Содержание
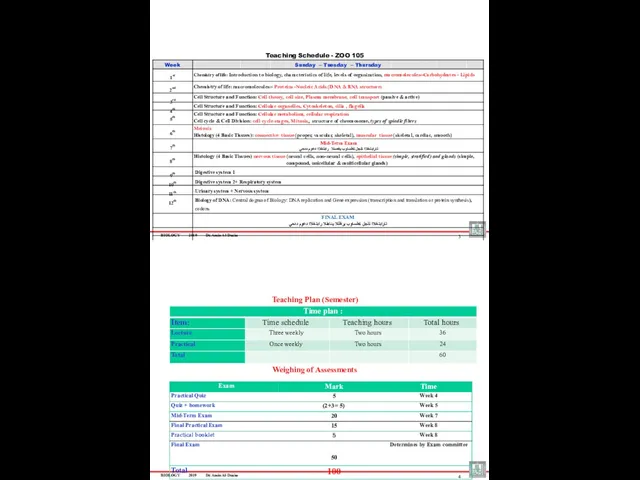
- 2. BIOLOGY 2019 Dr. Amin Al-Doaiss 3 Week Sunday – Tuesday – Thursday 1st Chemistry of life:
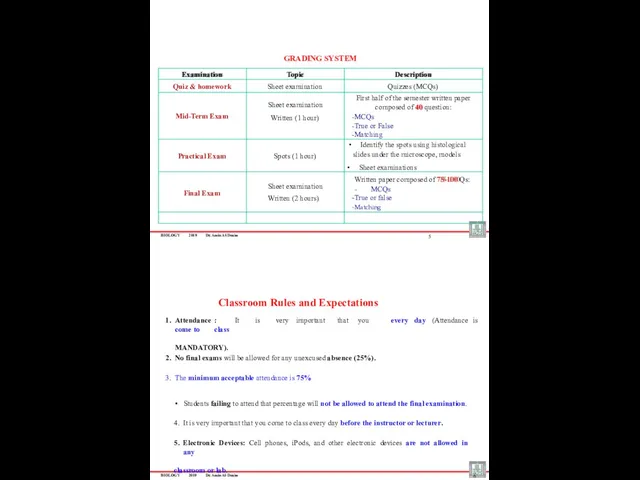
- 3. BIOLOGY 2019 Dr. Amin Al-Doaiss Examination Topic Description Quiz & homework Sheet examination Quizzes (MCQs) Mid-Term
- 4. BIOLOGY 2019 Dr. Amin Al-Doaiss Introduction to biology Biology (the study of life) The science of
- 5. BIOLOGY 2019 Dr. Amin Al-Doaiss Chemistry of Life 9 Dr. AMIN ABDULLAH AL-DOAISS From atoms, molecules
- 6. BIOLOGY 2019 Dr. Amin Al-Doaiss Matter is anything that takes up space and has mass. Matter
- 7. BIOLOGY 2019 Dr. Amin Al-Doaiss 13 Levels of organization Molecules of Life “ MACROMOLECULES “ BIOLOGY
- 8. Macromolecules MOLECULES There are two types of molecules : Inorganic molecules: e.g., salts (e.g., NaCl) and
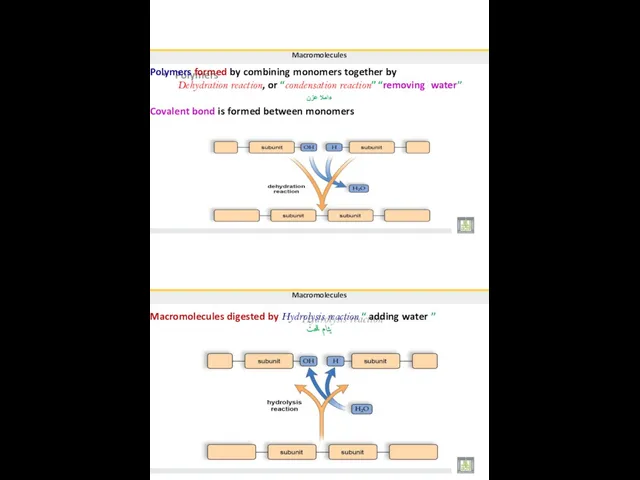
- 9. Macromolecules Polymers formed by combining monomers together by Dehydration reaction, or “condensation reaction” “removing water” ءاملا
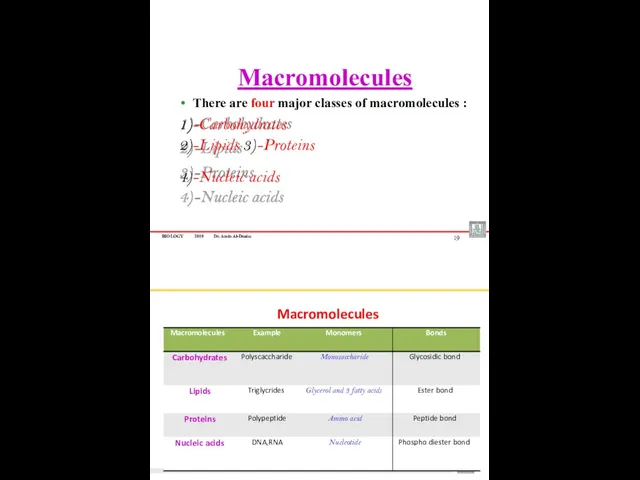
- 10. BIOLOGY 2019 Dr. Amin Al-Doaiss Macromolecules There are four major classes of macromolecules : 1)-Carbohydrates 2)-Lipids
- 11. Chemical Bond Macromolecules Building blocks of the cell Monosaccharaides FATTY ACIDS AMINO ACIDS NUCLEOTIDES Larger units
- 12. BIOLOGY 2019 Dr. Amin Al-Doaiss Fuel and Building Materials Are organic macromolecules composed of monomers (simple
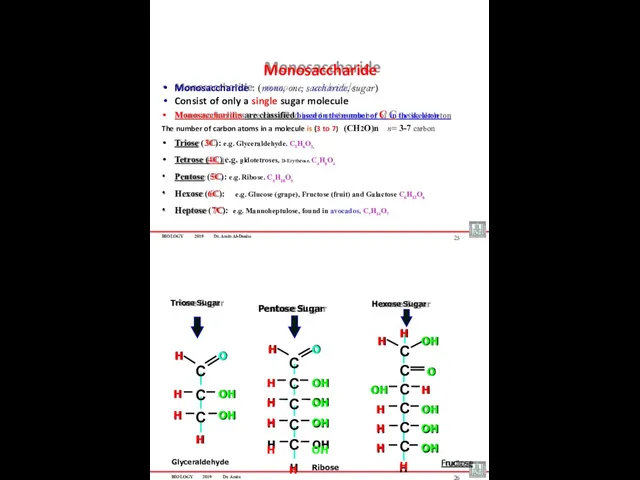
- 13. BIOLOGY 2019 Dr. Amin Al-Doaiss Monosaccharide : (mono, one; saccharide, sugar) Consist of only a single
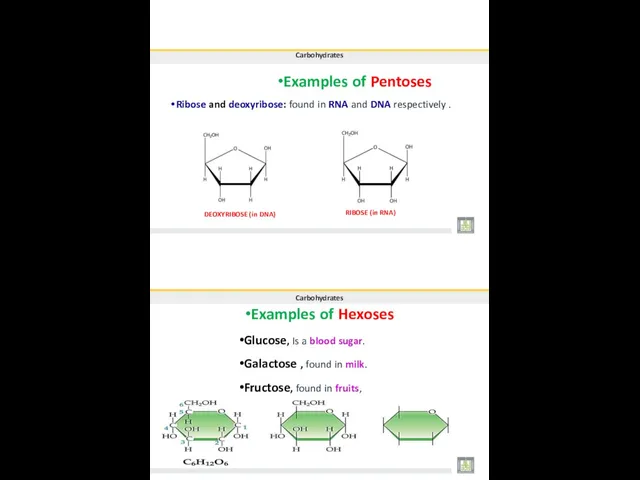
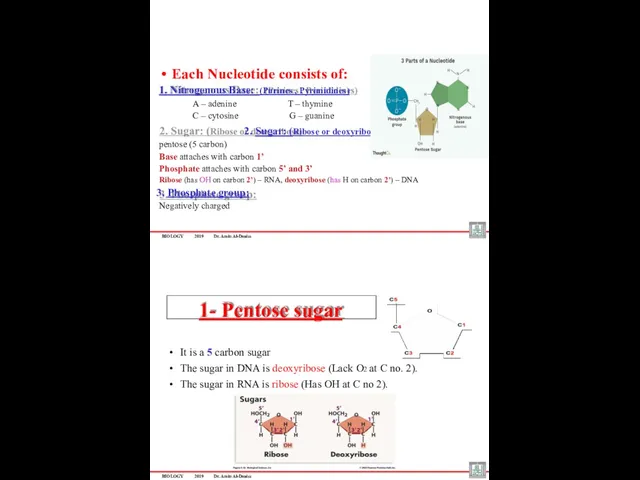
- 14. •Examples of Pentoses Carbohydrates Ribose and deoxyribose: found in RNA and DNA respectively . DEOXYRIBOSE (in
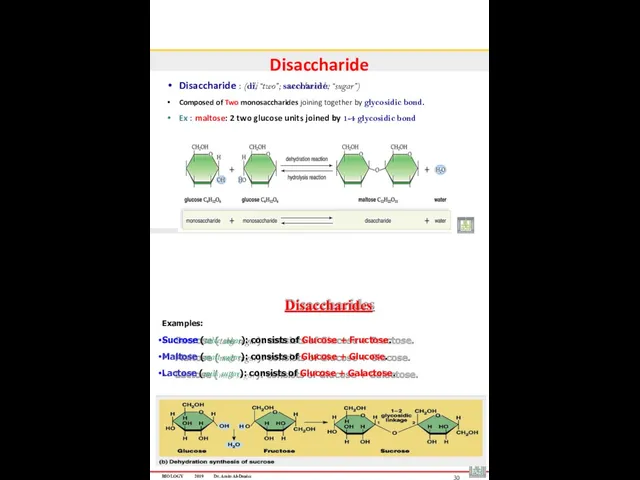
- 15. Disaccharide Disaccharide : (di, “two”; saccharide, “sugar”) Composed of Two monosaccharides joining together by glycosidic bond.
