Содержание
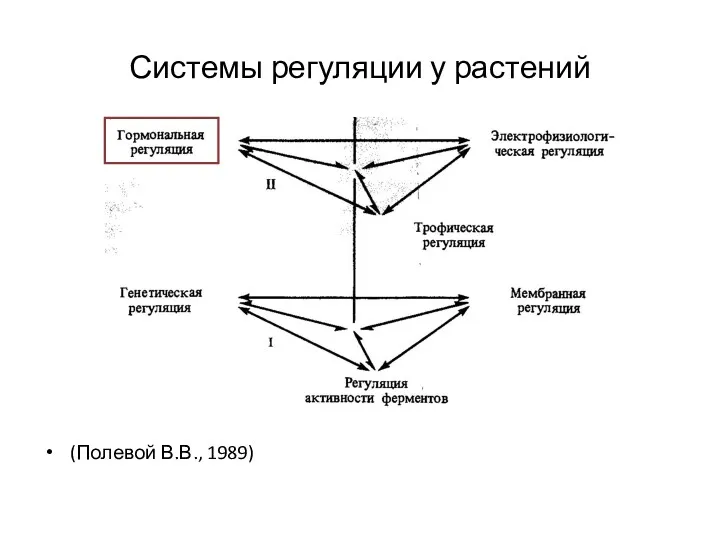
- 2. Системы регуляции у растений (Полевой В.В., 1989)
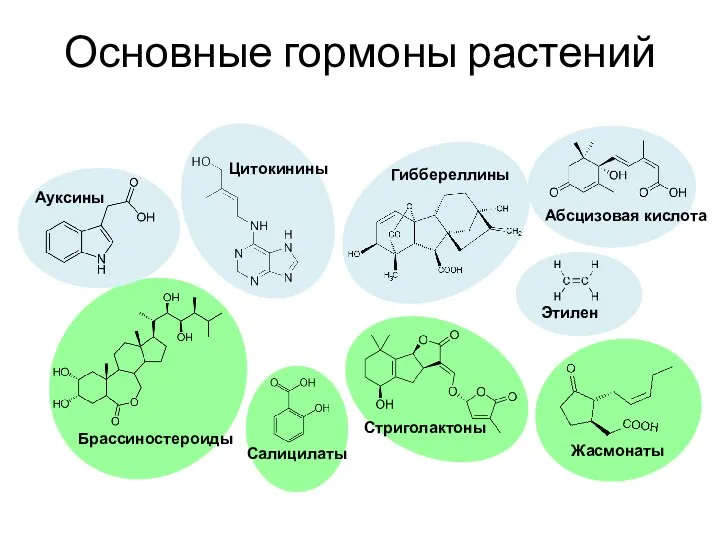
- 3. Основные гормоны растений
- 4. Общие свойства гормонов растений Специфический ответ Наличие специфических рецепторов Концентрации 10-6-10-12 М Мультифункциональность Потенциально могут быть
- 5. Нарушение синтеза некоторых гормонов отражается на росте растений Lester, D.R., Ross, J.J., Davies, P.J., and Reid,
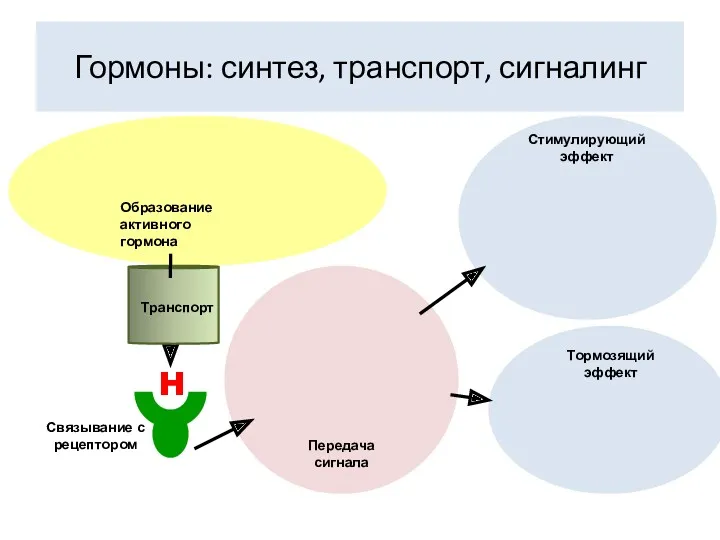
- 6. Гормоны: синтез, транспорт, сигналинг
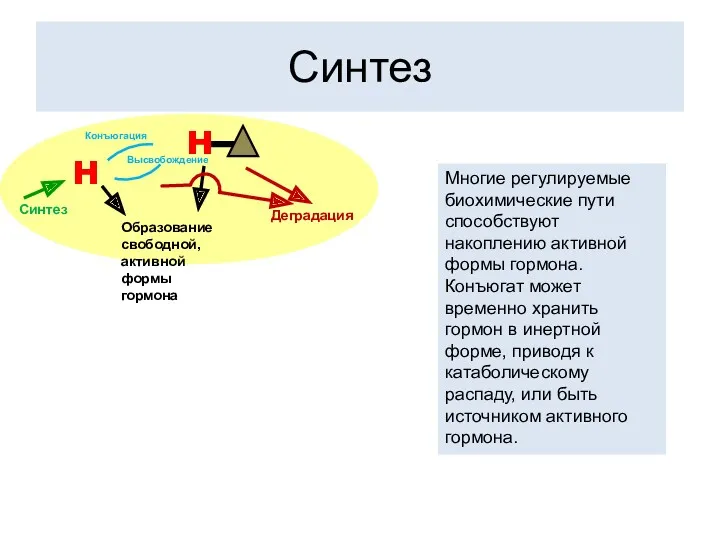
- 7. Синтез Многие регулируемые биохимические пути способствуют накоплению активной формы гормона. Конъюгат может временно хранить гормон в
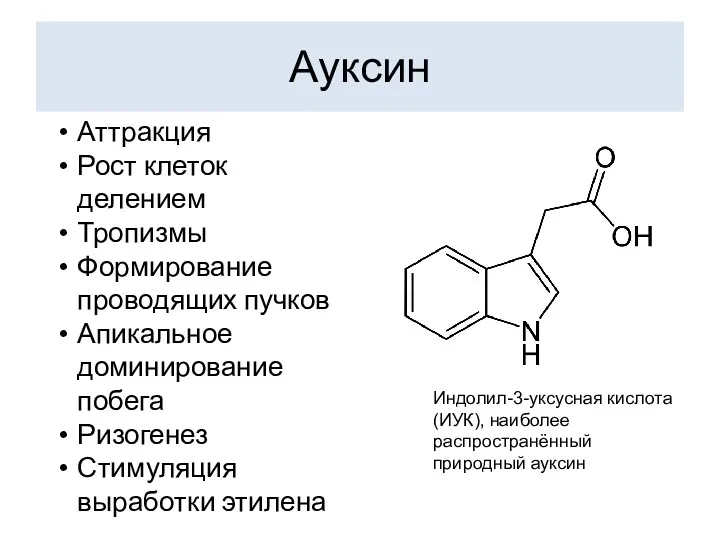
- 8. Ауксин Индолил-3-уксусная кислота (ИУК), наиболее распространённый природный ауксин Аттракция Рост клеток делением Тропизмы Формирование проводящих пучков
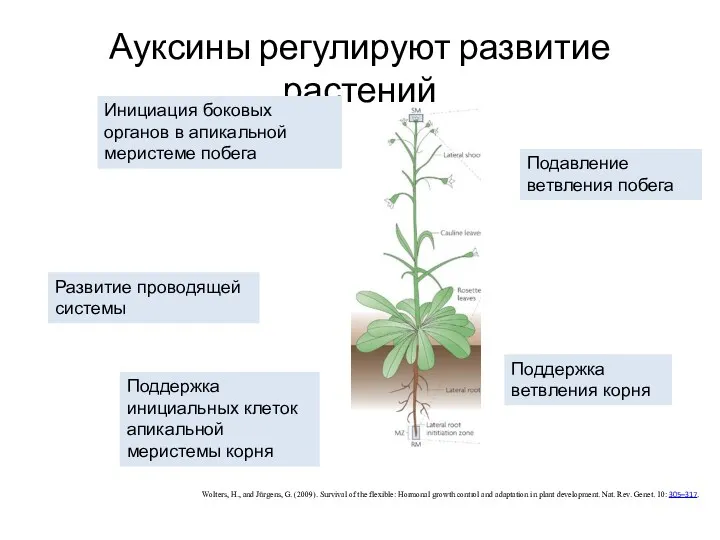
- 9. Ауксины регулируют развитие растений Wolters, H., and Jürgens, G. (2009). Survival of the flexible: Hormonal growth
