Содержание
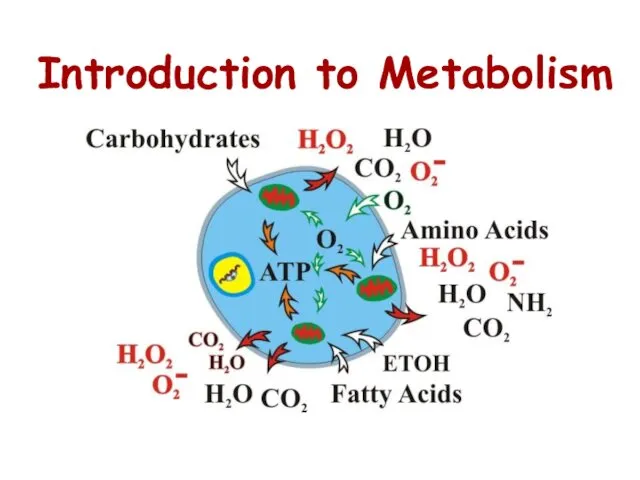
- 2. Introduction to Metabolism
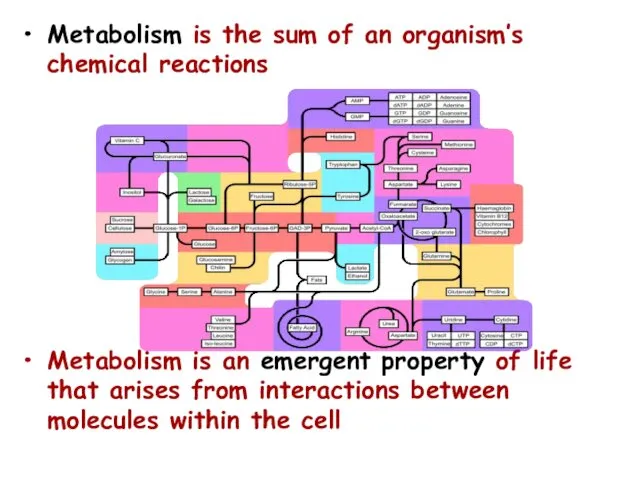
- 3. Metabolism is the sum of an organism’s chemical reactions Metabolism is an emergent property of life
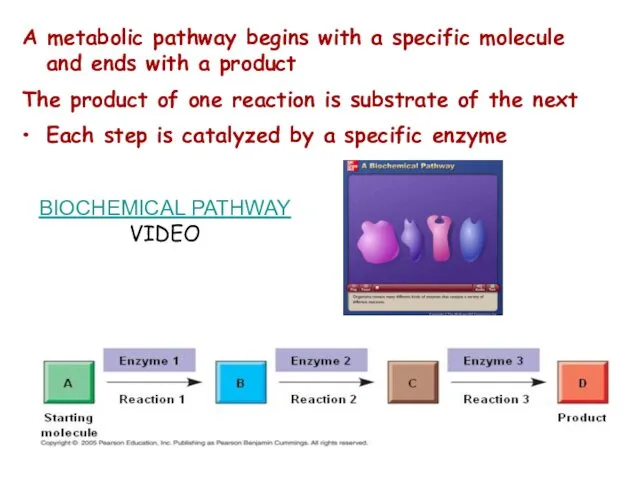
- 4. A metabolic pathway begins with a specific molecule and ends with a product The product of
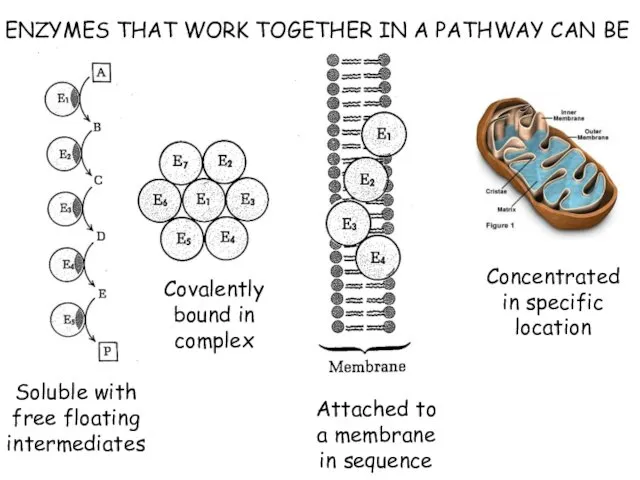
- 5. ENZYMES THAT WORK TOGETHER IN A PATHWAY CAN BE Soluble with free floating intermediates Covalently bound
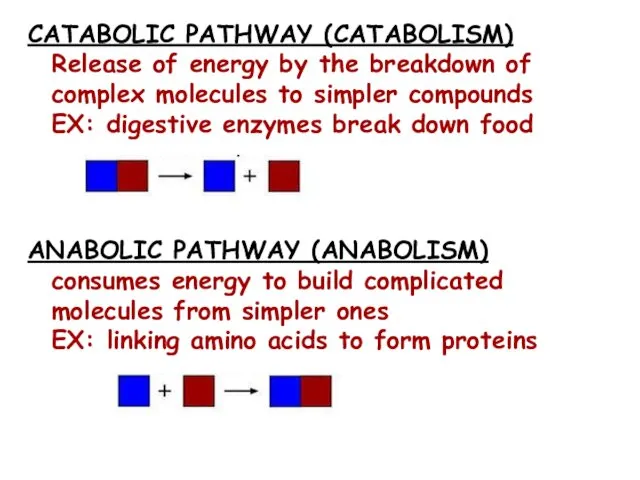