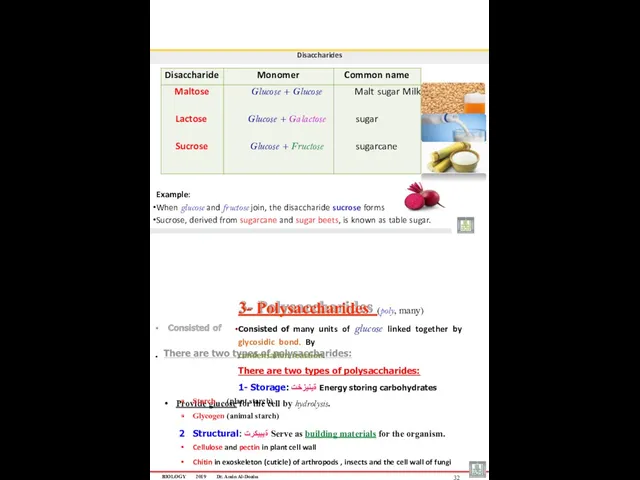
- 16. Disaccharides Example: When glucose and fructose join, the disaccharide sucrose forms Sucrose, derived from sugarcane and
- 17. Carbohydrates STARCH : It is a Long chain of glucose (up to 4,000 units) linked together
- 18. Carbohydrates CELLULOSE : It is a structural form of polysaccharide, supports the plant cell wall Cellulose
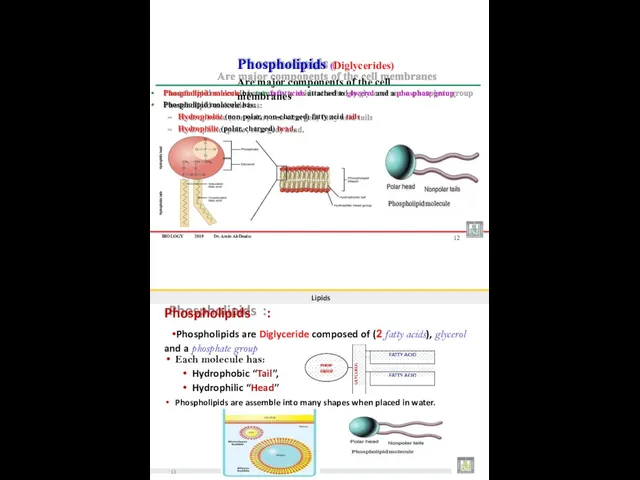
- 19. BIOLOGY 2019 Dr. Amin Al-Doaiss 2 Lipids Phospholipids are type of lipids that form the major
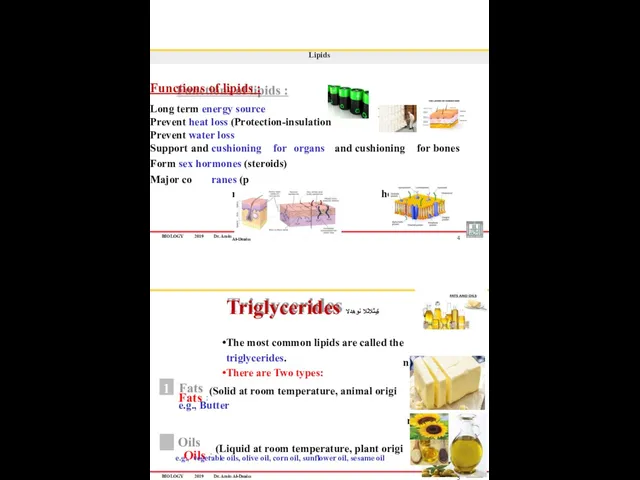
- 20. BIOLOGY 2019 Dr. Amin mponent of cell memb Al-Doaiss 4 Lipids Functions of lipids : Long
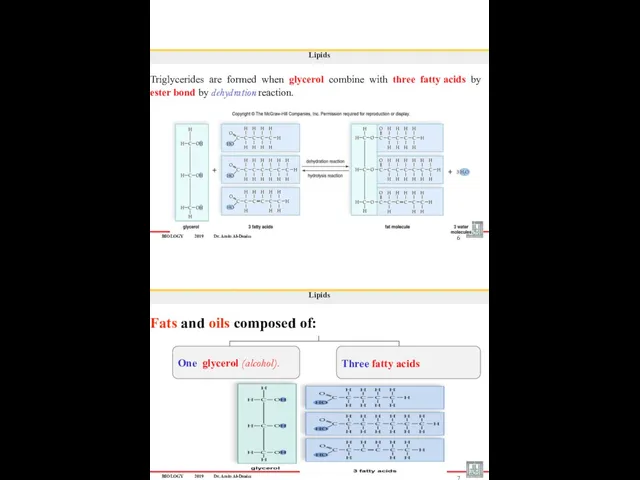
- 21. BIOLOGY 2019 Dr. Amin Al-Doaiss 6 Lipids Triglycerides are formed when glycerol combine with three fatty
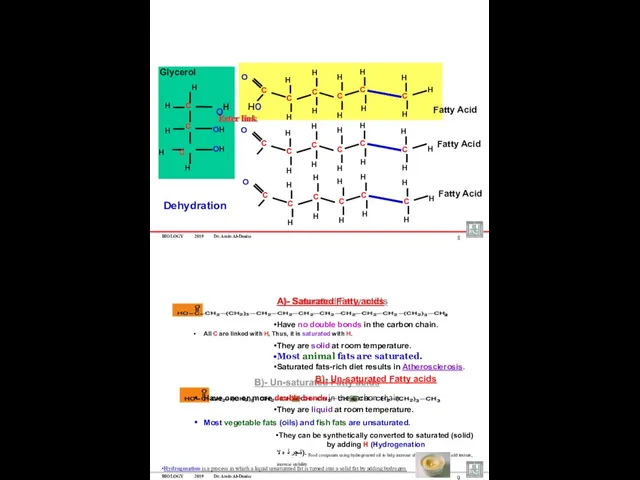
- 22. BIOLOGY 2019 Dr. Amin Al-Doaiss OH H H C C C C C C H H
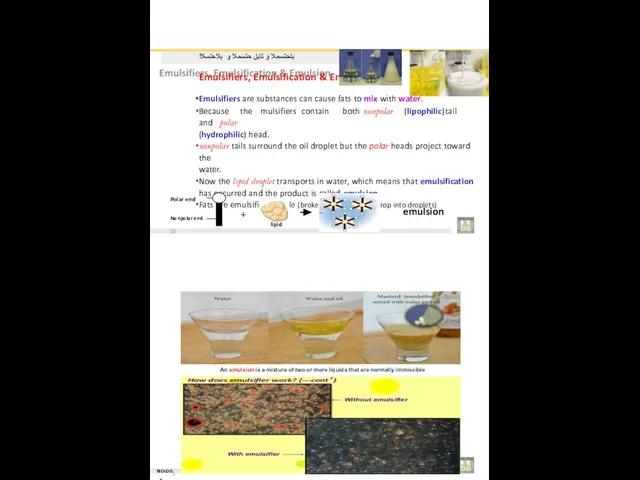
- 23. بلحتسملا و تابلِ حتسملا و بلاحتسلاا Emulsifiers, Emulsification & Emulsion Emulsifiers are substances can cause fats
- 24. BIOLOGY 2019 Dr. Amin Al-Doaiss Phospholipid molecule has two fatty acids attached to glycerol and a
- 25. Lipids Steroids : Steroids are lipids that have a backbone of four fused carbon rings (Cholesterol)
- 26. BIOLOGY 2019 Dr. Amin Al-Doaiss The Omega-3 Fatty Acids A special class of unsaturated fatty acids,
- 27. - The side chain R links with different compounds - Differences in R groups produce the
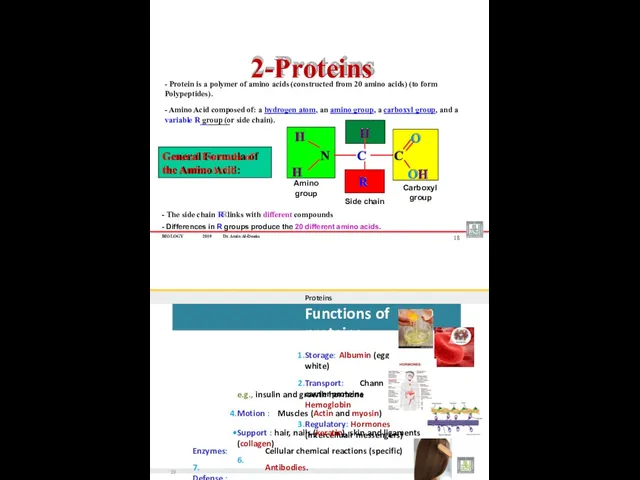
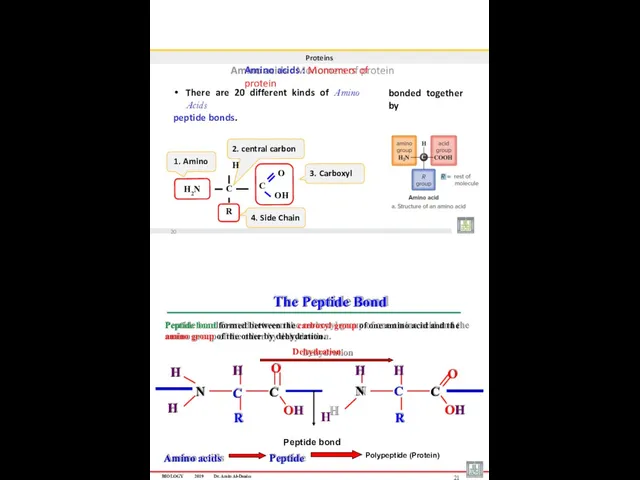
- 28. Proteins Amino acids : Monomers of protein There are 20 different kinds of Amino Acids peptide
- 29. Proteins Peptides : When two amino acids join together a dipeptide is formed. • • Three