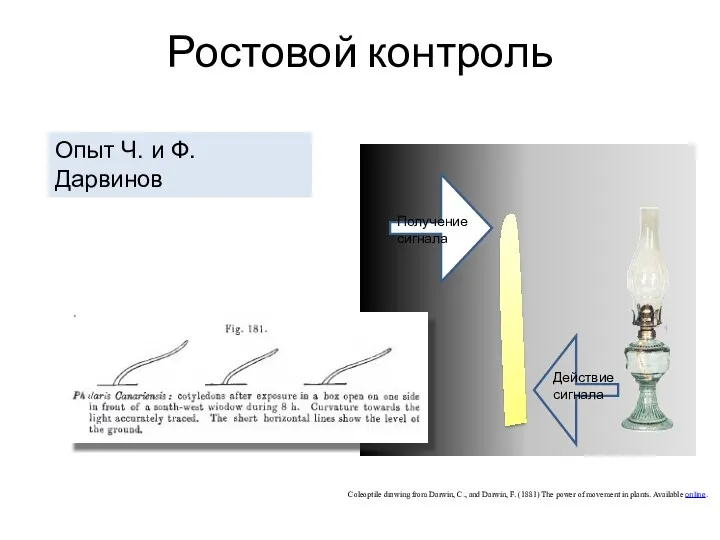
- 10. Ростовой контроль Опыт Ч. и Ф. Дарвинов Coleoptile drawing from Darwin, C., and Darwin, F. (1881)
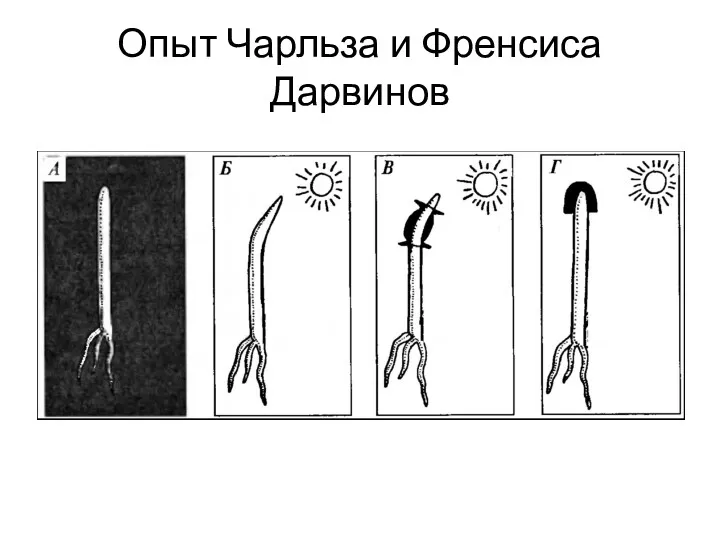
- 11. Опыт Чарльза и Френсиса Дарвинов
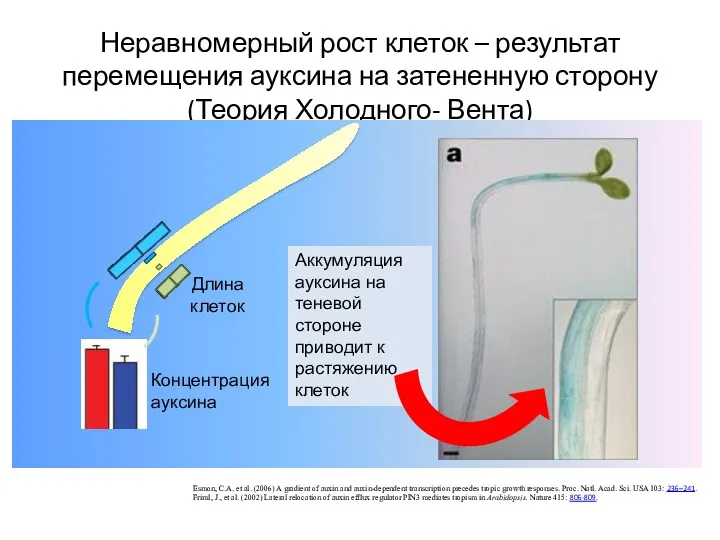
- 12. Неравномерный рост клеток – результат перемещения ауксина на затененную сторону (Теория Холодного- Вента) Esmon, C.A. et
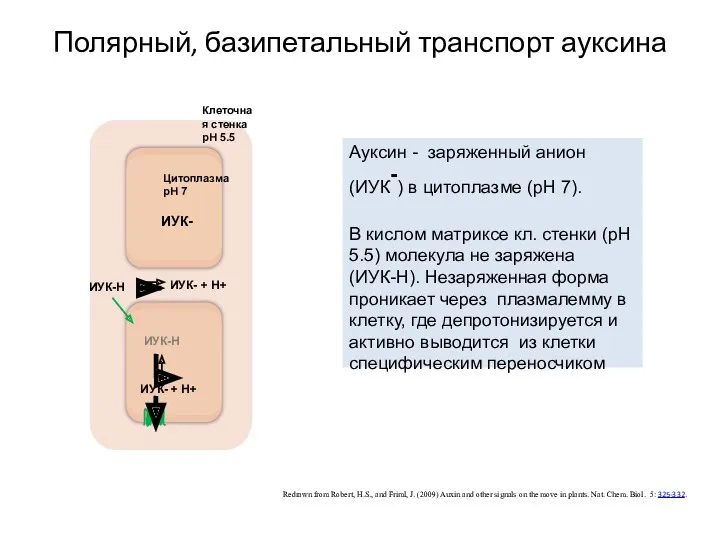
- 13. Полярный, базипетальный транспорт ауксина Redrawn from Robert, H.S., and Friml, J. (2009) Auxin and other signals
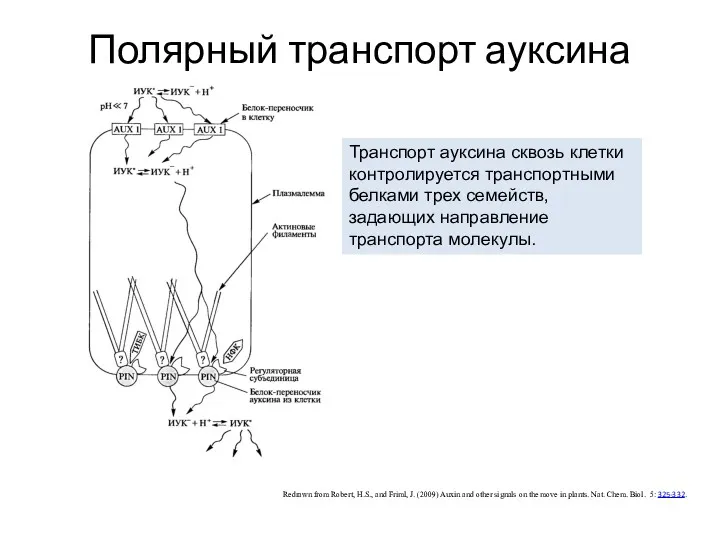
- 14. Полярный транспорт ауксина Redrawn from Robert, H.S., and Friml, J. (2009) Auxin and other signals on
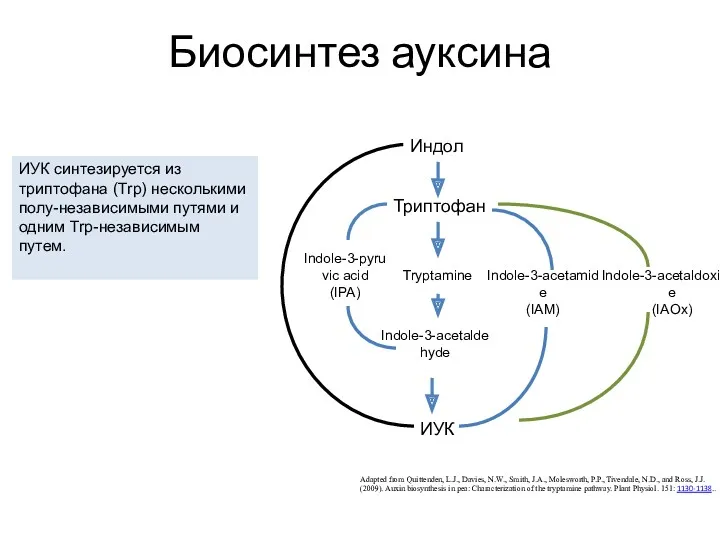
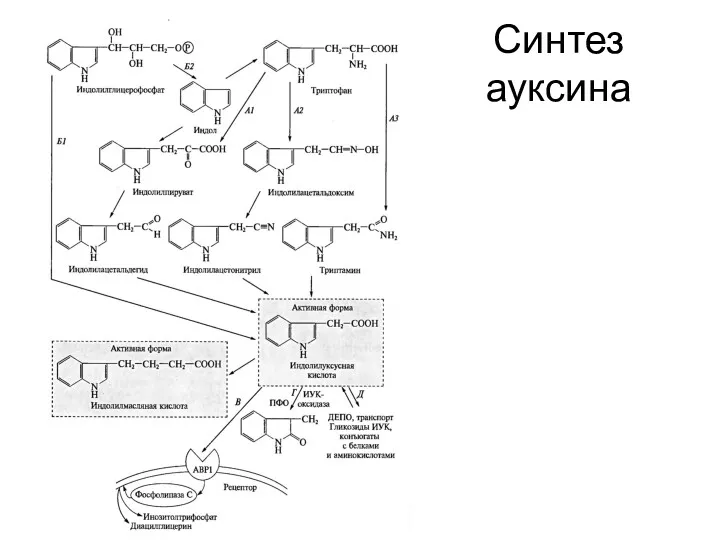
- 15. Биосинтез ауксина Adapted from Quittenden, L.J., Davies, N.W., Smith, J.A., Molesworth, P.P., Tivendale, N.D., and Ross,
- 16. Синтез ауксина
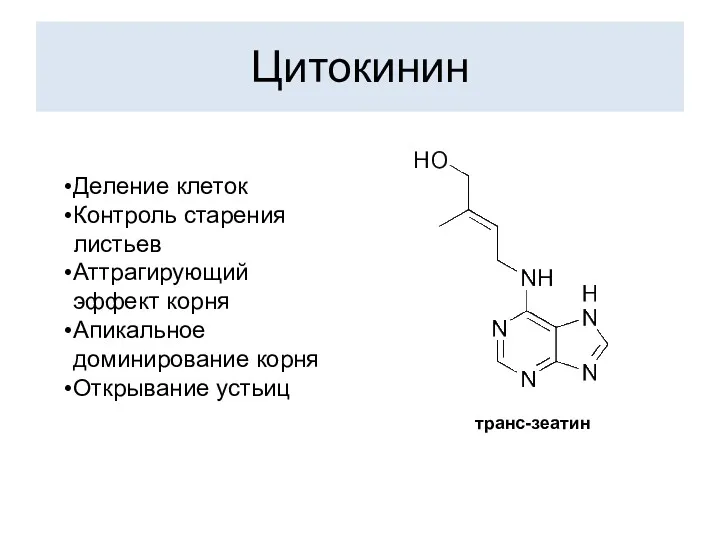
- 17. Цитокинин
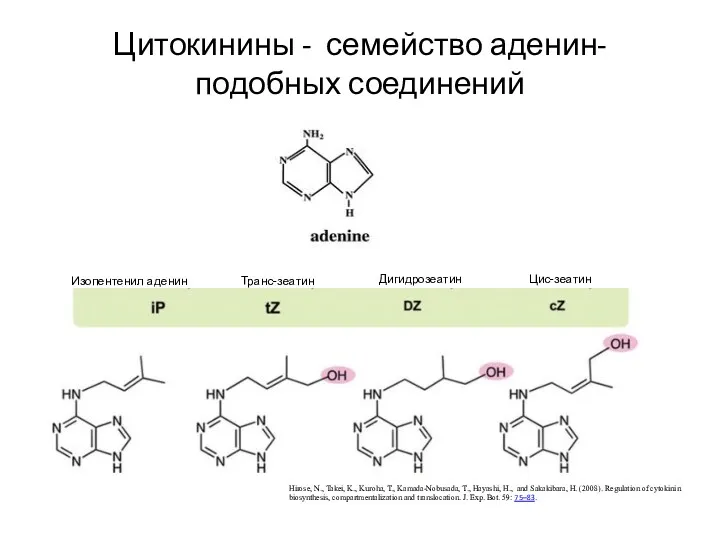
