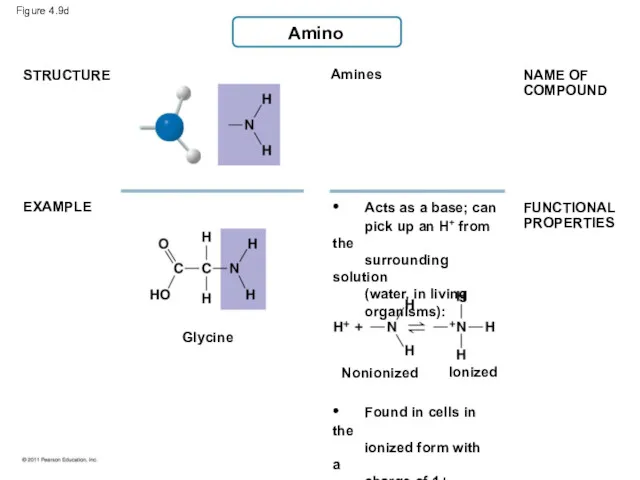
- 43. Amino Amines Glycine STRUCTURE EXAMPLE • Acts as a base; can pick up an H+ from
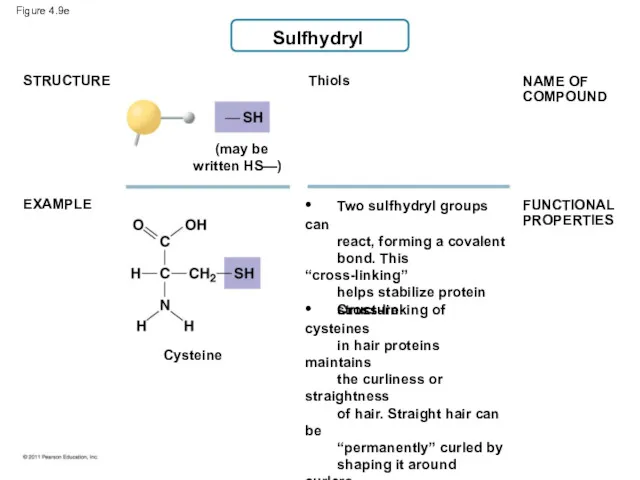
- 44. Sulfhydryl Thiols (may be written HS—) STRUCTURE EXAMPLE • Two sulfhydryl groups can react, forming a
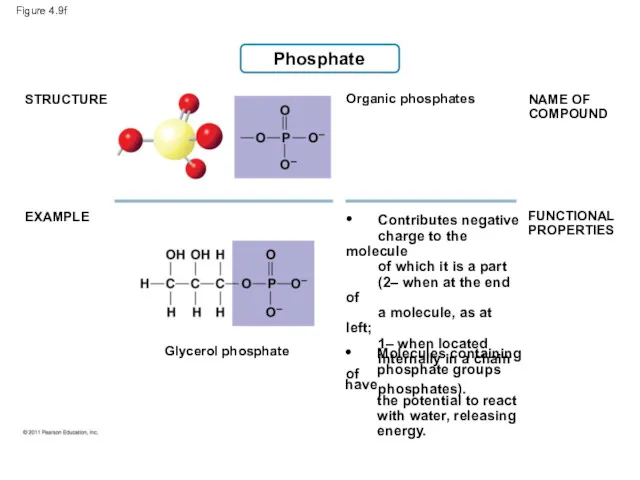
- 45. Figure 4.9f Phosphate STRUCTURE EXAMPLE NAME OF COMPOUND FUNCTIONAL PROPERTIES Organic phosphates Glycerol phosphate • Contributes