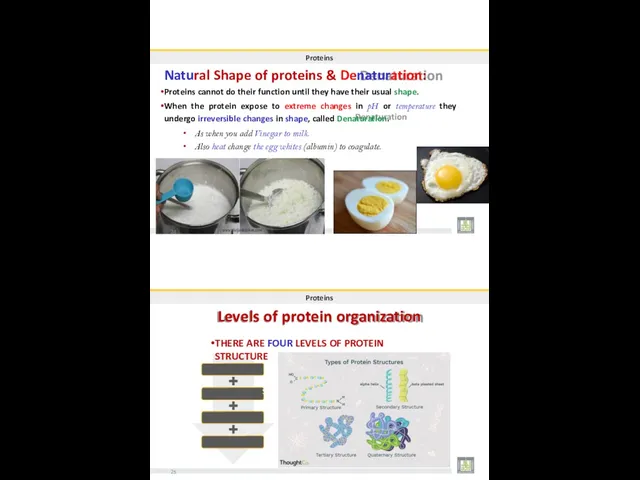
- 30. Proteins Natural Shape of proteins & Denaturation: Proteins cannot do their function until they have their
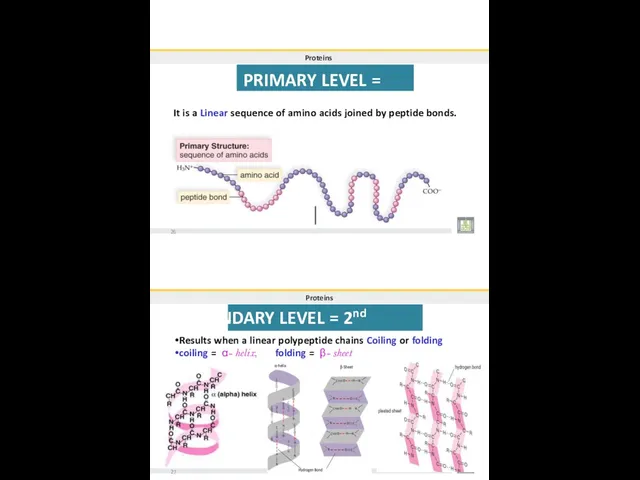
- 31. Proteins PRIMARY LEVEL = 1st It is a Linear sequence of amino acids joined by peptide
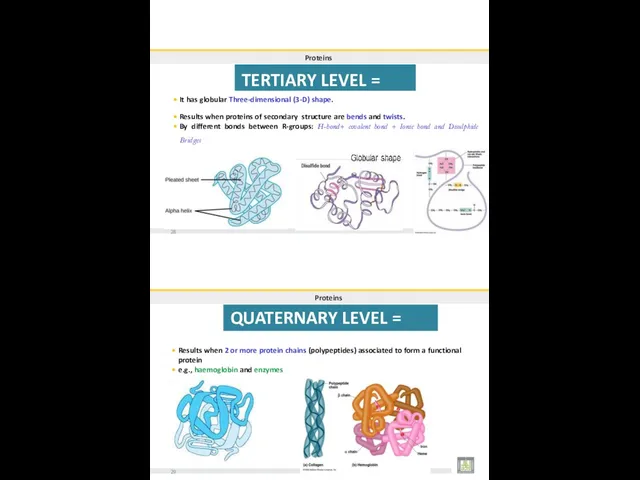
- 32. Proteins TERTIARY LEVEL = 3rd It has globular Three-dimensional (3-D) shape. Results when proteins of secondary
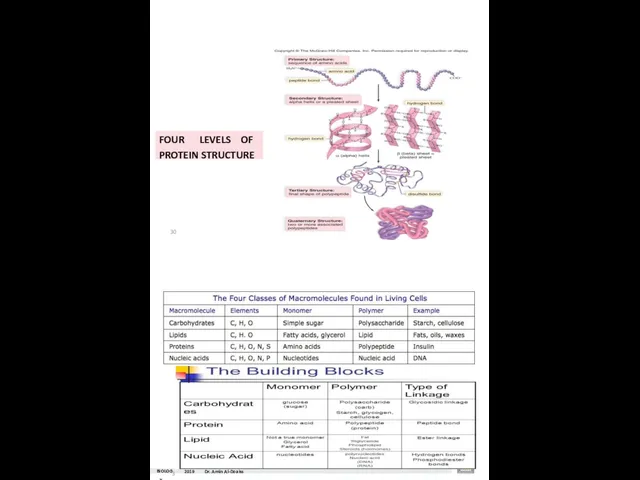
- 33. FOUR LEVELS OF PROTEIN STRUCTURE 30 BIOLOG31Y 2019 Dr. Amin Al-Doaiss
- 34. BIOLO3G2Y 2019 Dr. Ami n Al-Doaiss Dr. AMIN ABDULLAH AL-DOAISS
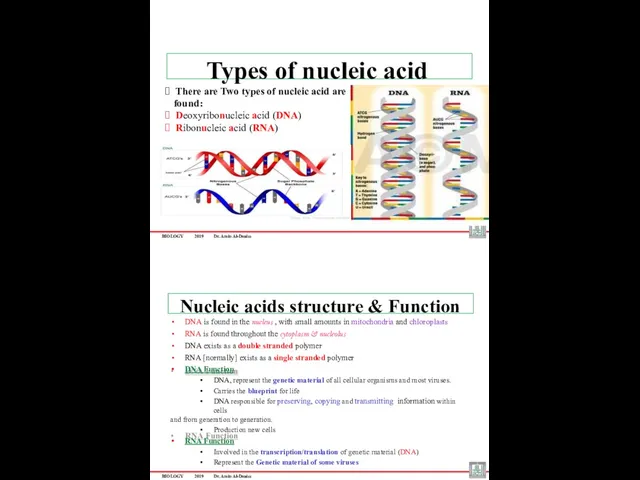
- 35. BIOLOGY 2019 Dr. Amin Al-Doaiss ? There are Two types of nucleic acid are found: ?
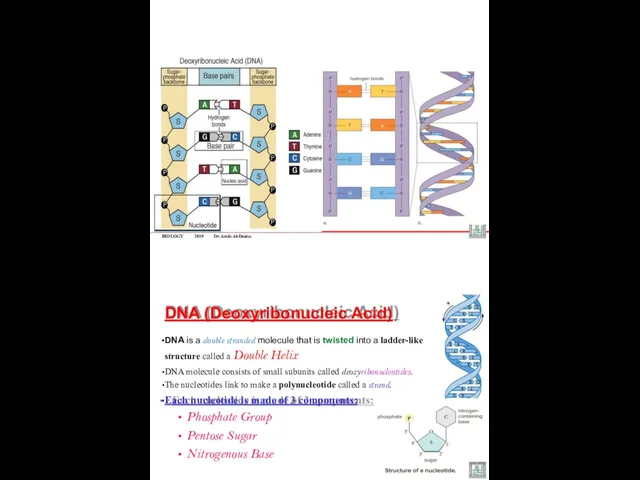
- 36. DNA (Deoxyribonucleic Acid) 1953- Watson and Crick created a 3-D model of structure called the double
- 37. BIOLOGY 2019 Dr. Amin Al-Doaiss (at King Saud University, Riyadh, Saudi Arabia, 2012) James Watson Amin
- 38. BIOLOGY 2019 Dr. Amin Al-Doaiss DNA (Deoxyribonucleic Acid) DNA is a double stranded molecule that is
- 39. BIOLOGY 2019 Dr. Amin Al-Doaiss Each Nucleotide consists of: 1. Nitrogenous Base: (Purines , Pyrimidines) A
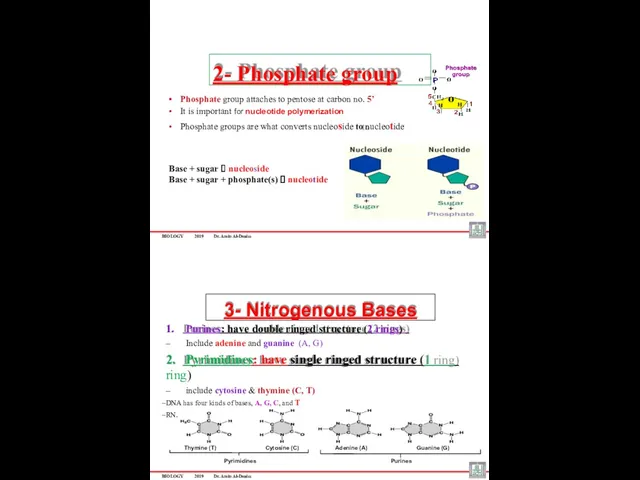
- 40. BIOLOGY 2019 Dr. Amin Al-Doaiss Phosphate group attaches to pentose at carbon no. 5’ It is
- 41. DNA Structure and Function There are four different nitrogen bases in DNA: Adenine (A) Guanine (G)
- 42. BIOLOGY 2019 Dr. Amin Al-Doaiss RiboNucleic Acid = RNA RNA is a Single stranded molecule made
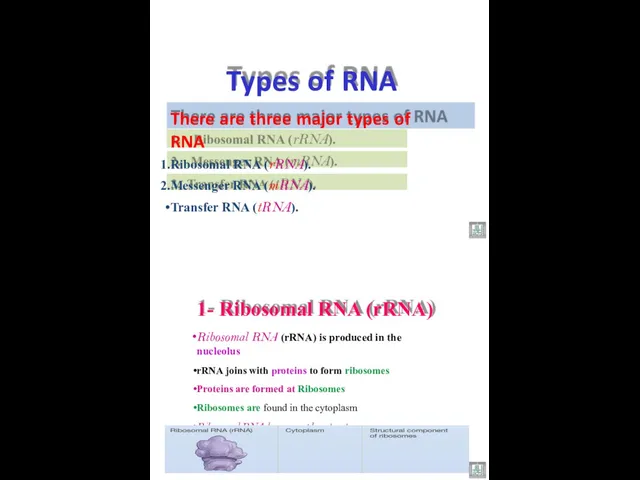
- 43. There are three major types of RNA Ribosomal RNA (rRNA). Messenger RNA (mRNA). Transfer RNA (tRNA).