- 18. Цитокинины - семейство аденин-подобных соединений Hirose, N., Takei, K., Kuroha, T., Kamada-Nobusada, T., Hayashi, H., and
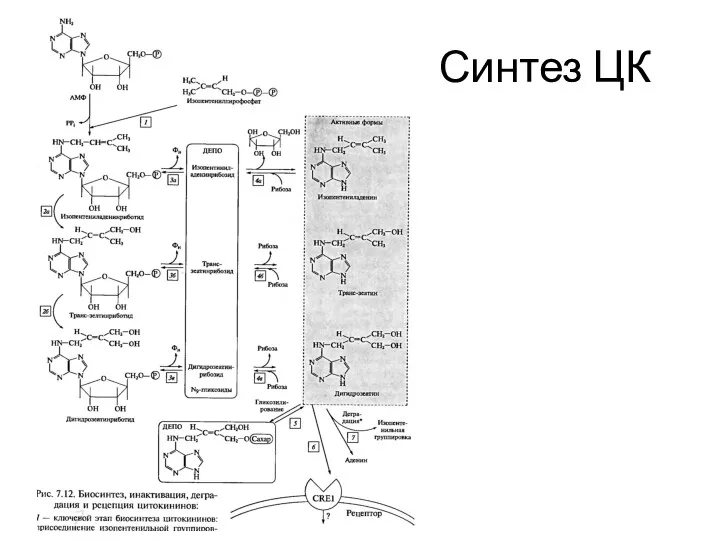
- 19. Синтез ЦК
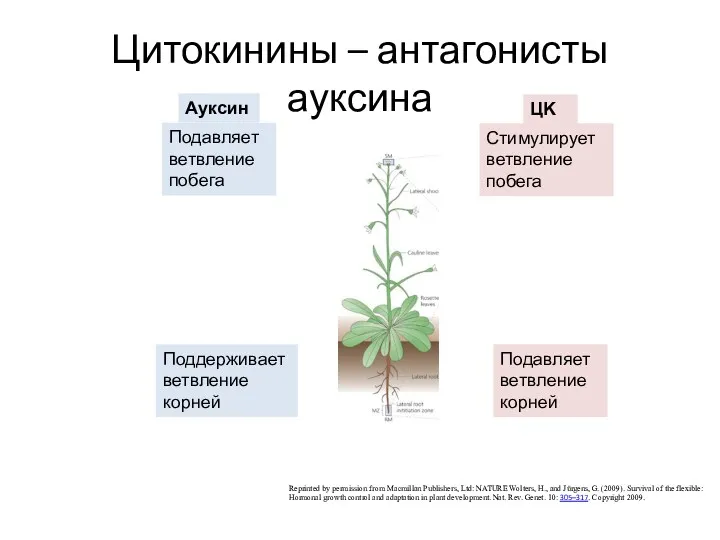
- 20. Цитокинины – антагонисты ауксина Reprinted by permission from Macmillan Publishers, Ltd: NATURE Wolters, H., and Jürgens,
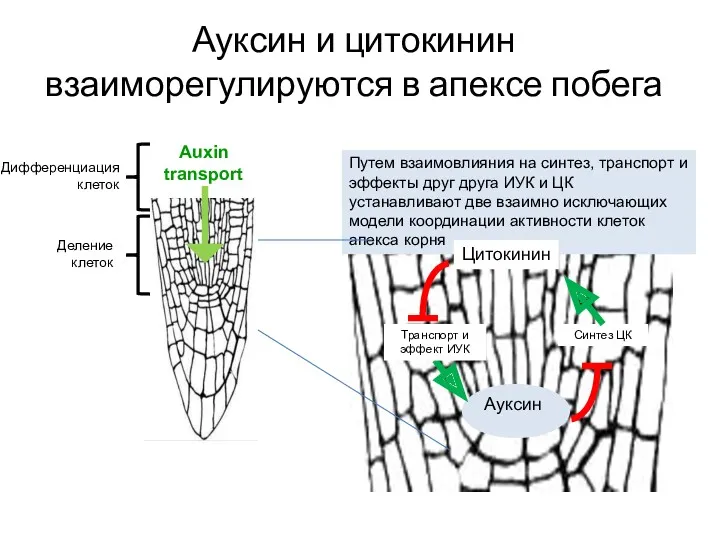
- 21. Ауксин и цитокинин взаиморегулируются в апексе побега
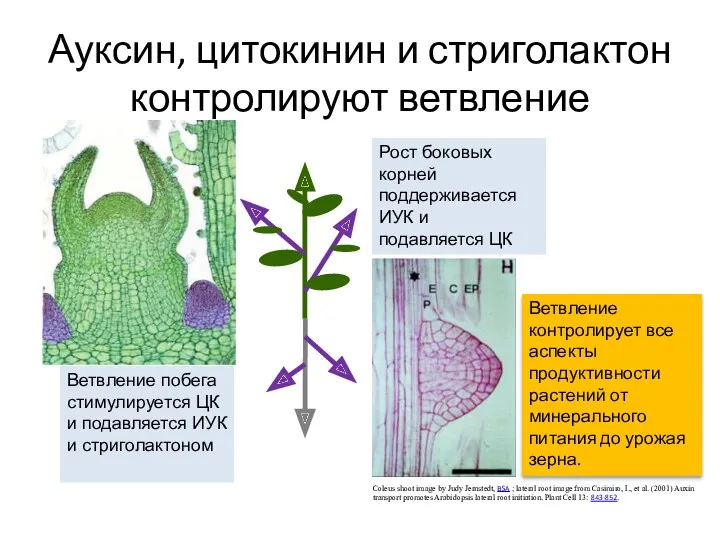
- 22. Ауксин, цитокинин и стриголактон контролируют ветвление Coleus shoot image by Judy Jernstedt, BSA ; lateral root
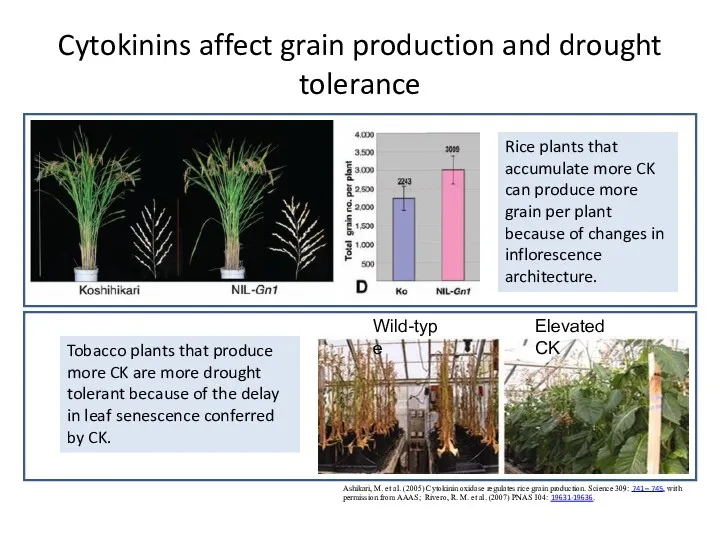
- 23. Cytokinins affect grain production and drought tolerance Ashikari, M. et al. (2005) Cytokinin oxidase regulates rice
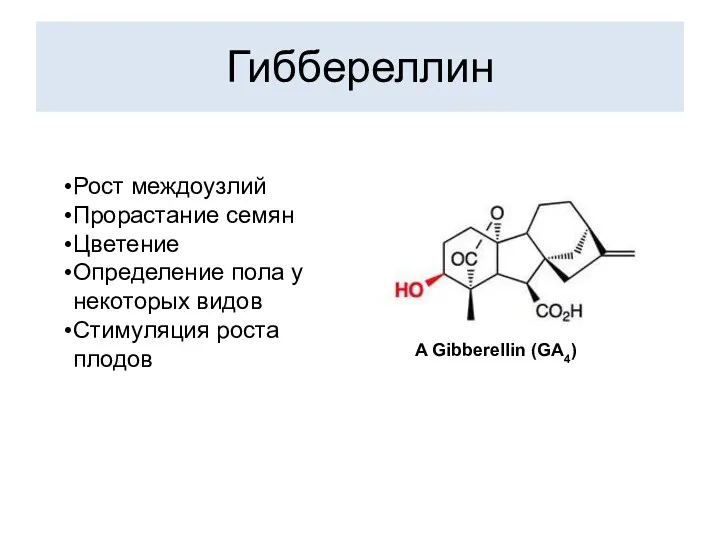
- 24. Гиббереллин
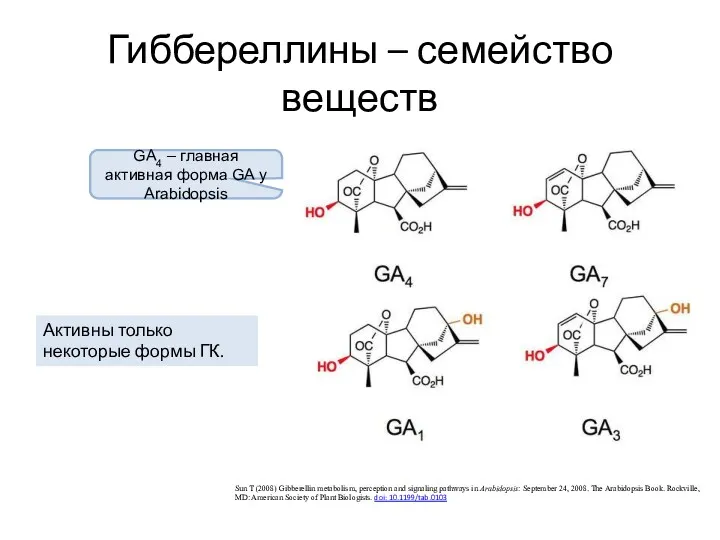
- 25. Гиббереллины – семейство веществ Sun T (2008) Gibberellin metabolism, perception and signaling pathways in Arabidopsis: September
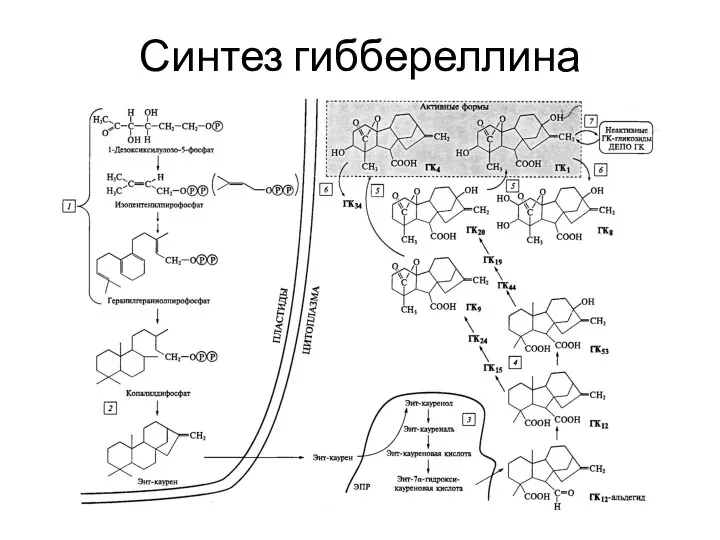