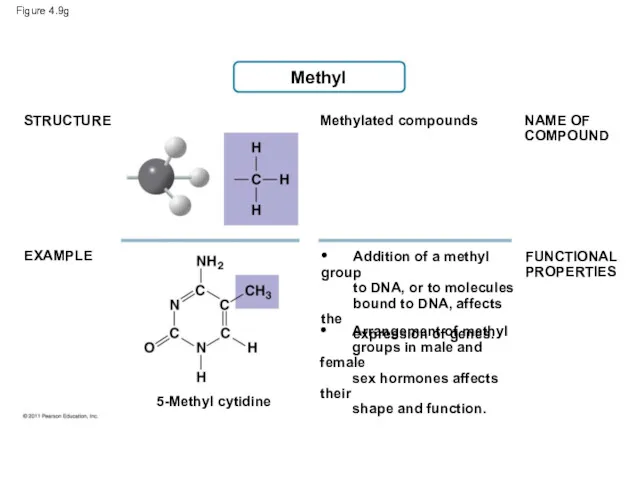
- 46. Figure 4.9g Methyl STRUCTURE EXAMPLE NAME OF COMPOUND FUNCTIONAL PROPERTIES Methylated compounds 5-Methyl cytidine • Addition
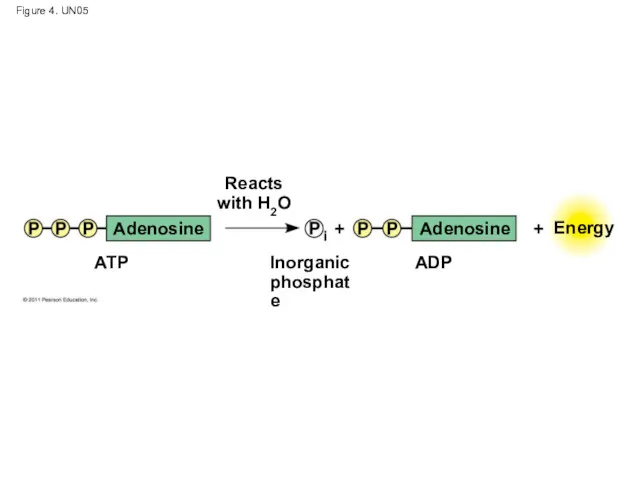
- 47. ATP: An Important Source of Energy for Cellular Processes One phosphate molecule, adenosine triphosphate (ATP), is
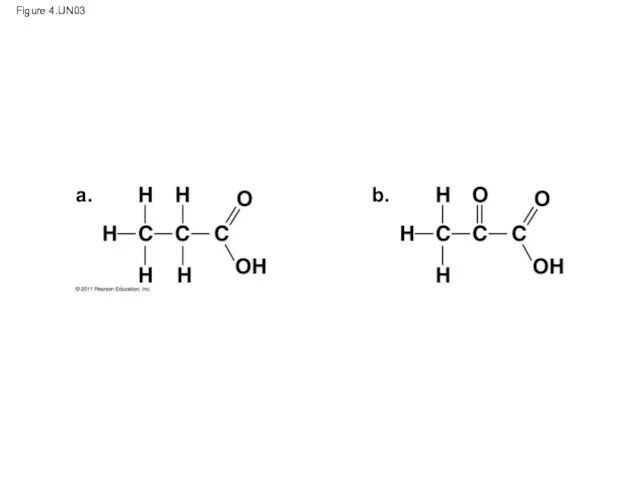
- 48. Figure 4.UN03 a. b.