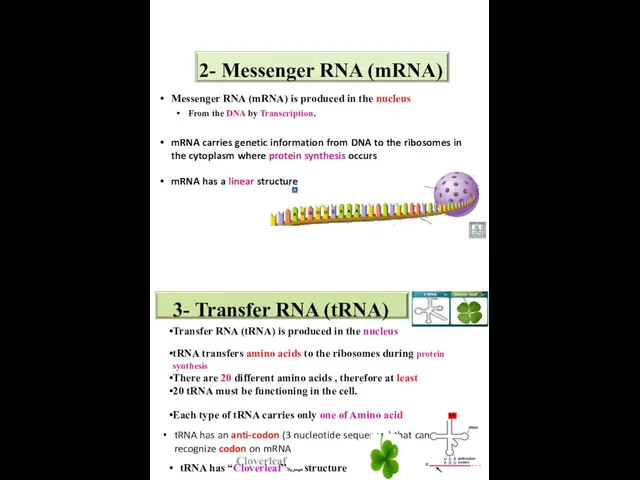
- 44. 2- Messenger RNA (mRNA) Messenger RNA (mRNA) is produced in the nucleus From the DNA by
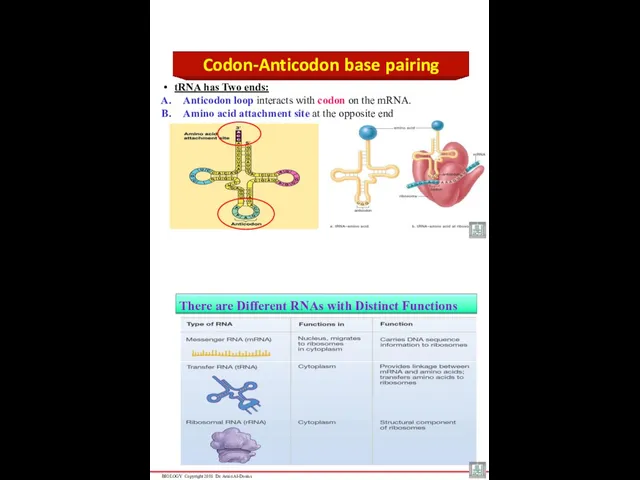
- 45. Codon-Anticodon base pairing tRNA has Two ends: Anticodon loop interacts with codon on the mRNA. Amino
- 46. DNA-RNA Similarities and differences Dr. AMIN ABDULLAH AL-DOAISS BIOLOGY 2019 Dr. Amin Al-Doaiss 1
- 47. BIOLOGY 2019 Dr. Amin Al-Doaiss 2 THE CELL The term of “ cell” comes from the
- 48. BIOLOGY 2019 Dr. Amin Al-Doaiss CELL THEORY Cell theory refers to an idea that said: cells
- 49. BIOLOGY 2019 Dr. Amin Al-Doaiss 6 1 mm 2 mm Cell size, why are the cells
- 50. BIOLOGY 2019 Dr. Amin Al-Doaiss Cell Types Cells can be classify into two categories: Prokaryotes (prokaryotic
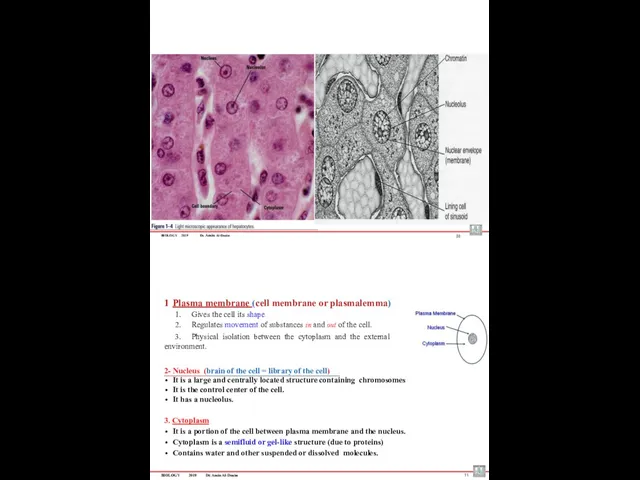
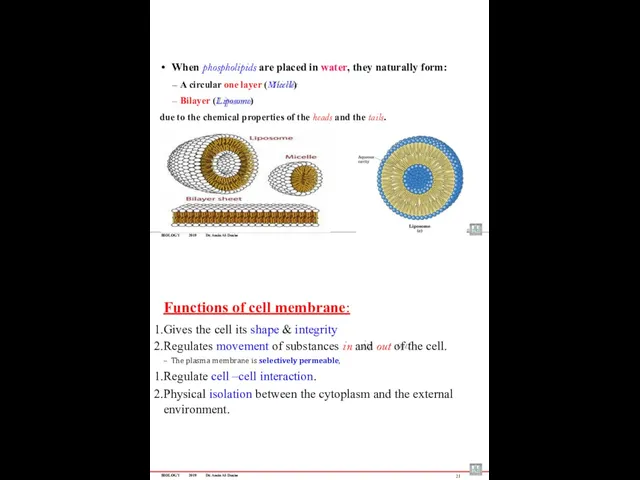
- 51. BIOLOGY 2019 Dr. Amin Al-Doaiss 10 Plasma membrane (cell membrane or plasmalemma) Gives the cell its
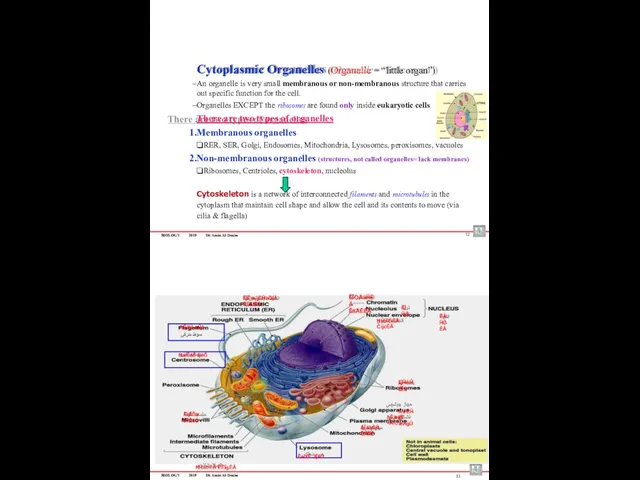
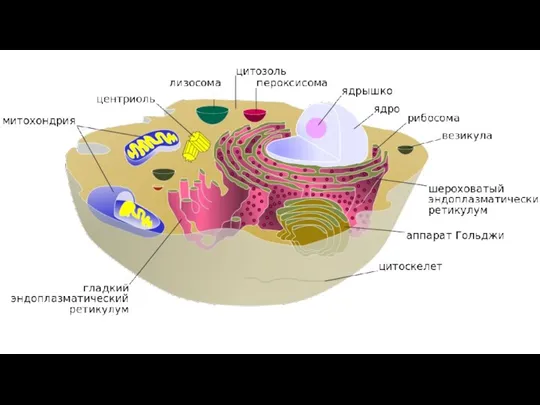
- 52. BIOLOGY 2019 Dr. Amin Al-Doaiss 12 Cytoplasmic Organelles (Organelle = “little organ”) An organelle is very
- 53. BIOLOGY 2019 Dr. Amin Al-Doaiss ËĪæĒäĚ ÊĤÖĊ ءÄäöÞ ÊàĪÎêijÈ ĦĤėÞĖÄ äÄàÖĖÄ ËĪĞĪÈ ÆĤďÒ 14 BIOLOGY 2019
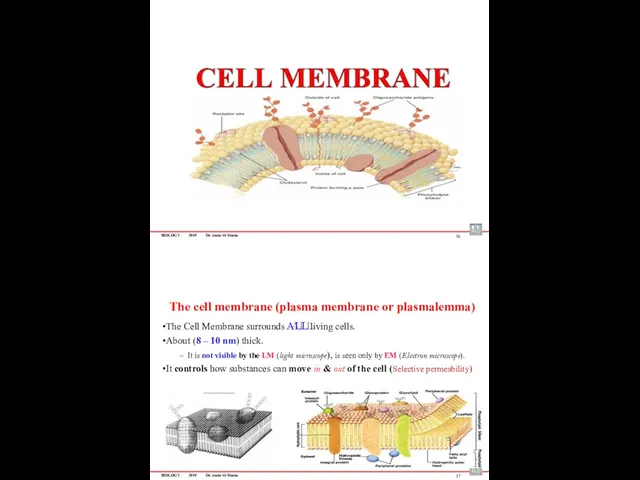
- 54. BIOLOGY 2019 Dr. Amin Al-Doaiss 16 The cell membrane (plasma membrane or plasmalemma) The Cell Membrane