- 26. Синтез гиббереллина
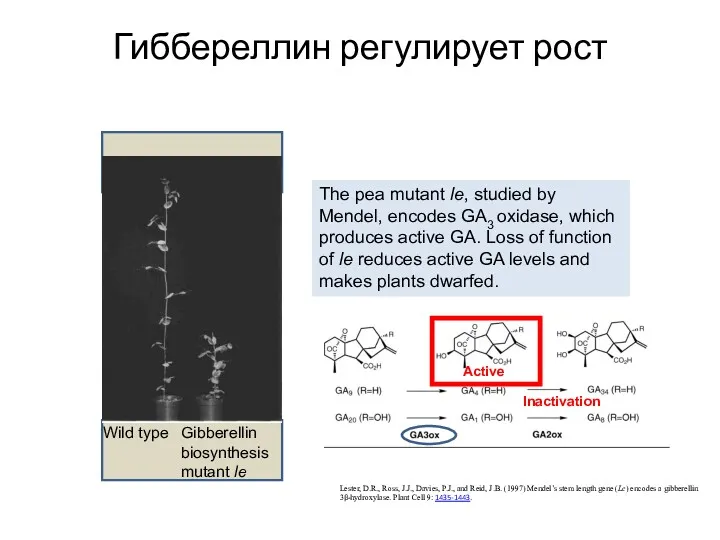
- 27. Гиббереллин регулирует рост Lester, D.R., Ross, J.J., Davies, P.J., and Reid, J.B. (1997) Mendel’s stem length
- 28. Гены, контролирующие синтез ГК оказались важны для «зеленой революции» Photos courtesy of S. Harrison, LSU Ag
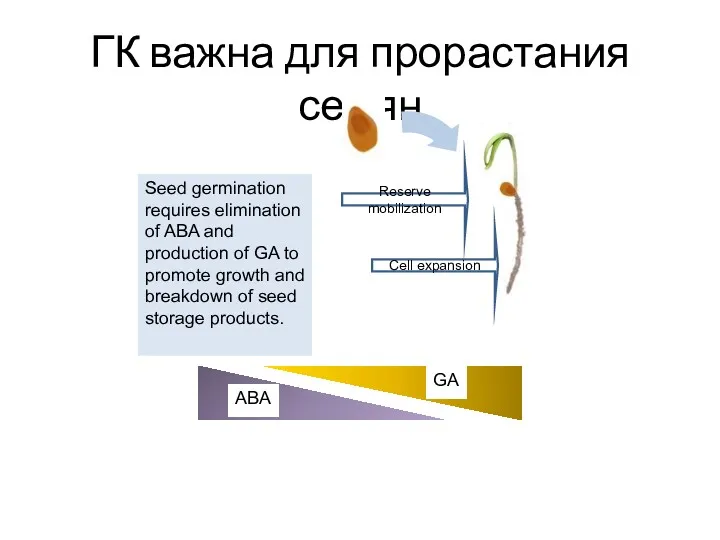
- 29. ГК важна для прорастания семян
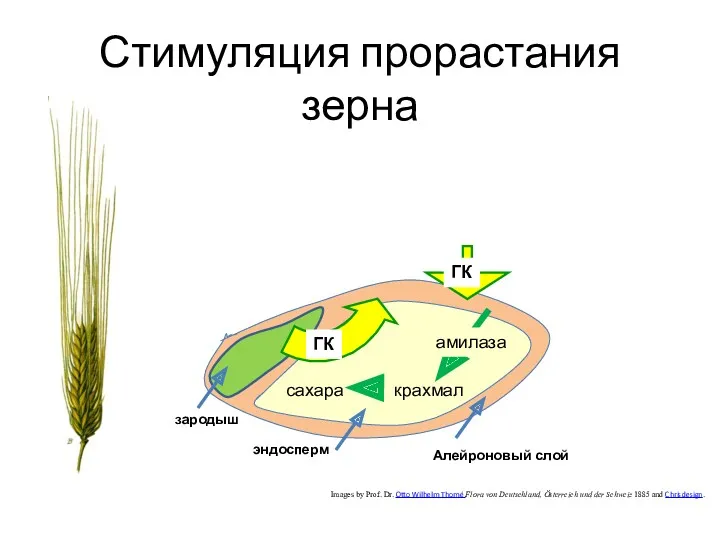
- 30. Стимуляция прорастания зерна Images by Prof. Dr. Otto Wilhelm Thomé Flora von Deutschland, Österreich und der
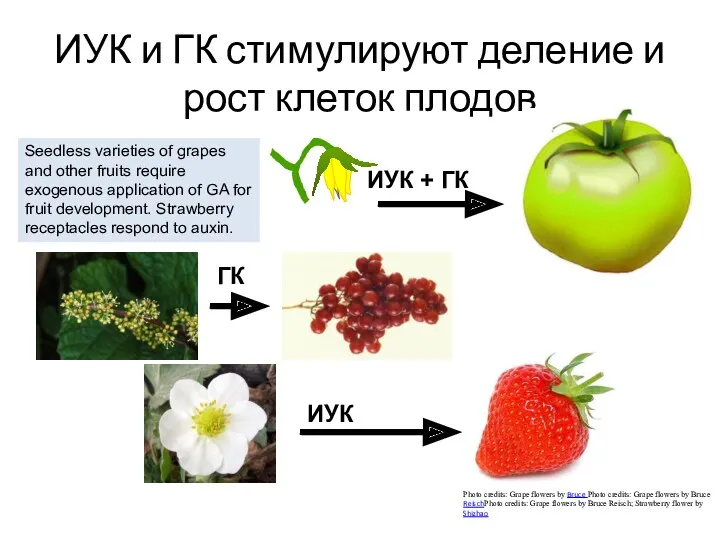
- 31. ИУК и ГК стимулируют деление и рост клеток плодов Seedless varieties of grapes and other fruits
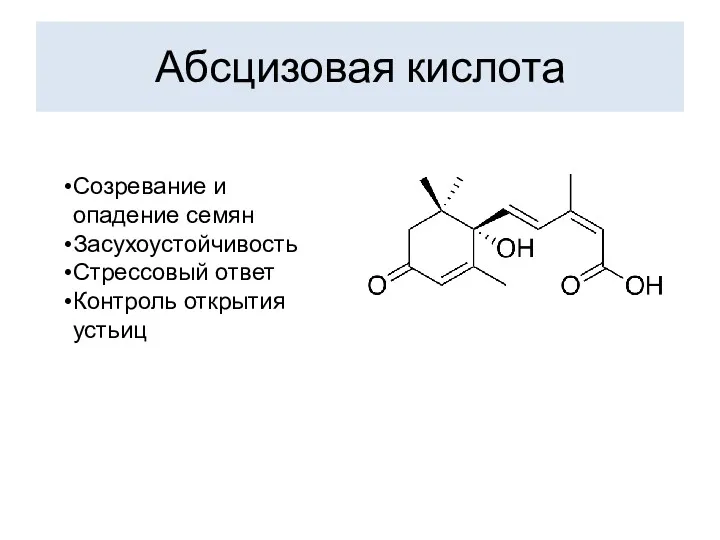
- 32. Абсцизовая кислота Созревание и опадение семян Засухоустойчивость Стрессовый ответ Контроль открытия устьиц
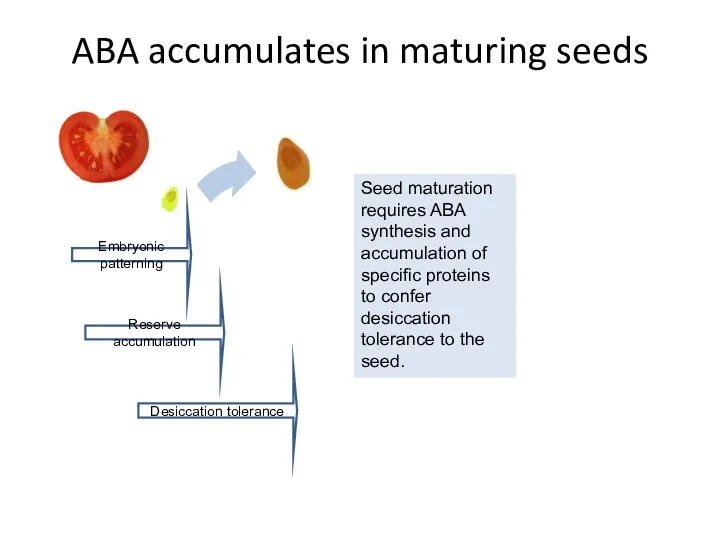
- 33. ABA accumulates in maturing seeds
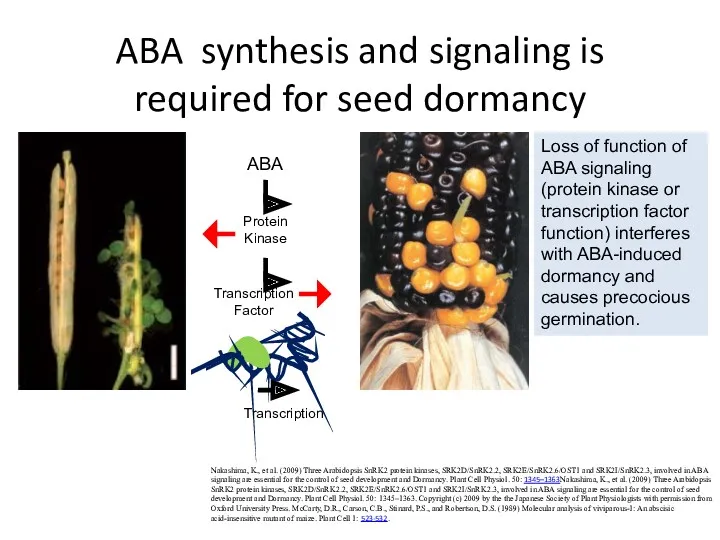
- 34. ABA synthesis and signaling is required for seed dormancy Nakashima, K., et al. (2009) Three Arabidopsis