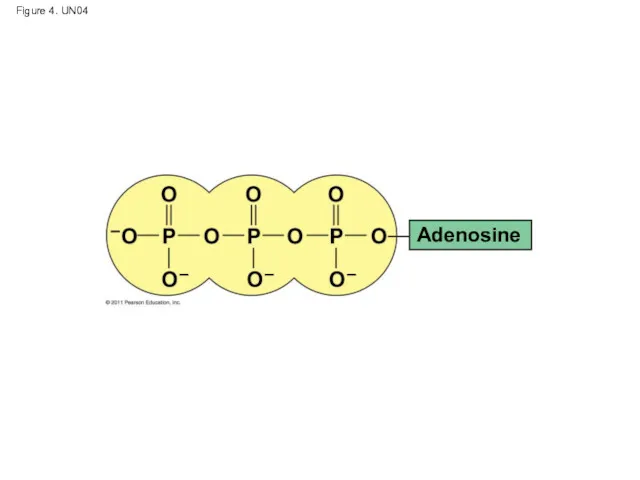
- 49. Figure 4. UN04 Adenosine
- 50. The Chemical Elements of Life: A Review The versatility of carbon makes possible the great diversity
- 51. Figure 4. UN05 Adenosine Adenosine Reacts with H2O Inorganic phosphate ATP ADP Energy
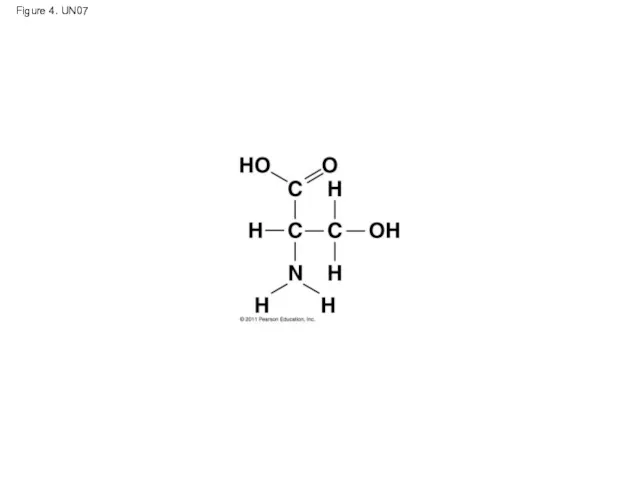
- 52. Figure 4. UN07
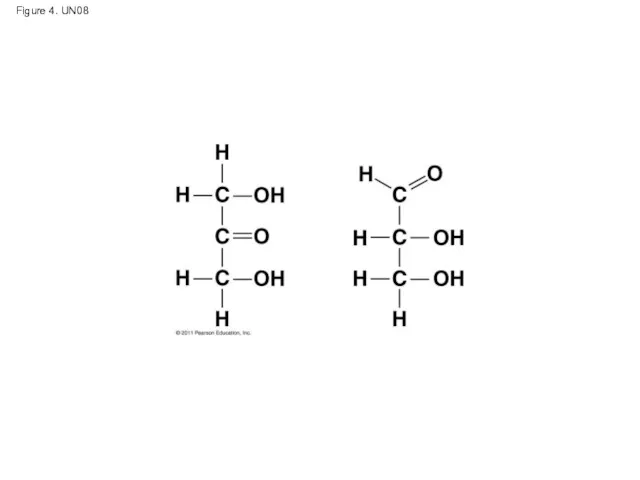
- 53. Figure 4. UN08
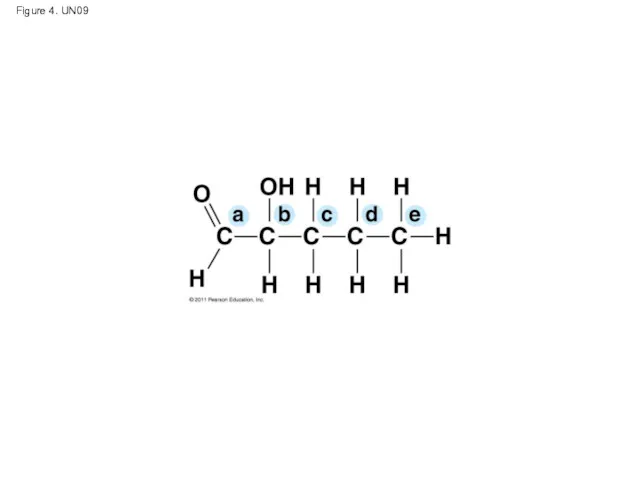
- 54. Figure 4. UN09
- 55. Figure 4. UN10
- 56. Figure 4. UN11
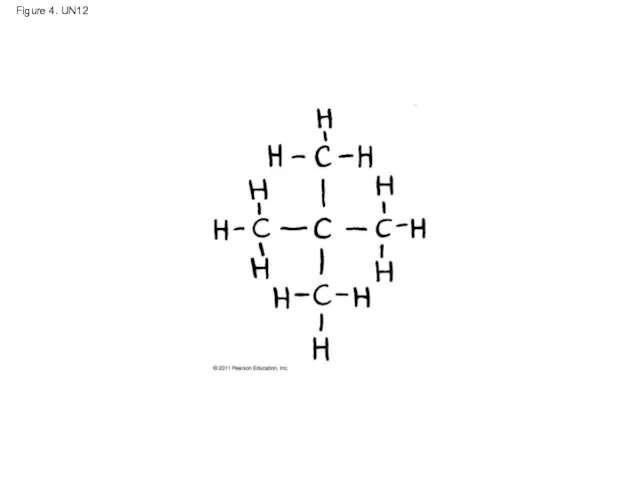
- 57. Figure 4. UN12
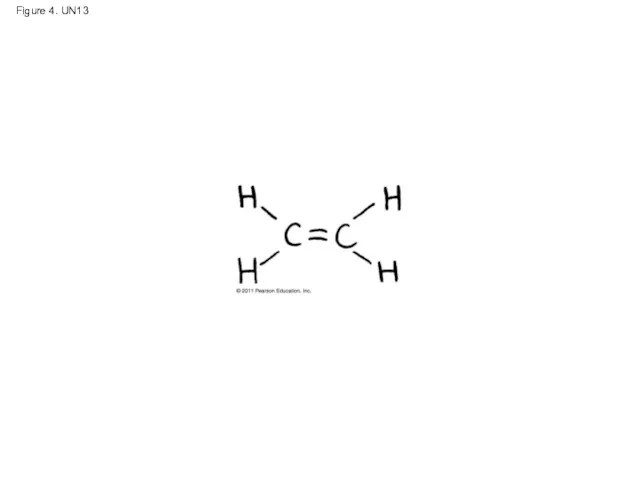
- 58. Figure 4. UN13
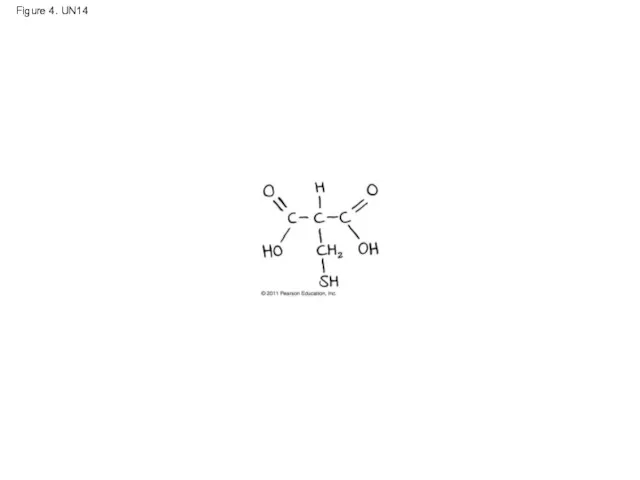
- 59. Figure 4. UN14
- 61. Скачать презентацию










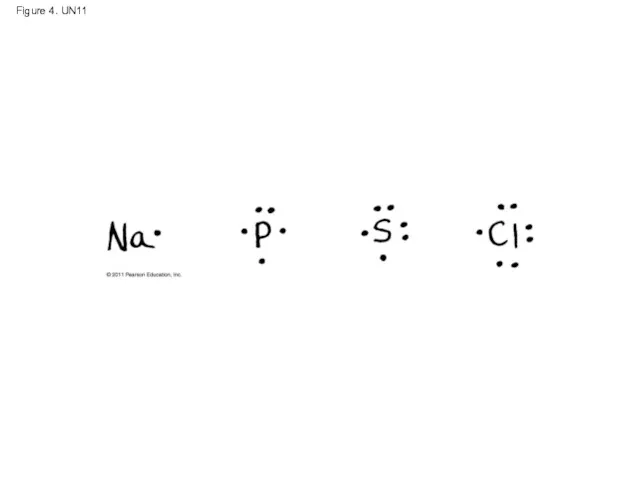
 Почему осенью осыпаются листья
Почему осенью осыпаются листья Среды жизни и места обитания животных. Взаимосвязь животных в природе
Среды жизни и места обитания животных. Взаимосвязь животных в природе Эмбриогенез амфибий