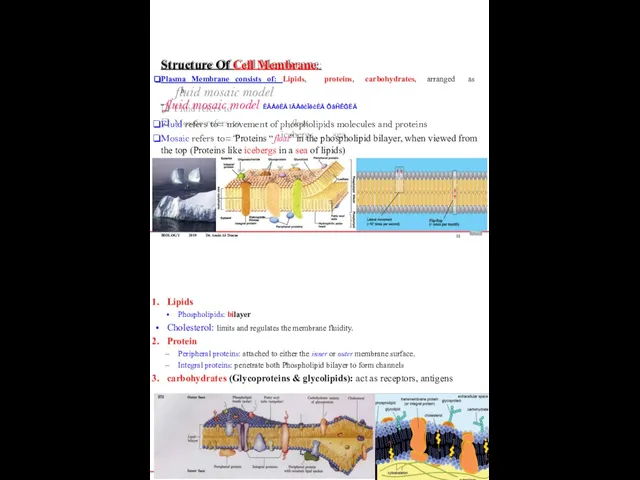
- 55. BIOLOGY 2019 Dr. Amin Al-Doaiss Structure Of Cell Membrane: Plasma Membrane consists of: Lipids, proteins, carbohydrates,
- 56. BIOLOGY 2019 Dr. Amin Al-Doaiss When phospholipids are placed in water, they naturally form: A circular
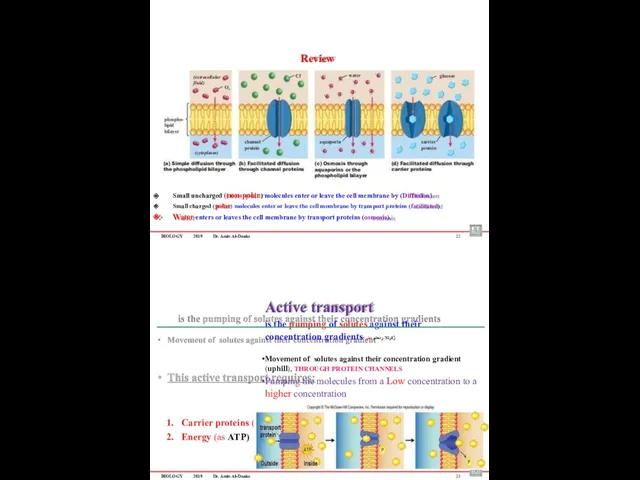
- 57. BIOLOGY 2019 Dr. Amin Al-Doaiss Selective permeability : – Means that some substances move freely across
- 58. BIOLOGY 2019 Dr. Amin Al-Doaiss Cell Transport is moving materials into, out of, or within the
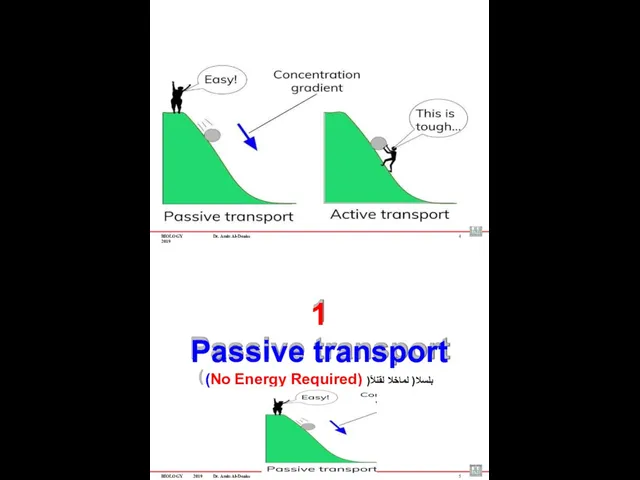
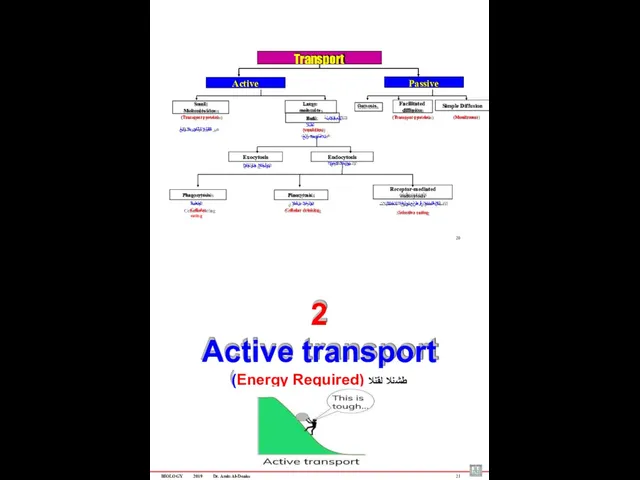
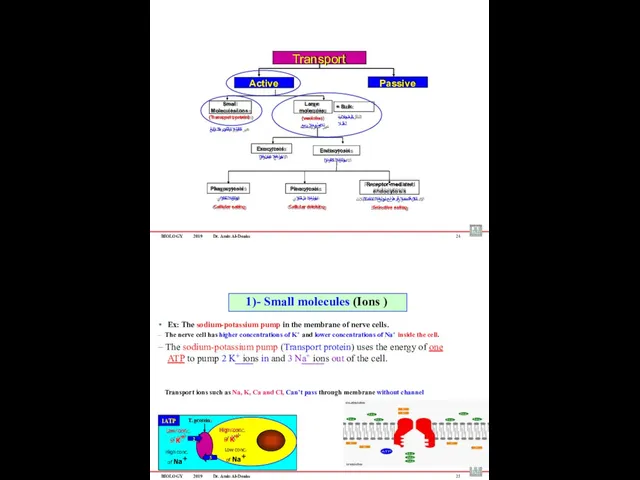
- 59. BIOLOGY 2019 Dr. Amin Al-Doaiss 4 1 Passive transport (No Energy Required) )ًبلسلا( لماخلا لقنلا BIOLOGY
- 60. BIOLOGY 2019 Dr. Amin Al-Doaiss Passive transport includes: Simple diffusion طٌسبلا راشتنلاا Facilitated diffusion رسٌملا لقنلا
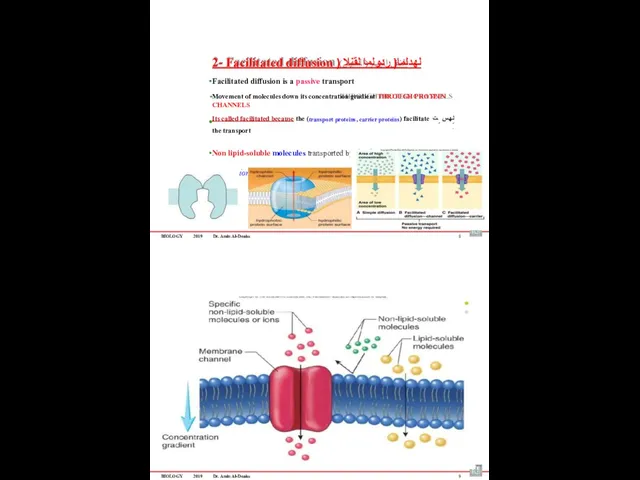
- 61. BIOLOGY 2019 Dr. Amin Al-Doaiss 2- Facilitated diffusion ) لهدلما( ردولما لقنلا Facilitated diffusion is a
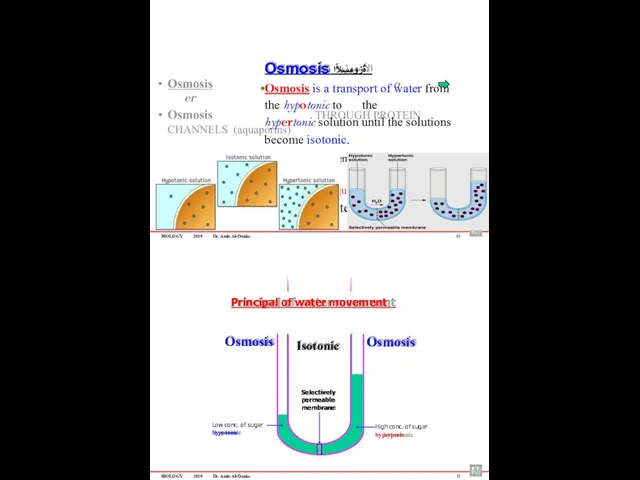
- 62. BIOLOGY 2019 Dr. Amin Al-Doaiss Osmosis ةٌزومسلأا Osmosis is a transport of water from the hypotonic
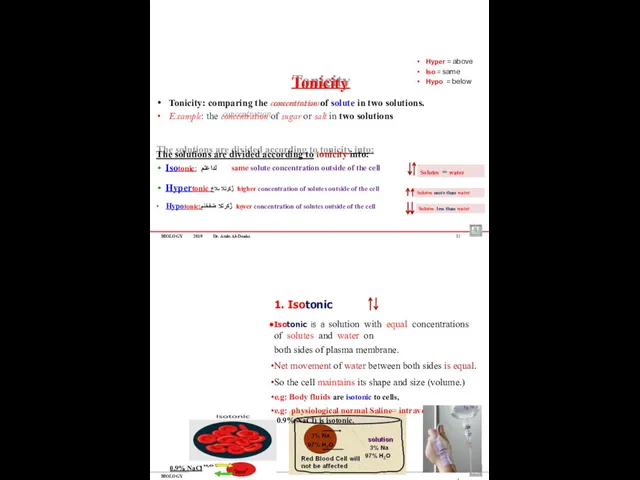
- 63. BIOLOGY 2019 Dr. Amin Al-Doaiss 12 Tonicity Tonicity: comparing the concentration of solute in two solutions.