- 35. Once dormant and dry, seeds can remain viable for very long times From Sallon, S., et
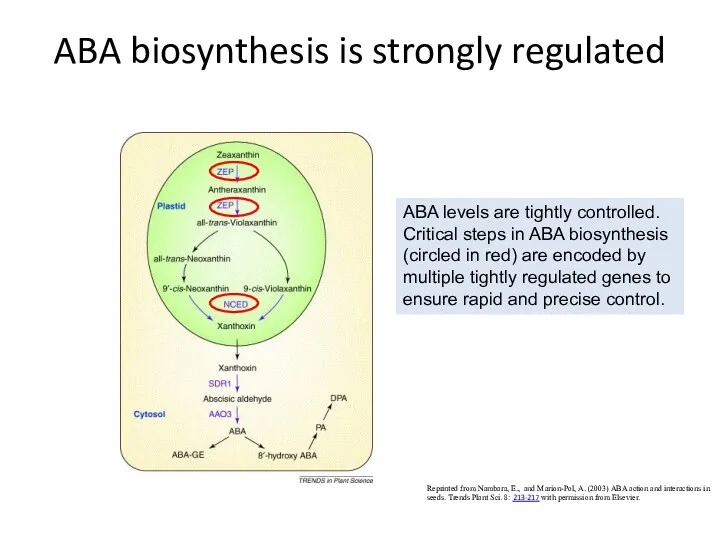
- 36. ABA biosynthesis is strongly regulated Reprinted from Nambara, E., and Marion-Pol, A. (2003) ABA action and
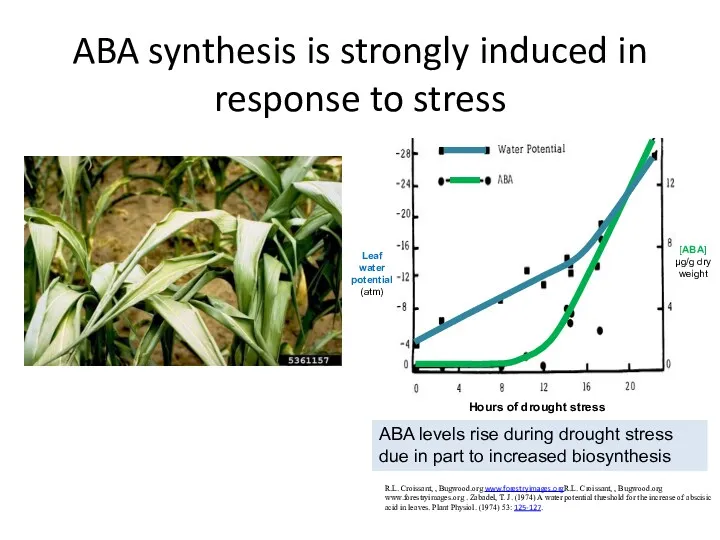
- 37. ABA synthesis is strongly induced in response to stress R.L. Croissant, , Bugwood.org www.forestryimages.orgR.L. Croissant, ,
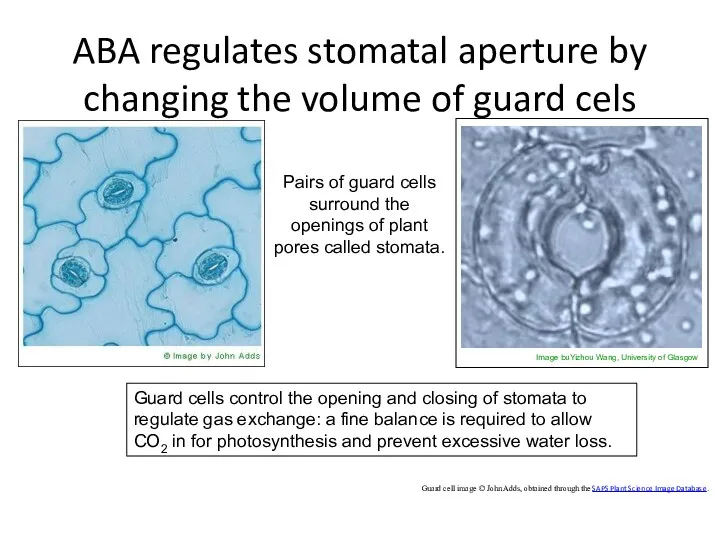
- 38. ABA regulates stomatal aperture by changing the volume of guard cels Guard cell image © John
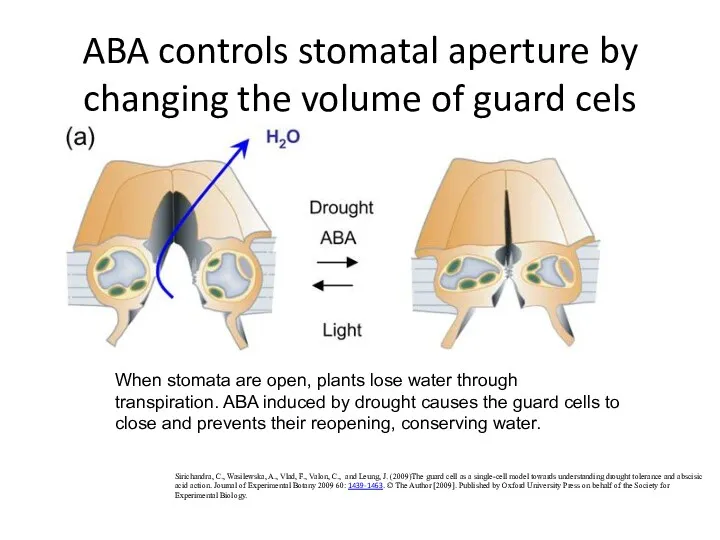
- 39. ABA controls stomatal aperture by changing the volume of guard cels When stomata are open, plants
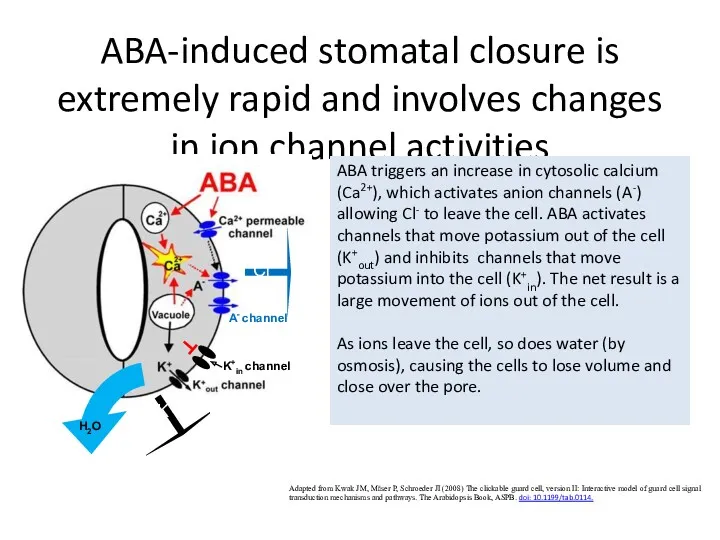
- 40. ABA-induced stomatal closure is extremely rapid and involves changes in ion channel activities ABA triggers an
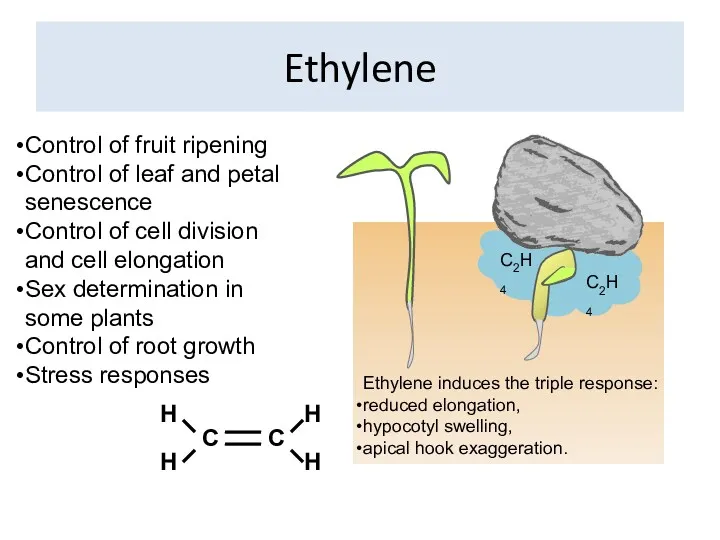
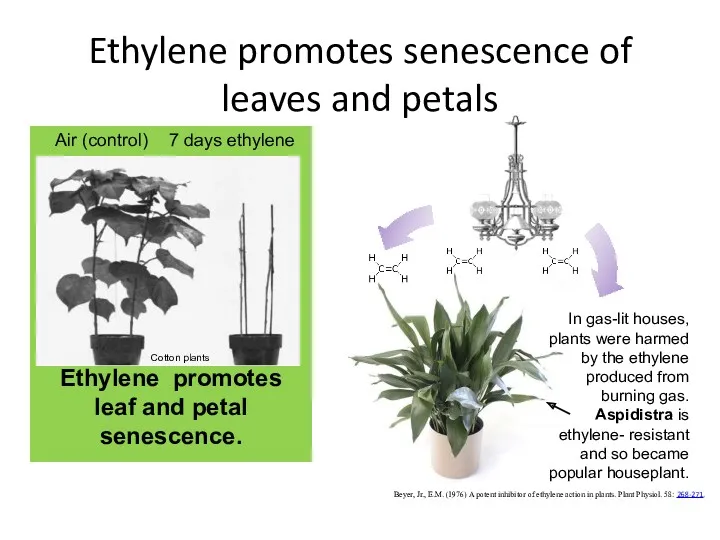
- 41. Ethylene
- 42. Beyer, Jr., E.M. (1976) A potent inhibitor of ethylene action in plants. Plant Physiol. 58: 268-271.