Эмбриогенез амфибий Обобщающий урок – КВН Общее знакомство с цветковыми растениями Клеточное строение растительного организма
Обобщающий урок – КВН Общее знакомство с цветковыми растениями Клеточное строение растительного организма Индивидуальное развитие человека, или онтогенез
Индивидуальное развитие человека, или онтогенез презентация ГМО
презентация ГМО Клеточные. Эукариоты. Царство Животные. Подцарство Позвоночные. Тип Хордовые. Подтип Черепные. Класс Земноводные
Клеточные. Эукариоты. Царство Животные. Подцарство Позвоночные. Тип Хордовые. Подтип Черепные. Класс Земноводные Внешнее строение листа
Внешнее строение листа Органеллы клетки
Органеллы клетки Жизненные циклы растений
Жизненные циклы растений Животные весной
Животные весной Методы микробиологического исследования
Методы микробиологического исследования Анатомия человека
Анатомия человека Грибы-паразиты
Грибы-паразиты Биологическая роль алюминия в организме человека
Биологическая роль алюминия в организме человека Система виділення. Механізми утворення сечі
Система виділення. Механізми утворення сечі Слагаемые биотехнологического производства
Слагаемые биотехнологического производства Эволюция (1 часть)
Эволюция (1 часть) Наследственность и среда. Тема 3
Наследственность и среда. Тема 3 Природные опасности. Ядовитые животные и растения
Природные опасности. Ядовитые животные и растения Механика и энергетика мышц
Механика и энергетика мышц Семейства: Костенцовые, Гиполеписовые, Многоножковые, Сальвиниевые, Ужовниковидные, Сосновые, Кипарисовые, Эфедровые
Семейства: Костенцовые, Гиполеписовые, Многоножковые, Сальвиниевые, Ужовниковидные, Сосновые, Кипарисовые, Эфедровые Пищевые потребности прокариот
Пищевые потребности прокариот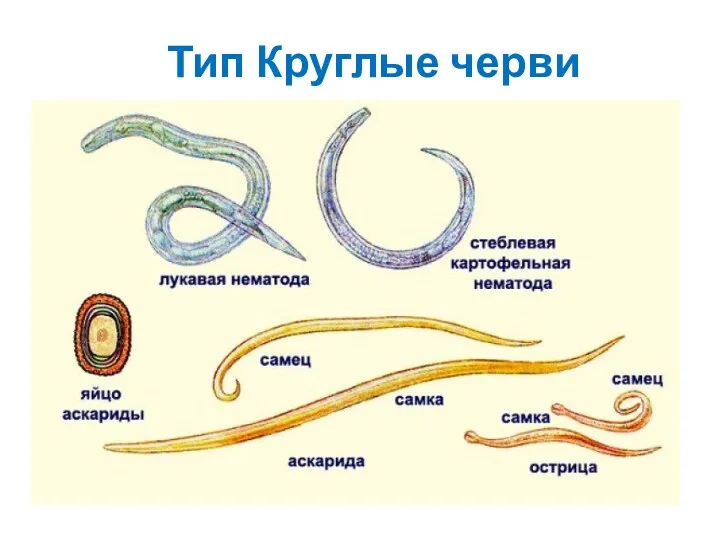 Тип Круглые черви
Тип Круглые черви Разбор заданий ВПР 5 класс
Разбор заданий ВПР 5 класс Мышцы . Лекция 3
Мышцы . Лекция 3 Самые - самые растения
Самые - самые растения Общая биология. Вводная 10 класс
Общая биология. Вводная 10 класс