- 64. BIOLOGY 2019 Dr. Amin Al-Doaiss 14 2. Hypotonic Hypotonic solution has lower solute concentration and higher
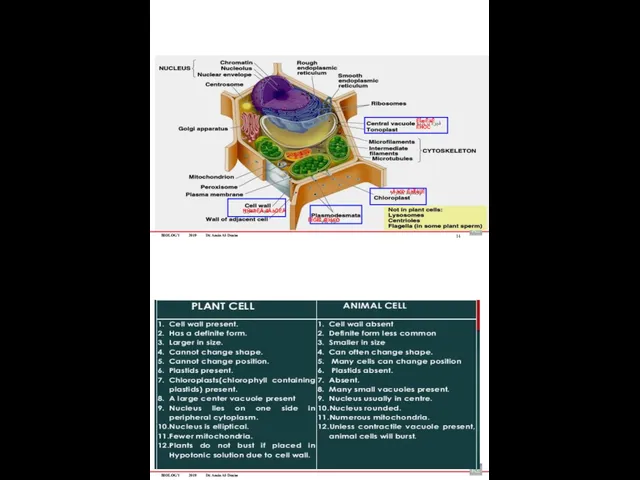
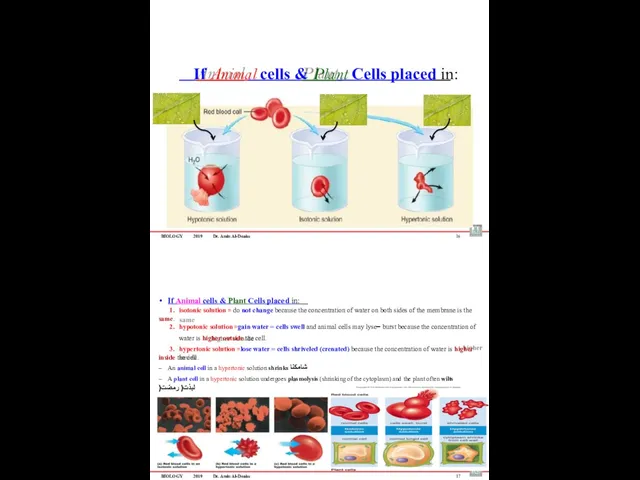
- 65. BIOLOGY 2019 Dr. Amin Al-Doaiss If Animal cells & Plant Cells placed in: 16 If Animal
- 66. BIOLOGY 2019 Dr. Amin Al-Doaiss 18 Normal plant cells Plasmolytic plant cells BIOLOGY 2019 Dr. Amin
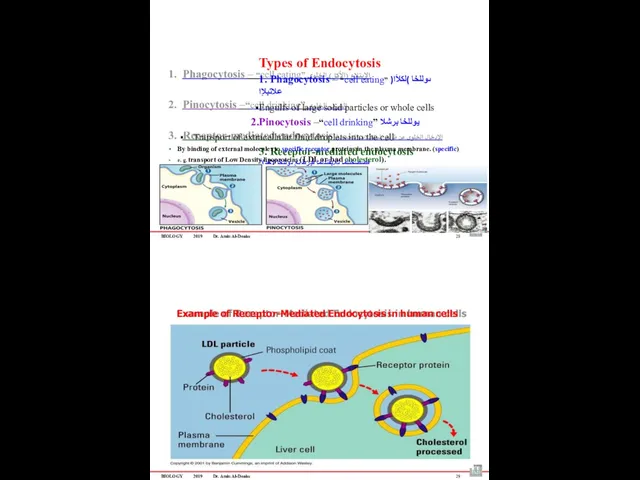
- 67. Phagocytosis Pinocytosis Receptor-mediated endocytosis ةمعلبلا Cellular eating يولخلا برشلا Cellular drinking تلابقتسملا قٌرط نع يولخلا لاخدلاا
- 68. BIOLOGY 2019 Dr. Amin Al-Doaiss 22 Small uncharged (non-polar) molecules enter or leave the cell membrane
- 69. BIOLOGY 2019 Dr. Amin Al-Doaiss 24 Phagocytosis Pinocytosis Receptor-mediated endocytosis يولخلا لكلاا Cellular eating يولخلا برشلا
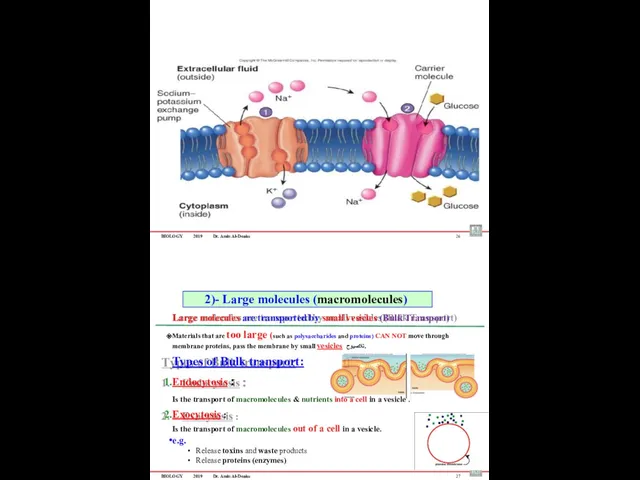
- 70. BIOLOGY 2019 Dr. Amin Al-Doaiss 26 Large molecules are transported by small vesicles (Bulk Transport) Materials
- 71. BIOLOGY 2019 Dr. Amin Al-Doaiss Types of Endocytosis 1. Phagocytosis – “cell eating” ىوللخا )لكلأا( علاتبلإا
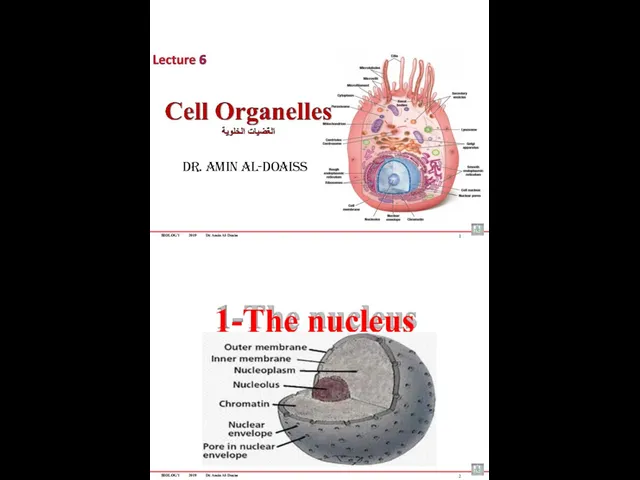
- 72. BIOLOGY 2019 Dr. Amin Al-Doaiss Dr. Amin Al-Doaiss 1 1-The nucleus BIOLOGY 2019 Dr. Amin Al-Doaiss
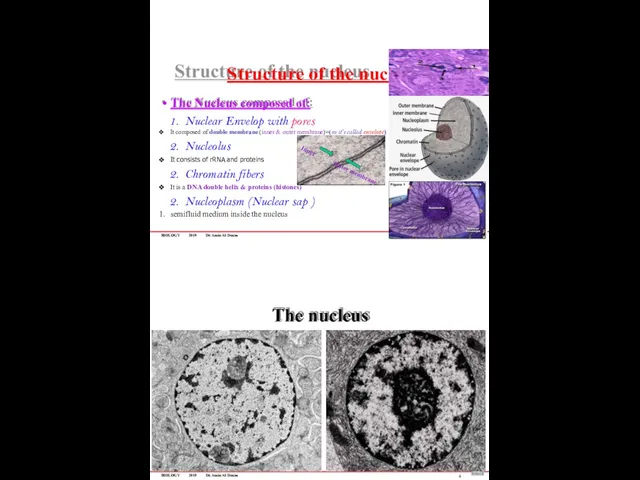
- 73. BIOLOGY 2019 Dr. Amin Al-Doaiss Structure of the nucleus 3 The Nucleus composed of: Nuclear Envelop
- 74. BIOLOGY 2019 Dr. Amin Al-Doaiss Chromatin Is a nucleoprotein structure composed of DNA double helix wrapped
- 75. BIOLOGY 2019 Dr. Amin Al-Doaiss Types of Ribosomes according their location & function 1) Free ribosomes
- 76. BIOLOGY 2019 Dr. Amin Al-Doaiss The Endomembrane system يلخادلا يئاشغلا زاهجلا The endomembrane system: (endo- =
- 77. BIOLOGY 2019 Dr. Amin Al-Doaiss 11 The cisternae of the (RER) they are flat membranes …...whereas
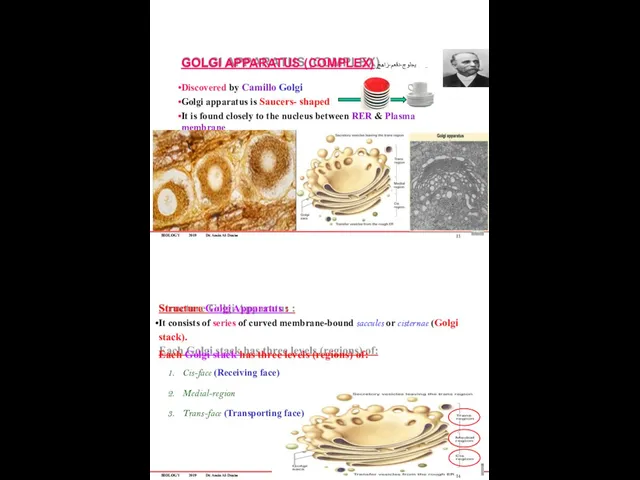
- 78. BIOLOGY 2019 Dr. Amin Al-Doaiss GOLGI APPARATUS (COMPLEX) يجلوج-دقعم-زاهج Discovered by Camillo Golgi Golgi apparatus is