- 6. CATABOLIC PATHWAY (CATABOLISM) Release of energy by the breakdown of complex molecules to simpler compounds EX:
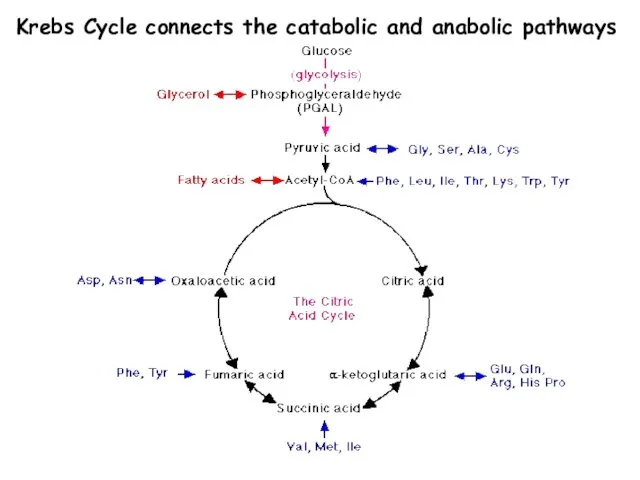
- 7. Krebs Cycle connects the catabolic and anabolic pathways
- 8. Forms of Energy ENERGY = capacity to cause change Energy exists in various forms (some of
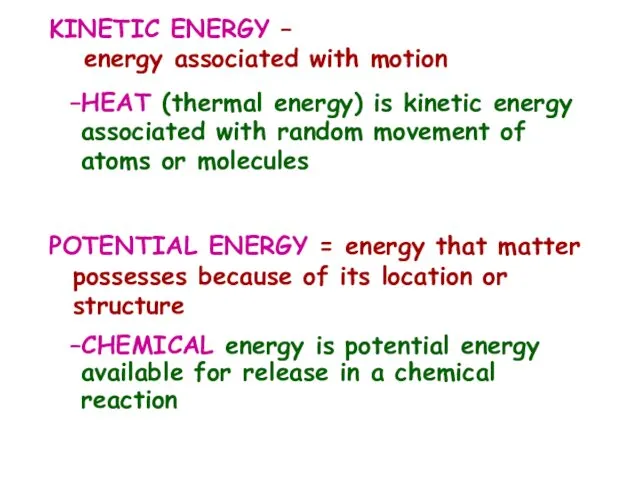
- 9. KINETIC ENERGY – energy associated with motion HEAT (thermal energy) is kinetic energy associated with random
- 10. On the platform, the diver has more potential energy. Diving converts potential energy to kinetic energy.