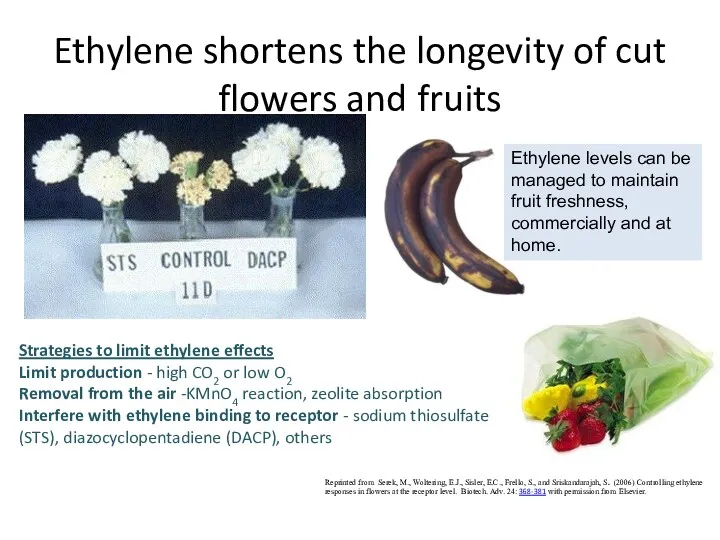
- 43. Ethylene shortens the longevity of cut flowers and fruits Reprinted from Serek, M., Woltering, E.J., Sisler,
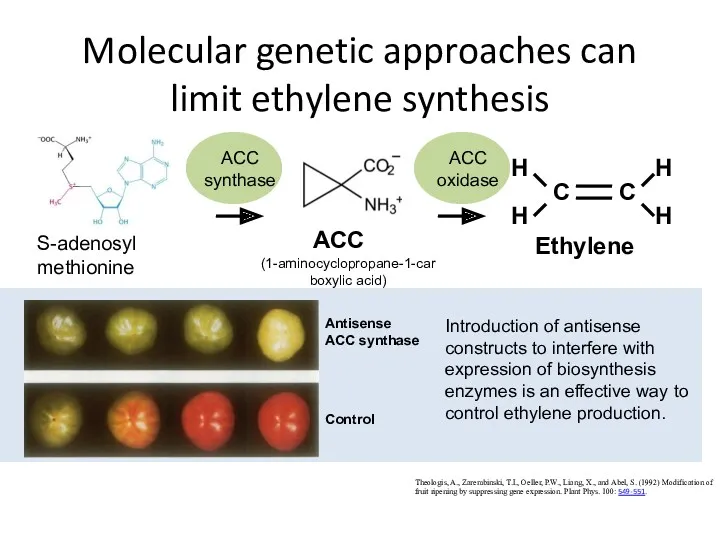
- 44. Molecular genetic approaches can limit ethylene synthesis Theologis, A., Zarembinski, T.I., Oeller, P.W., Liang, X., and
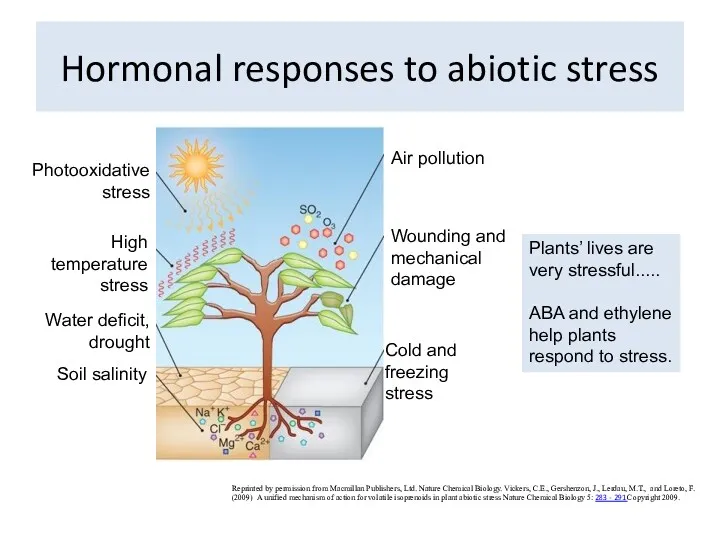
- 45. Hormonal responses to abiotic stress Reprinted by permission from Macmillan Publishers, Ltd. Nature Chemical Biology. Vickers,
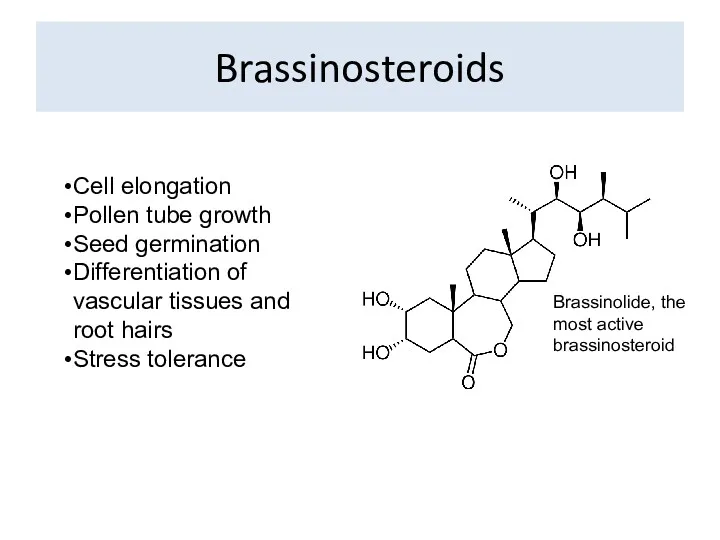
- 46. Brassinosteroids Brassinolide, the most active brassinosteroid Cell elongation Pollen tube growth Seed germination Differentiation of vascular
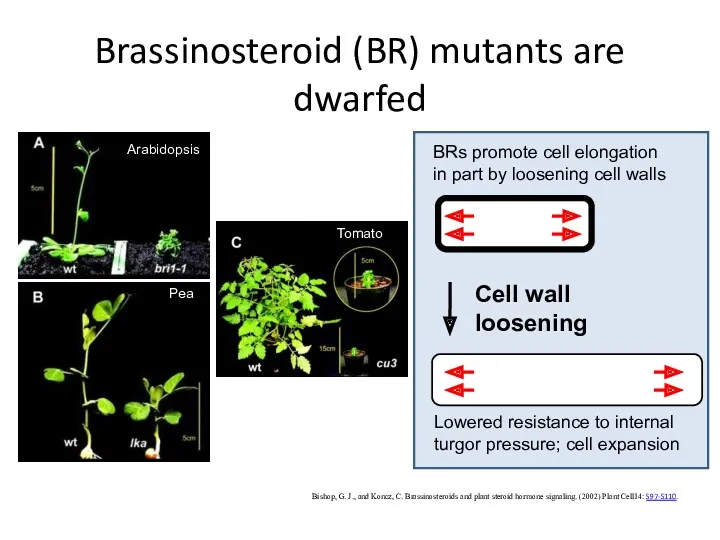
- 47. Brassinosteroid (BR) mutants are dwarfed Bishop, G. J., and Koncz, C. Brassinosteroids and plant steroid hormone
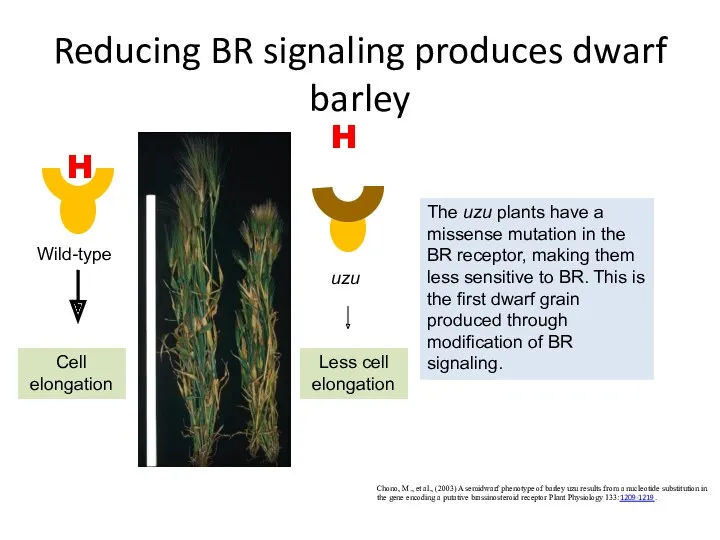
- 48. Reducing BR signaling produces dwarf barley Chono, M., et al., (2003) A semidwarf phenotype of barley
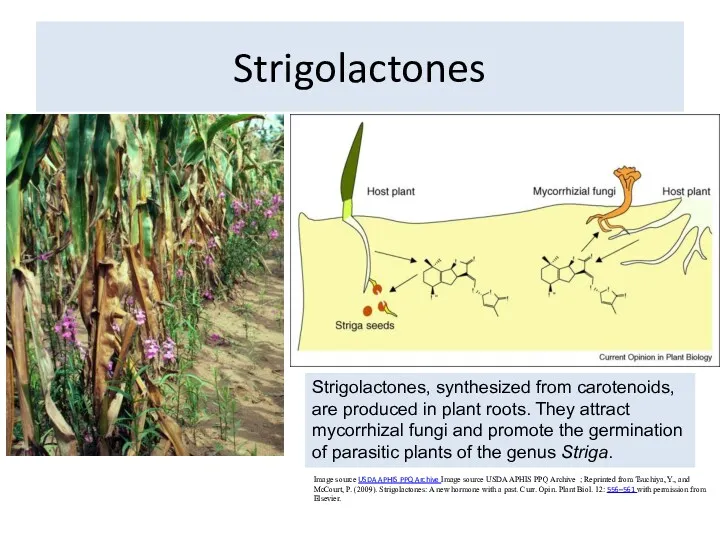
- 49. Strigolactones Image source USDA APHIS PPQ Archive Image source USDA APHIS PPQ Archive ; Reprinted from
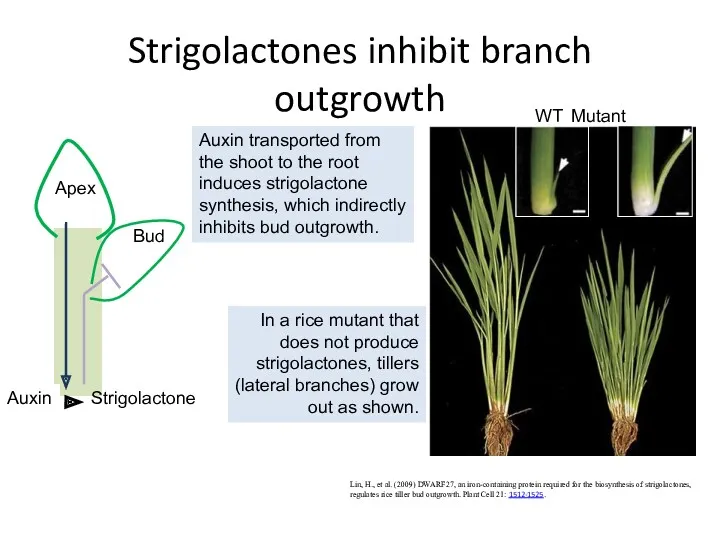
- 50. Strigolactones inhibit branch outgrowth Lin, H., et al. (2009) DWARF27, an iron-containing protein required for the
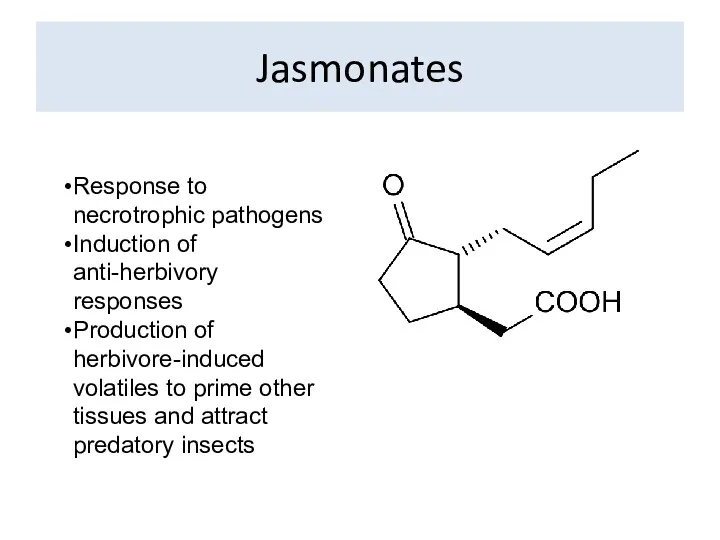
- 51. Jasmonates Response to necrotrophic pathogens Induction of anti-herbivory responses Production of herbivore-induced volatiles to prime other
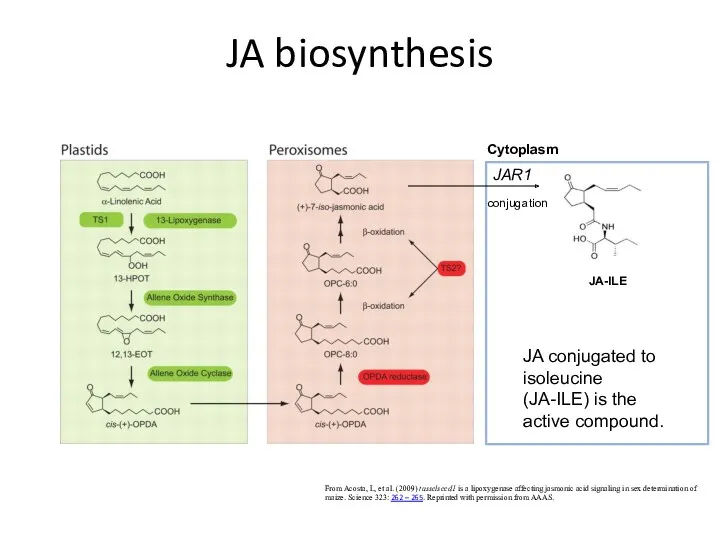
- 52. JA biosynthesis JA-ILE From Acosta, I., et al. (2009) tasselseed1 is a lipoxygenase affecting jasmonic acid
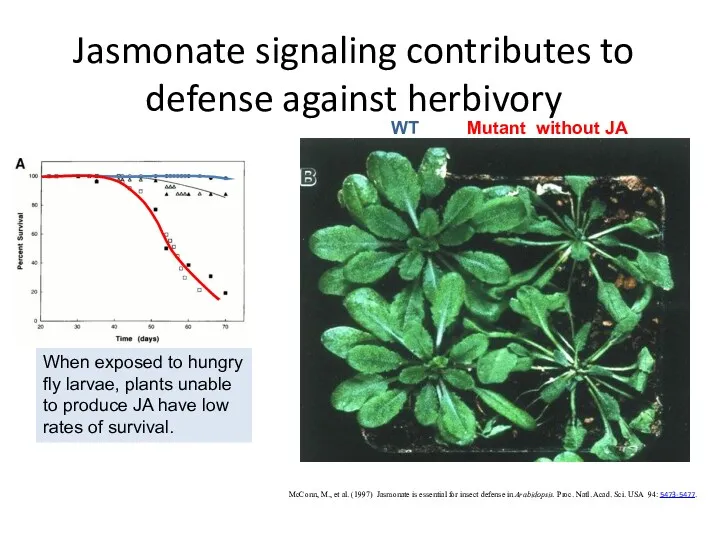
- 53. Jasmonate signaling contributes to defense against herbivory McConn, M., et al. (1997) Jasmonate is essential for
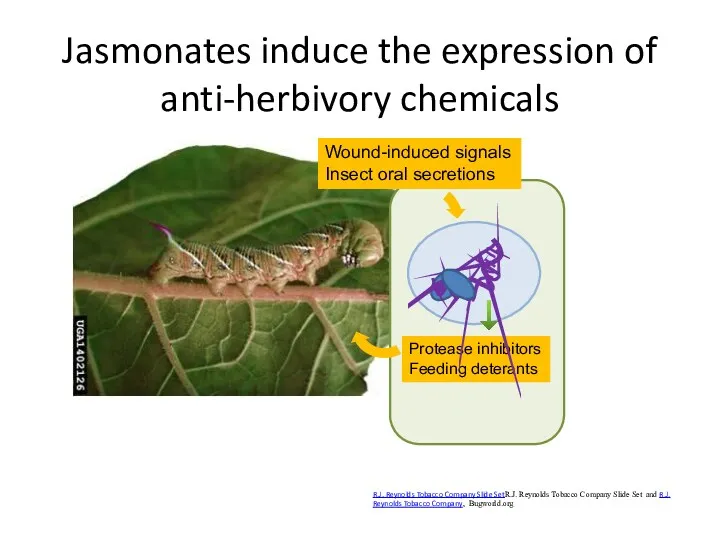
- 54. Jasmonates induce the expression of anti-herbivory chemicals R.J. Reynolds Tobacco Company Slide SetR.J. Reynolds Tobacco Company
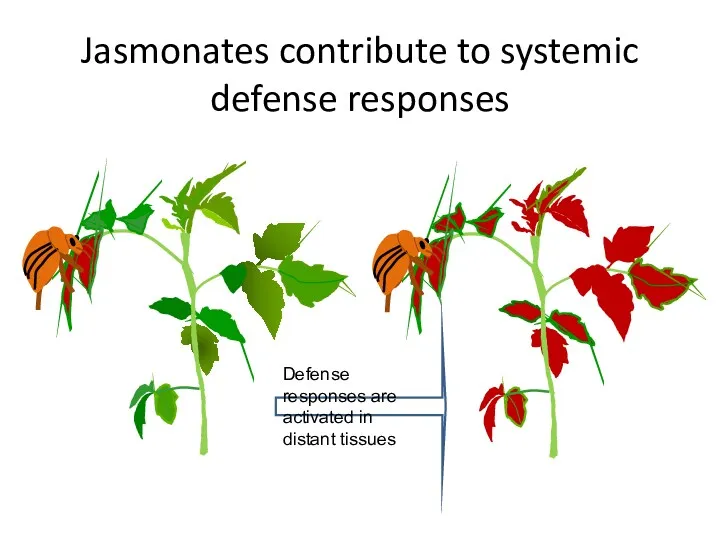