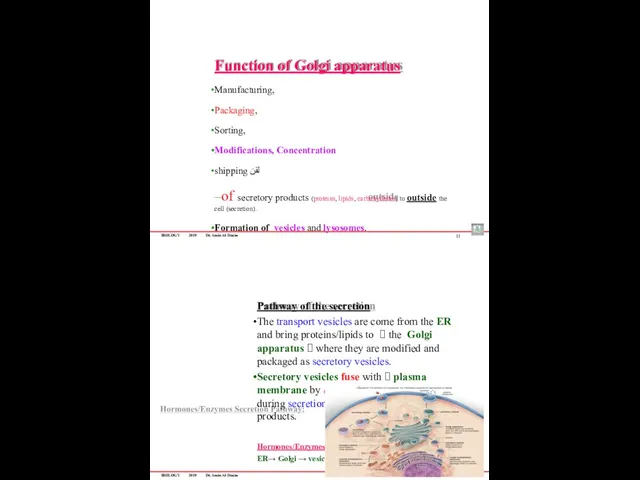
- 79. BIOLOGY 2019 Dr. Amin Al-Doaiss Function of Golgi apparatus Manufacturing, Packaging, Sorting, Modifications, Concentration shipping لقن
- 80. BIOLOGY 2019 Dr. Amin Al-Doaiss THE LYSOSOME Digestive bodies Trash collector of the cell 17 ة
- 81. BIOLOGY 2019 Dr. Amin Al-Doaiss Energy-Related Organelles The two energy-related organelles of eukaryotes are chloroplasts and
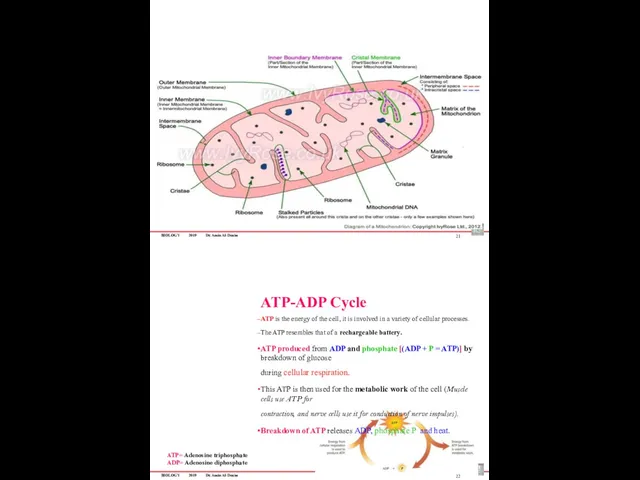
- 82. BIOLOGY 2019 Dr. Amin Al-Doaiss 21 ATP-ADP Cycle ATP is the energy of the cell, it
- 83. BIOLOGY 2019 Dr. Amin Al-Doaiss ATP molecule Adenosine triphosphate The three parts of an ATP molecule
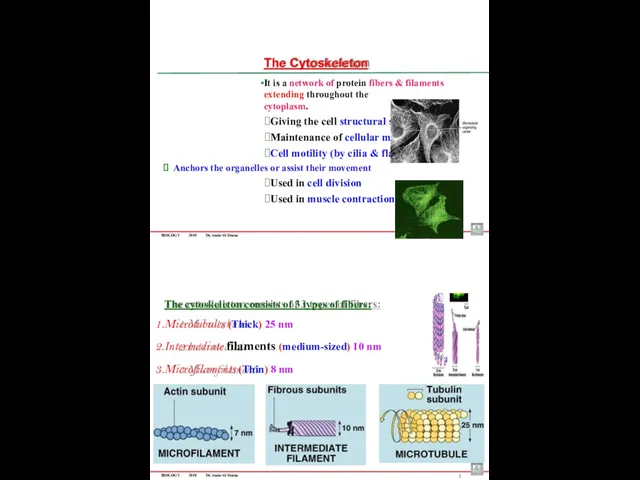
- 84. BIOLOGY 2019 Dr. Amin Al-Doaiss The Cytoskeleton It is a network of protein fibers & filaments
- 85. BIOLOGY 2019 Dr. Amin Al-Doaiss 4 5 1- Microfilaments (Actin filaments) The smallest & thinnest fibers
- 86. 6 BIOLOGY 2019 Dr. Amin Al-Doaiss Medium sized filaments They are rope-like filaments Composed of fibrous
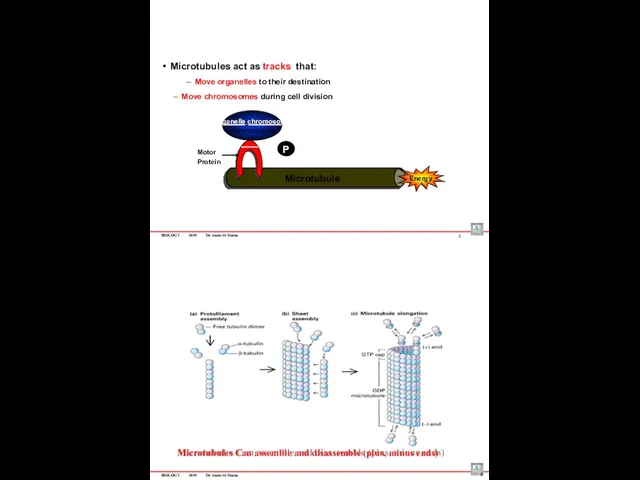
- 87. BIOLOGY 2019 Dr. Amin Al-Doaiss 8 Microtubule Motor Protein P Microtubules act as tracks that: Move
- 88. BIOLOGY 2019 Dr. Amin Al-Doaiss Cytoskeleton Tubular structure consist of α & β Tubulin protein Consist
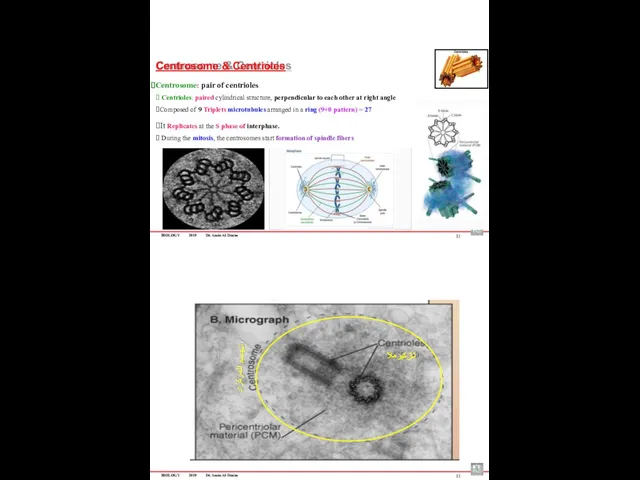
- 89. BIOLOGY 2019 Dr. Amin Al-Doaiss Centrosome & Centrioles Centrosome: pair of centrioles ⮚ Centrioles: paired cylindrical
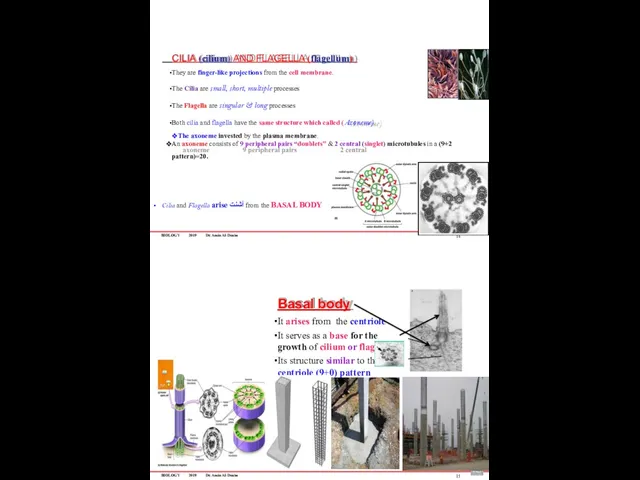
- 90. BIOLOGY 2019 Dr. Amin Al-Doaiss CILIA (cilium) AND FLAGELLA (flagellum) They are finger-like projections from the
- 91. BIOLOGY 2019 Dr. Amin Al-Doaiss Dr. Amin Al-Doaiss 1 Cellular metabolism The sum of all of
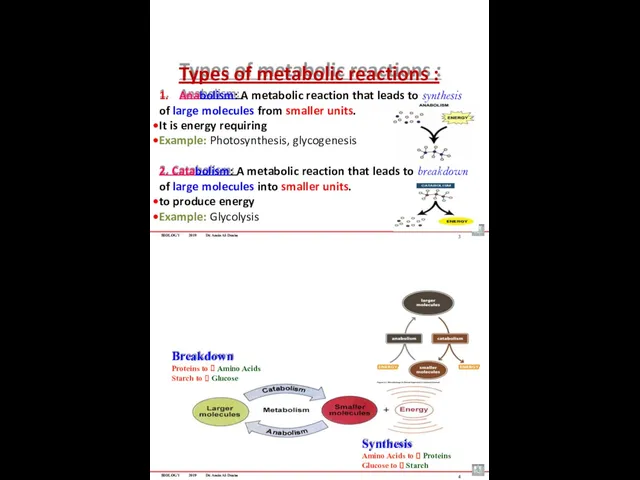
- 92. BIOLOGY 2019 Dr. Amin Al-Doaiss Types of metabolic reactions : 1. Anabolism: A metabolic reaction that
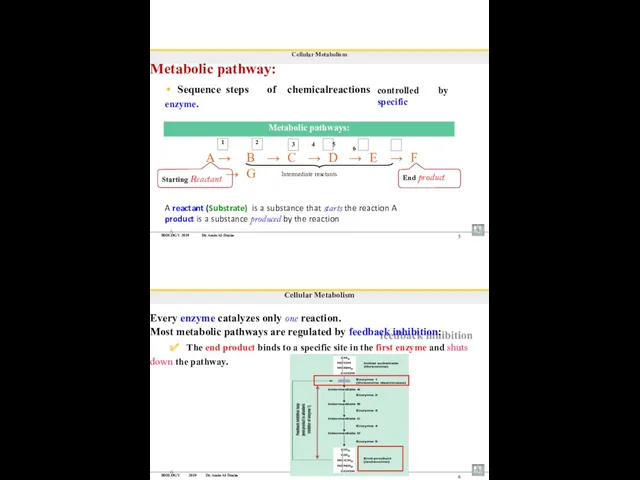
- 93. BIOLOGY 2019 Dr. Amin Al-Doaiss 5 Cellular Metabolism Metabolic pathway: Sequence steps of chemical reactions enzyme.