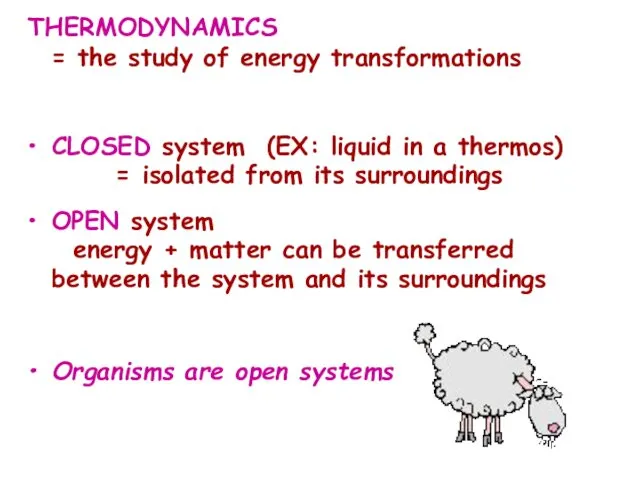
- 11. THERMODYNAMICS = the study of energy transformations CLOSED system (EX: liquid in a thermos) = isolated
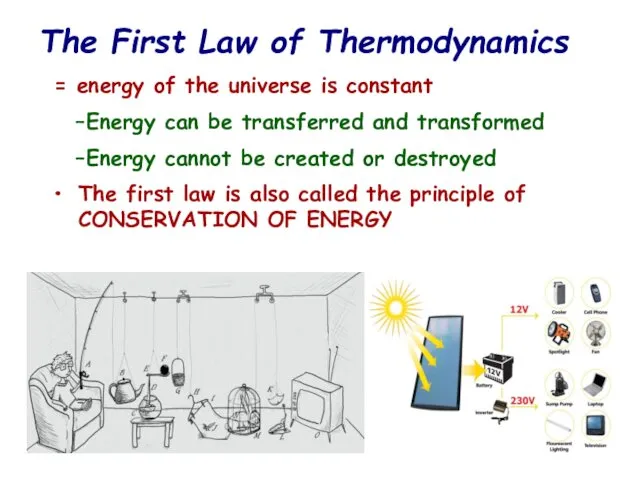
- 12. The First Law of Thermodynamics = energy of the universe is constant Energy can be transferred
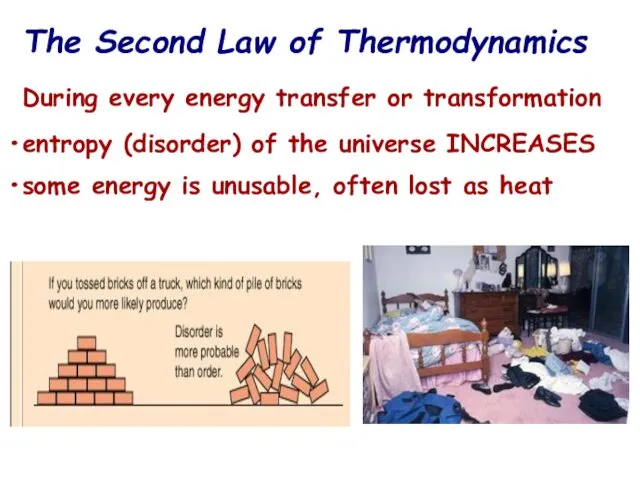
- 13. The Second Law of Thermodynamics During every energy transfer or transformation entropy (disorder) of the universe
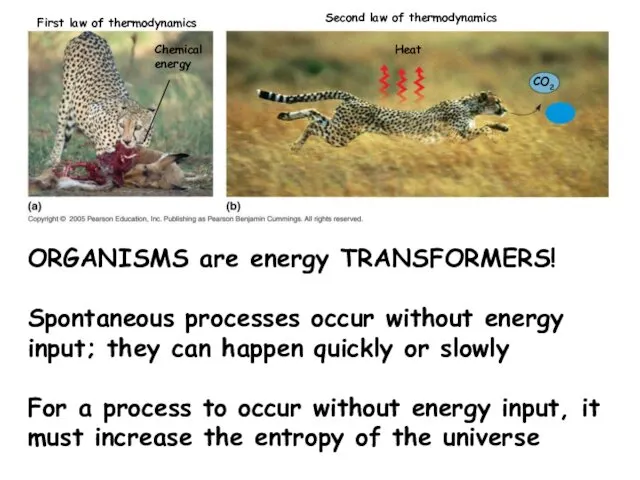
- 14. Chemical energy Heat CO2 First law of thermodynamics Second law of thermodynamics H2O ORGANISMS are energy
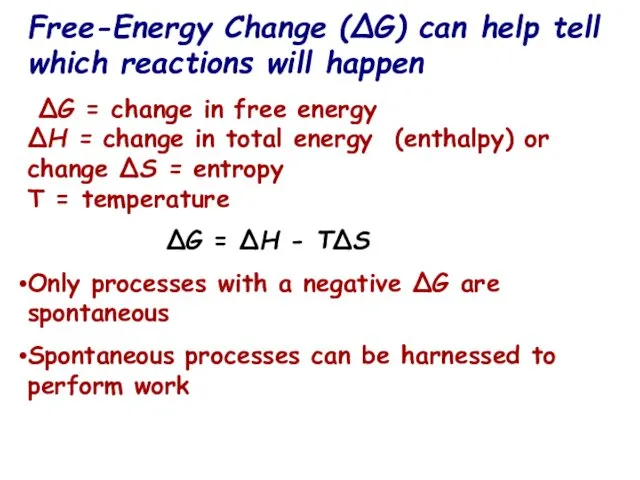
- 15. Free-Energy Change (ΔG) can help tell which reactions will happen ∆G = change in free energy
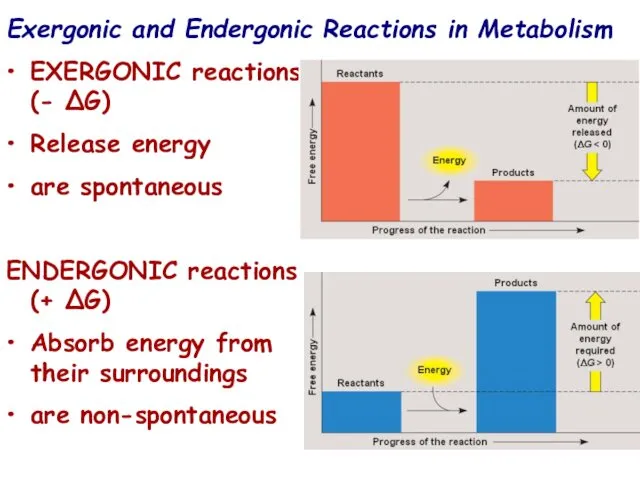
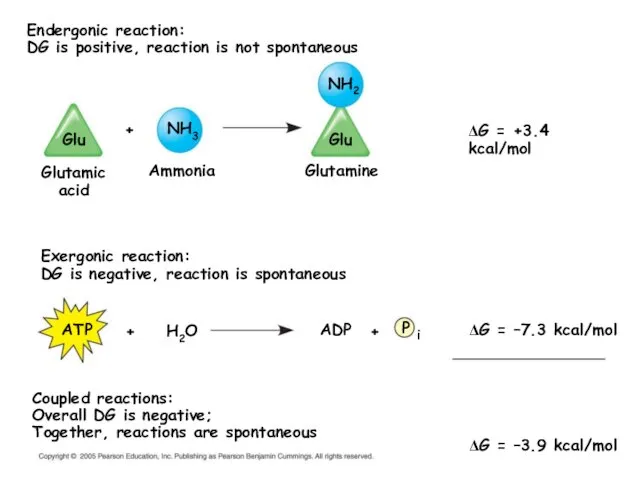
- 16. Exergonic and Endergonic Reactions in Metabolism EXERGONIC reactions (- ∆G) Release energy are spontaneous ENDERGONIC reactions