- 55. Jasmonates contribute to systemic defense responses
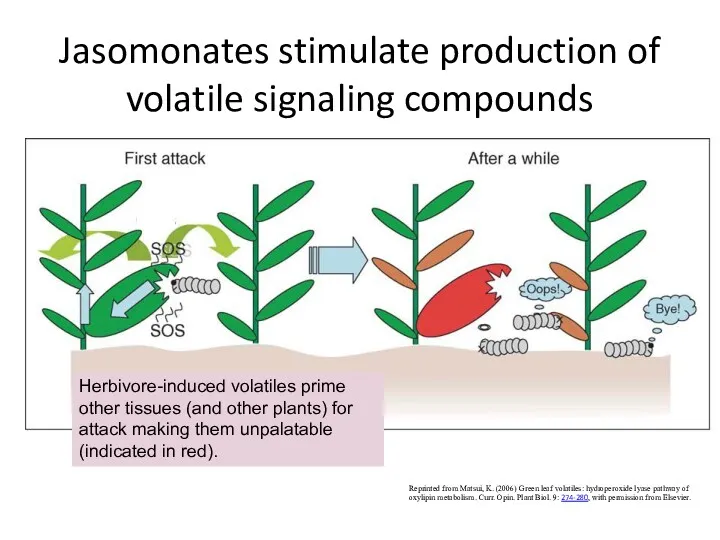
- 56. Jasomonates stimulate production of volatile signaling compounds Reprinted from Matsui, K. (2006) Green leaf volatiles: hydroperoxide
- 57. Herbivore-induced volatiles are recognized by carnivorous and parasitoid insects Tim HayeTim Haye, Universität Kiel, Germany Bugwood.org;
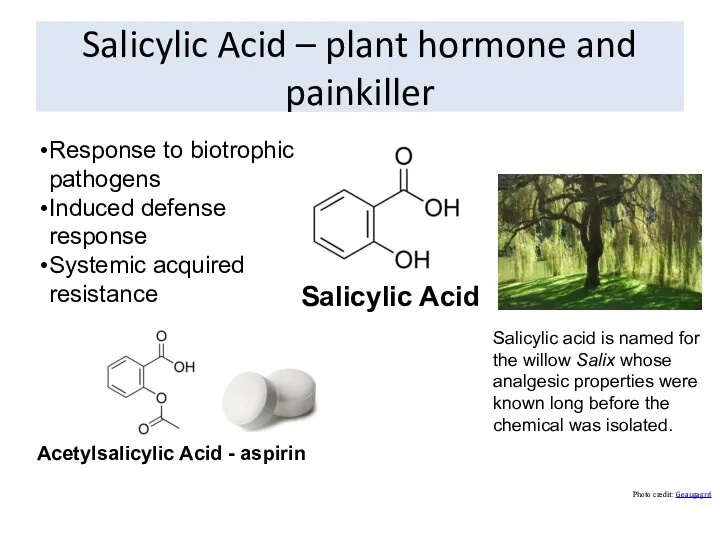
- 58. Salicylic Acid – plant hormone and painkiller Photo credit: Geaugagrrl Response to biotrophic pathogens Induced defense
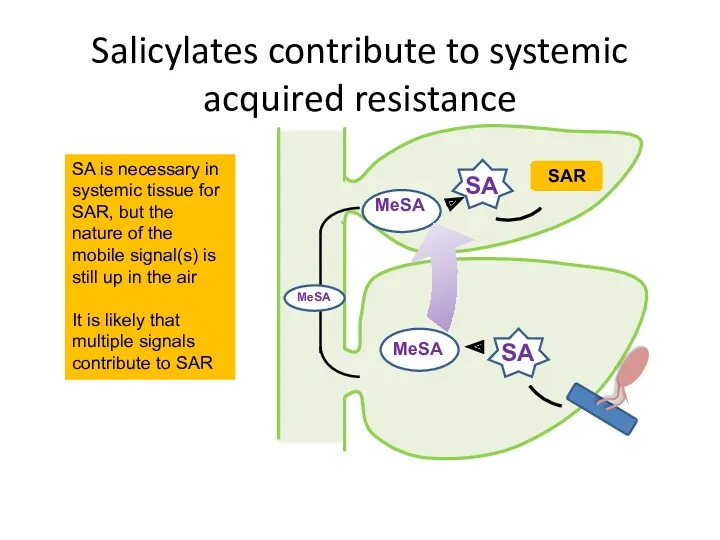
- 59. Salicylates contribute to systemic acquired resistance SA is necessary in systemic tissue for SAR, but the
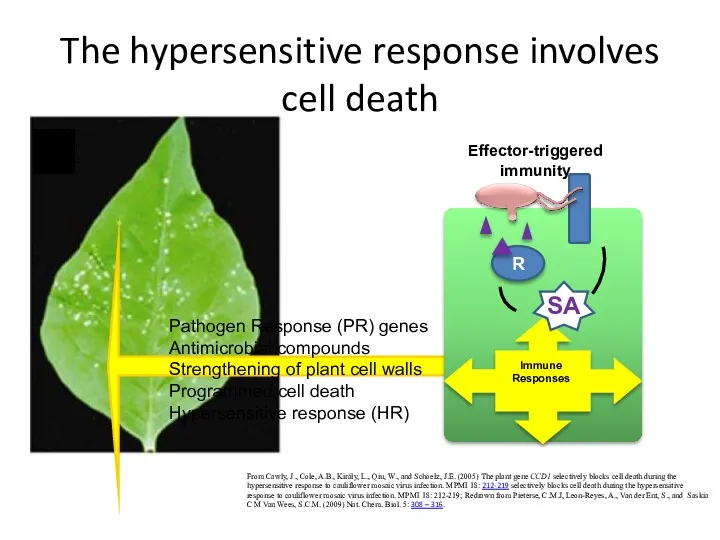
- 60. The hypersensitive response involves cell death From Cawly, J., Cole, A.B., Király, L., Qiu, W., and
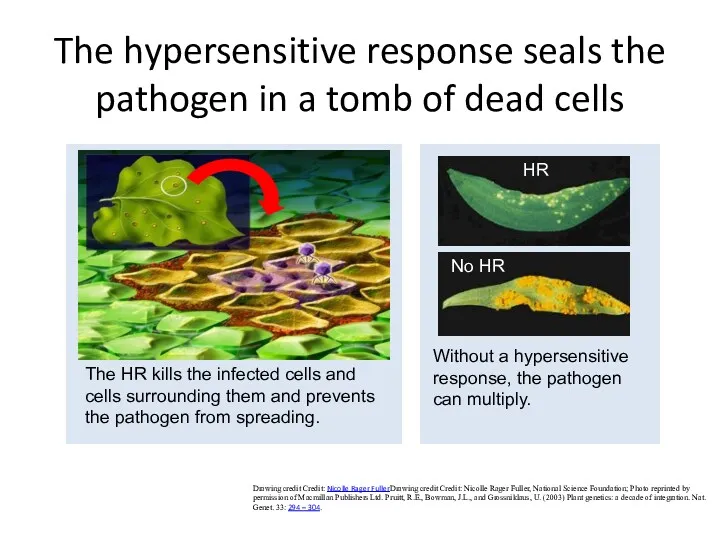
- 61. The hypersensitive response seals the pathogen in a tomb of dead cells Drawing credit Credit: Nicolle
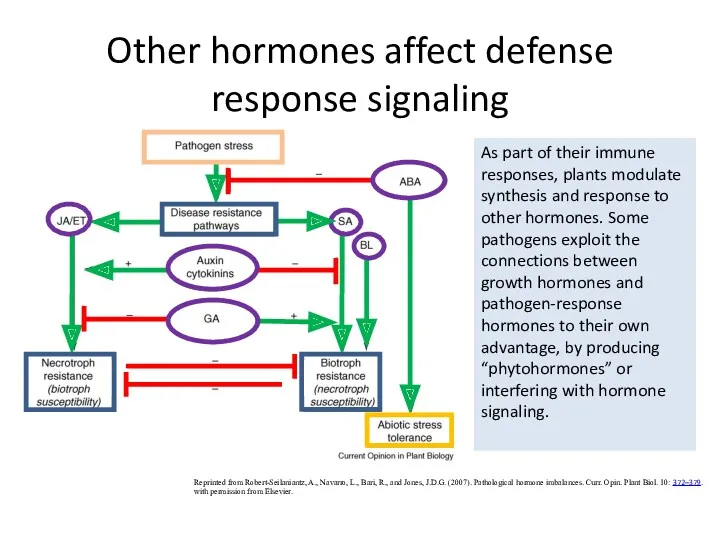
- 62. Other hormones affect defense response signaling Reprinted from Robert-Seilaniantz, A., Navarro, L., Bari, R., and Jones,
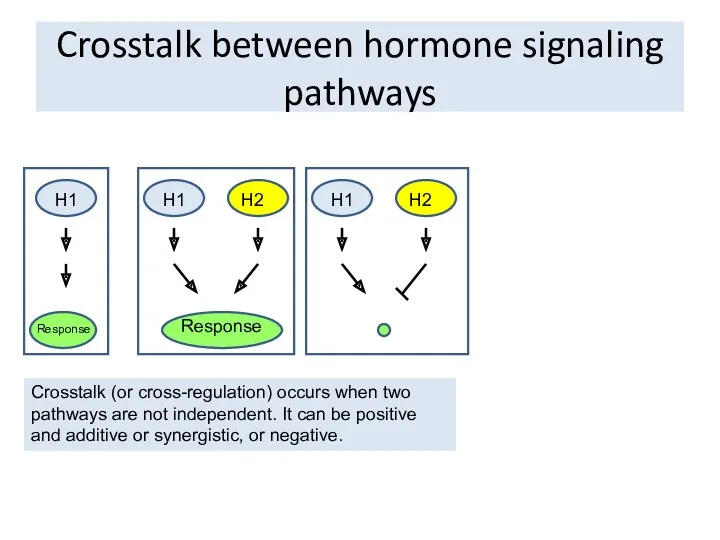
- 63. Crosstalk between hormone signaling pathways
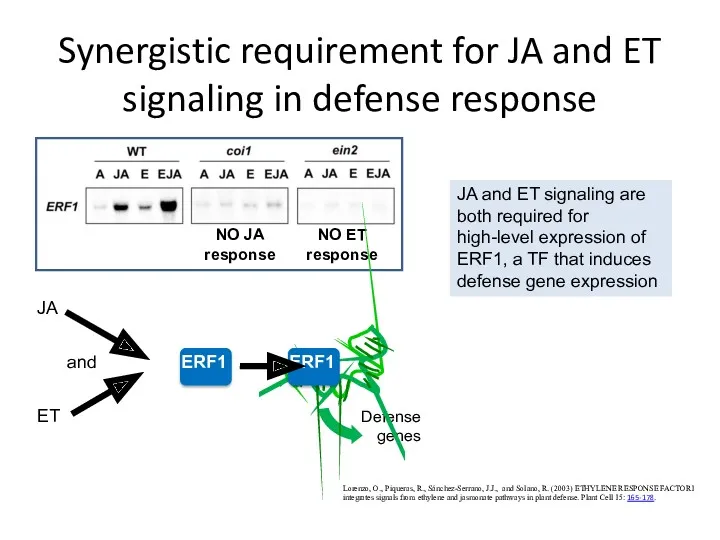
- 64. Synergistic requirement for JA and ET signaling in defense response Lorenzo, O., Piqueras, R., Sánchez-Serrano, J.J.,
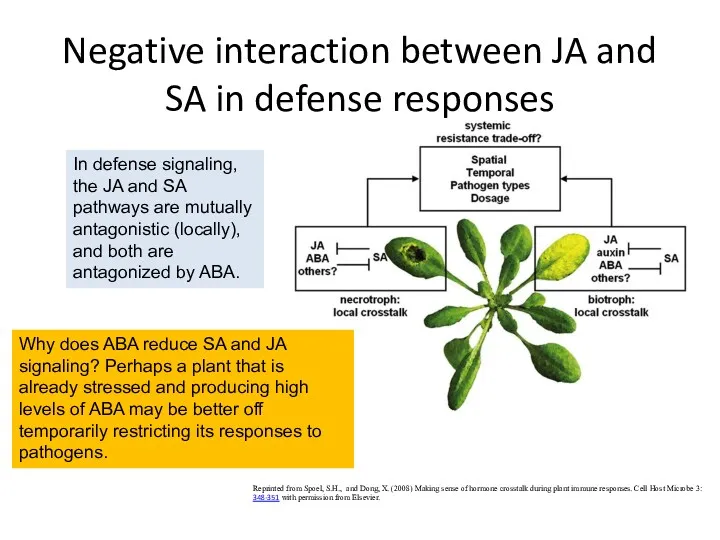
- 65. Negative interaction between JA and SA in defense responses Reprinted from Spoel, S.H., and Dong, X.
- 71. Скачать презентацию









 Зелена аптека (2 клас)
Зелена аптека (2 клас) Жұлын құрылысы
Жұлын құрылысы Жәндіктер, балықтар мен адамның тыныс алу мүшелері құрылысының ерекшеліктерін зерттеу және салыстыру
Жәндіктер, балықтар мен адамның тыныс алу мүшелері құрылысының ерекшеліктерін зерттеу және салыстыру Общая характеристика грибов
Общая характеристика грибов Эволюционная теория Ж.-Б. Ламарка. Урок биологии в 11 классе