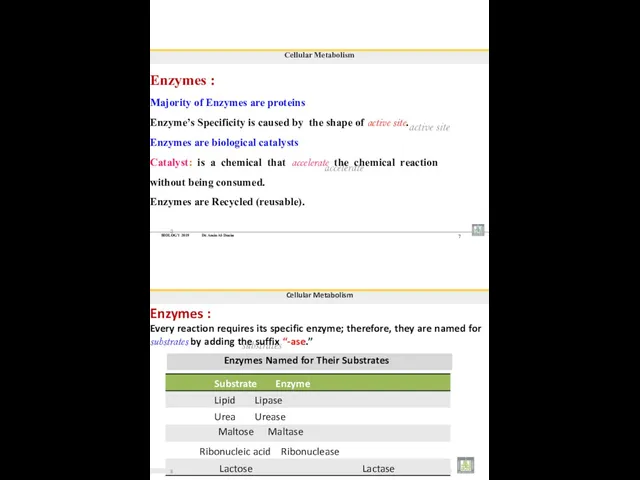
- 94. BIOLOGY 2019 Dr. Amin Al-Doaiss 7 Cellular Metabolism Enzymes : Majority of Enzymes are proteins Enzyme’s
- 95. BIOLOGY 2019 Dr. Amin Al-Doaiss 9 Cellular Metabolism Enzyme-Substrate Complex Enzymes form a complex with their
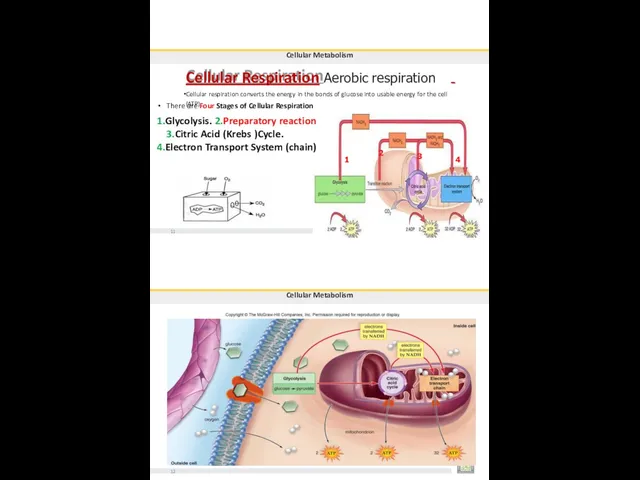
- 96. Cellular Respiration Aerobic respiration Cellular respiration converts the energy in the bonds of glucose into usable
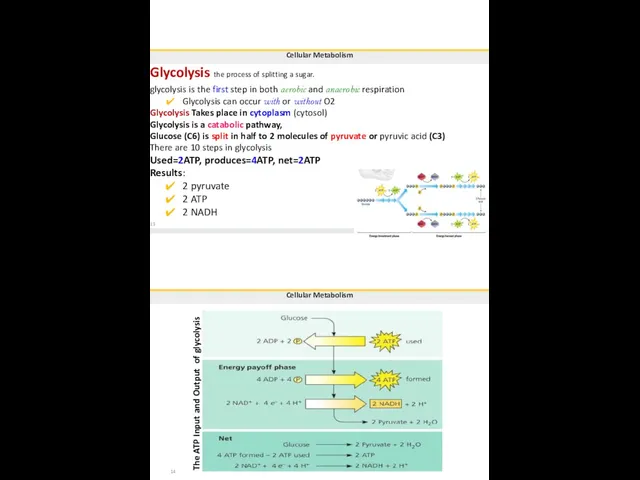
- 97. Cellular Metabolism Glycolysis the process of splitting a sugar. glycolysis is the first step in both
- 98. Cellular Metabolism Preparatory (transition, link) reaction Takes place in the matrix of the mitochondria. Pyruvate (3C)
- 99. Cellular Metabolism citric acid Input Acetic acid ADP Krebs Cycle 3 NAD+ FAD oxaloacetic acid 17
- 101. Скачать презентацию









































 Презентация к уроку биологии в 5 классе по теме: Живое и неживое: каковы особенности биологических систем? к учебнику В.А.Самкова, Д.И.Рокотова Биология 5 класс
Презентация к уроку биологии в 5 классе по теме: Живое и неживое: каковы особенности биологических систем? к учебнику В.А.Самкова, Д.И.Рокотова Биология 5 класс Царства живой природы
Царства живой природы Презентация к занятию по теме Аллергия
Презентация к занятию по теме Аллергия Жасуша теориясы
Жасуша теориясы Презентация про ЗОЖ для 5классов.
Презентация про ЗОЖ для 5классов. Russian desman
Russian desman Class Basidiomycetes. Class Deuteromyces
Class Basidiomycetes. Class Deuteromyces Нейрофизиология соматосенсорной системы
Нейрофизиология соматосенсорной системы Зоофагтарды, гербифагтарды және микроорганизмдерді қолдану әдістері. (Лекция 10)
Зоофагтарды, гербифагтарды және микроорганизмдерді қолдану әдістері. (Лекция 10) Птичий калейдоскоп. Внеклассное мероприятие
Птичий калейдоскоп. Внеклассное мероприятие Поучительные рассказы о животных от профессора Колобкова
Поучительные рассказы о животных от профессора Колобкова Большие личности миниатюрных пород
Большие личности миниатюрных пород Значение гомологии и аналогии в живом мире
Значение гомологии и аналогии в живом мире What is the engine of our body machine
What is the engine of our body machine Строение и функции кожи человека
Строение и функции кожи человека Тема навчального проекту. Біологія-це цікаво
Тема навчального проекту. Біологія-це цікаво Тип Кишечнополостные
Тип Кишечнополостные Общий путь катаболизма. (Лекция 9)
Общий путь катаболизма. (Лекция 9) Организационно-экономические основы формирования и развития устойчивой системы селекции и семеноводства в регионе
Организационно-экономические основы формирования и развития устойчивой системы селекции и семеноводства в регионе Құрамында антрацен туындылары бар дәрілік өсімдік шикізаттарын талдау
Құрамында антрацен туындылары бар дәрілік өсімдік шикізаттарын талдау Разнообразие растений
Разнообразие растений Физиология кровообращения
Физиология кровообращения Движение крови по сосудам. Причины движения крови по сосудам
Движение крови по сосудам. Причины движения крови по сосудам Испарение воды растением
Испарение воды растением Культура горох посевной
Культура горох посевной Возникновение и развитие эволюционных представлений
Возникновение и развитие эволюционных представлений Методы исследования генетики человека
Методы исследования генетики человека Споровые растения
Споровые растения