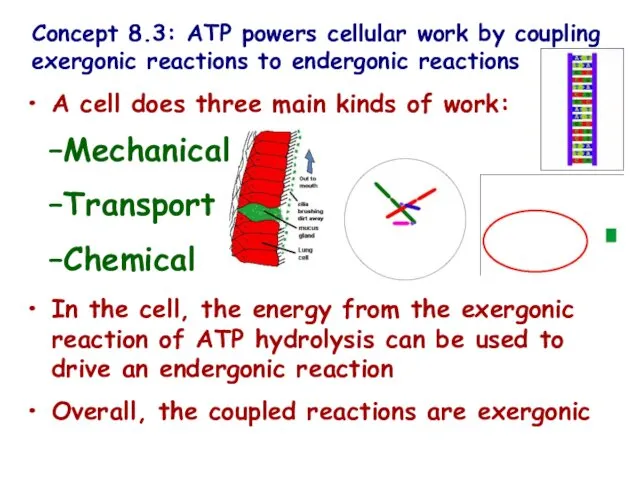
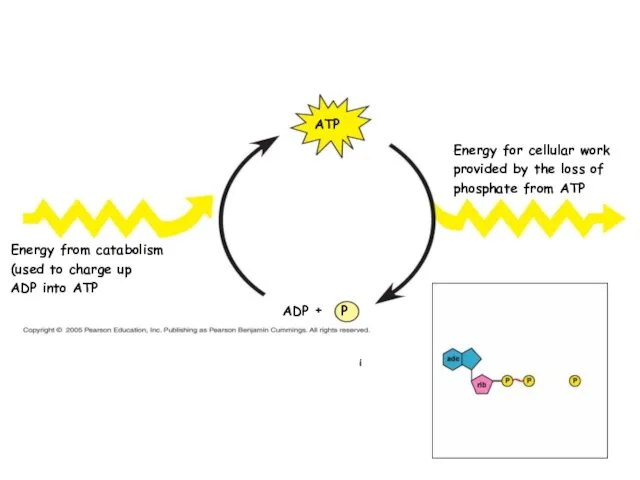
- 17. Concept 8.3: ATP powers cellular work by coupling exergonic reactions to endergonic reactions A cell does
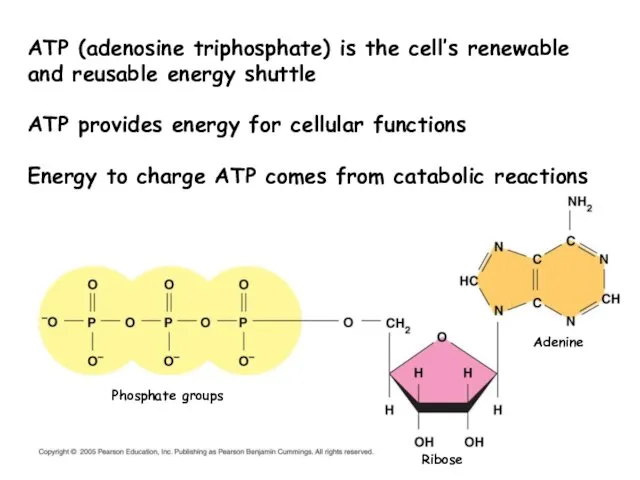
- 18. Phosphate groups Ribose Adenine ATP (adenosine triphosphate) is the cell’s renewable and reusable energy shuttle ATP
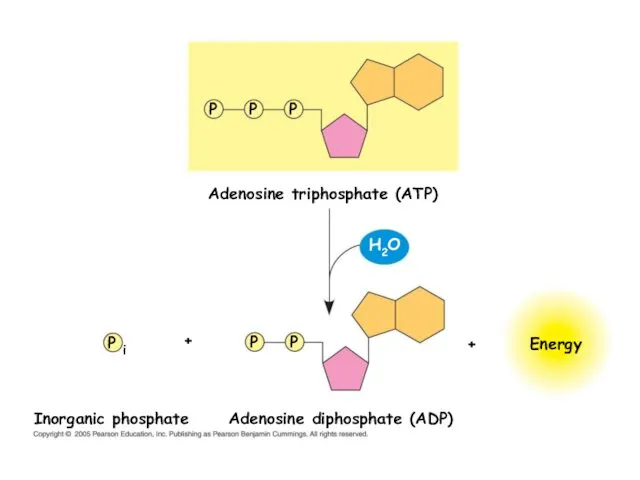
- 19. Adenosine triphosphate (ATP) Energy P P P P P P i Adenosine diphosphate (ADP) Inorganic phosphate
- 20. P i ADP Energy for cellular work provided by the loss of phosphate from ATP Energy
- 21. Endergonic reaction: DG is positive, reaction is not spontaneous Exergonic reaction: DG is negative, reaction is
- 23. Скачать презентацию

 Масличные культуры
Масличные культуры Отряды птиц: дневные хищные, совы, куриные
Отряды птиц: дневные хищные, совы, куриные Ткани. Гистология
Ткани. Гистология Скелетные ткани: хрящевые костные
Скелетные ткани: хрящевые костные Комнатные растения в интерьере квартире