Эволюционная теория Ж.-Б. Ламарка. Урок биологии в 11 классе презентация к урокам биологии и экологии Живые организмы как среда жизни
презентация к урокам биологии и экологии Живые организмы как среда жизни Биологические свойства воды
Биологические свойства воды Естественный отбор и его формы. (11 класс)
Естественный отбор и его формы. (11 класс) Структурна ботаніка: анатомія рослин
Структурна ботаніка: анатомія рослин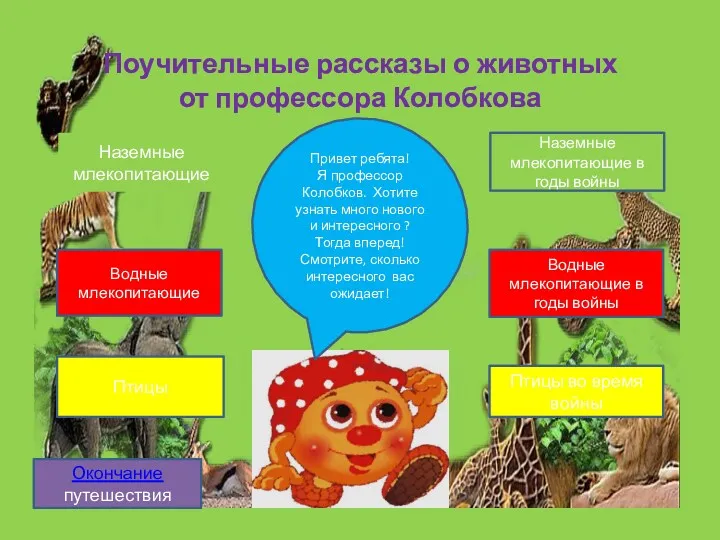 Поучительные рассказы о животных от профессора Колобкова
Поучительные рассказы о животных от профессора Колобкова Макро- и микроэлементы в питании человека
Макро- и микроэлементы в питании человека Образование новых видов - микроэволюция
Образование новых видов - микроэволюция Царство животные. Общая характеристика. Классификация животных
Царство животные. Общая характеристика. Классификация животных Введение в профиль Микробиология
Введение в профиль Микробиология Происхождение человека (антропогенез)
Происхождение человека (антропогенез) Тип Кольчатые черви
Тип Кольчатые черви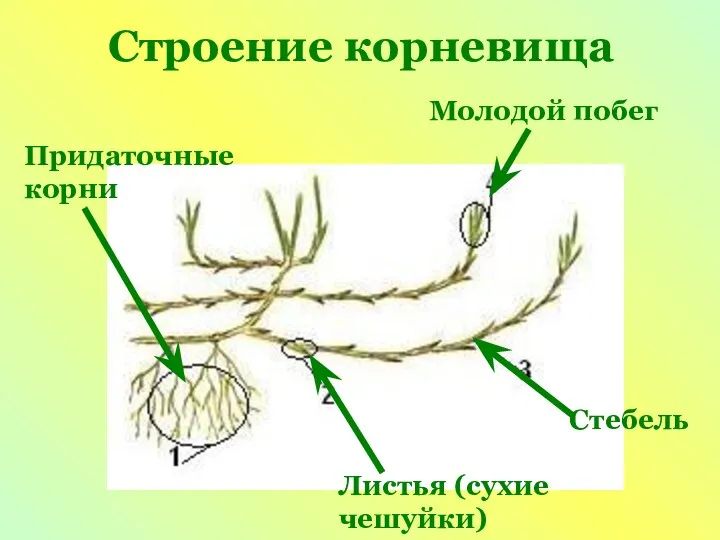 Строение корневища
Строение корневища Цветущий сад
Цветущий сад Биология как наука. Методы научного познания
Биология как наука. Методы научного познания Введение в генетику. Закономерности наследования на организменном уровне
Введение в генетику. Закономерности наследования на организменном уровне Вид. Критерии вида. Популяция
Вид. Критерии вида. Популяция Одомашнивание, как начальный этап селекции
Одомашнивание, как начальный этап селекции Приспособленность организмов и ее относительность. 9 класс
Приспособленность организмов и ее относительность. 9 класс Царство Plantae. Высшие споровые растения. Семенные растения. Отдел Голосеменные
Царство Plantae. Высшие споровые растения. Семенные растения. Отдел Голосеменные Помидор – полезный овощ. 2 класс
Помидор – полезный овощ. 2 класс Сова. Анатомические признаки сов
Сова. Анатомические признаки сов Эмбриология человека
Эмбриология человека Биофизика цветного зрения
Биофизика цветного зрения