Комнатные растения в интерьере квартире Изучение особенности строения шейных , грудных, поясничных, крестцовых и копчиковых позвонков на анатомических препаратах
Изучение особенности строения шейных , грудных, поясничных, крестцовых и копчиковых позвонков на анатомических препаратах Тест по биологии по теме Растения для 6-7 класса
Тест по биологии по теме Растения для 6-7 класса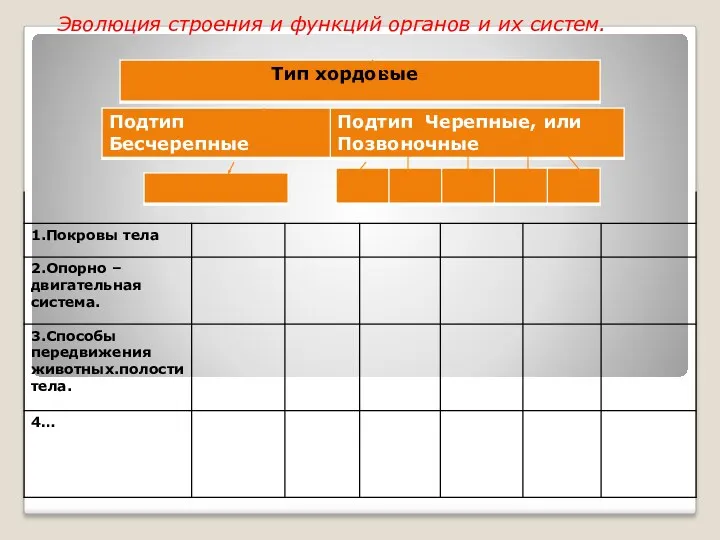 презентация к уроку Покровы тела .7 класс
презентация к уроку Покровы тела .7 класс Анализ и оценка антропогенных изменений в биосфере
Анализ и оценка антропогенных изменений в биосфере Декоративные качества комнатных растений
Декоративные качества комнатных растений Физические процессы в биологических мембранах. Лекция 8
Физические процессы в биологических мембранах. Лекция 8 Надтип Первичнополостные черви Aschelminthes
Надтип Первичнополостные черви Aschelminthes Голонасінні
Голонасінні Бурые водоросли
Бурые водоросли Дыхательная система
Дыхательная система Тварини рекордсмени
Тварини рекордсмени Многообразие живых организмов. Одноклеточные и многоклеточные организмы
Многообразие живых организмов. Одноклеточные и многоклеточные организмы Структура и функции клетки. Клеточная теория
Структура и функции клетки. Клеточная теория Ленинградский зоопарк
Ленинградский зоопарк Эндокринная система
Эндокринная система СПИД - сведи вероятность к нулю(презентация)
СПИД - сведи вероятность к нулю(презентация) Обмен веществ. Метаболизм
Обмен веществ. Метаболизм Презентация Лист. внешнее и внутреннее строение.
Презентация Лист. внешнее и внутреннее строение. Ластоногі. Група водних хижих ссавців
Ластоногі. Група водних хижих ссавців Презентация к уроку Движение крови по сосудам
Презентация к уроку Движение крови по сосудам Строение клетки. Цитоплазма
Строение клетки. Цитоплазма Метод Дригальского (метод выделения чистой культуры)
Метод Дригальского (метод выделения чистой культуры) Класс Круглоротые
Класс Круглоротые