Содержание
- 2. Some information about money and RNA In 2014, the monoclonal antibodies market had the highest growth
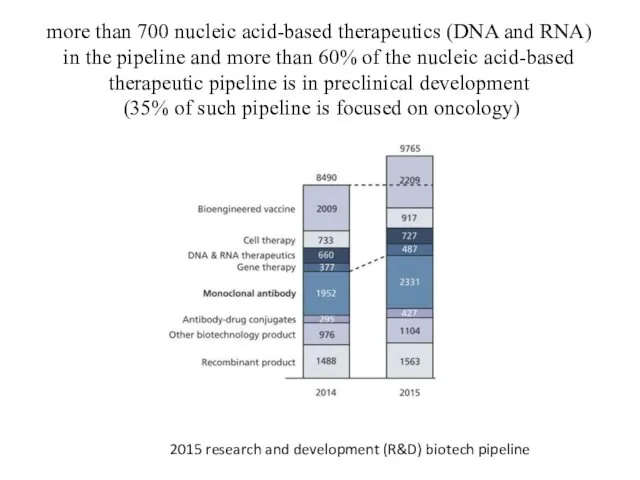
- 3. more than 700 nucleic acid-based therapeutics (DNA and RNA) in the pipeline and more than 60%
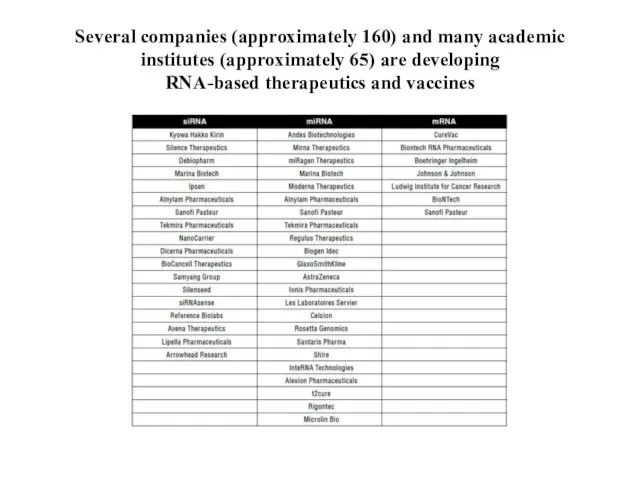
- 4. Several companies (approximately 160) and many academic institutes (approximately 65) are developing RNA-based therapeutics and vaccines
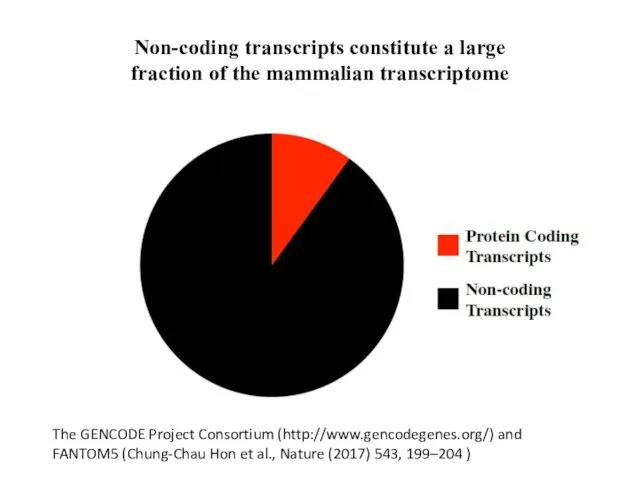
- 5. Non-coding transcripts constitute a large fraction of the mammalian transcriptome The GENCODE Project Consortium (http://www.gencodegenes.org/) and
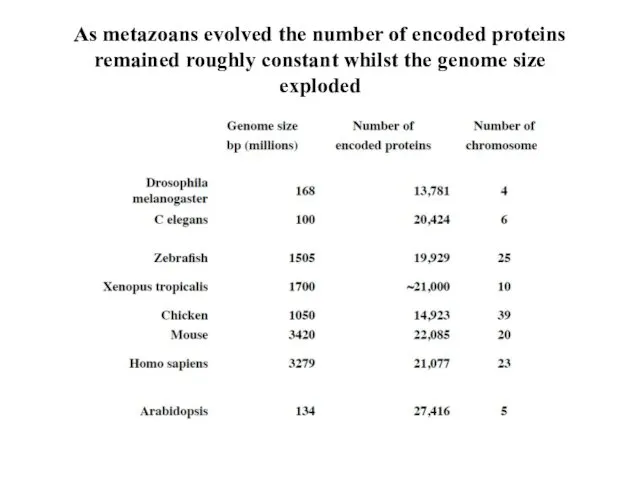
- 6. As metazoans evolved the number of encoded proteins remained roughly constant whilst the genome size exploded
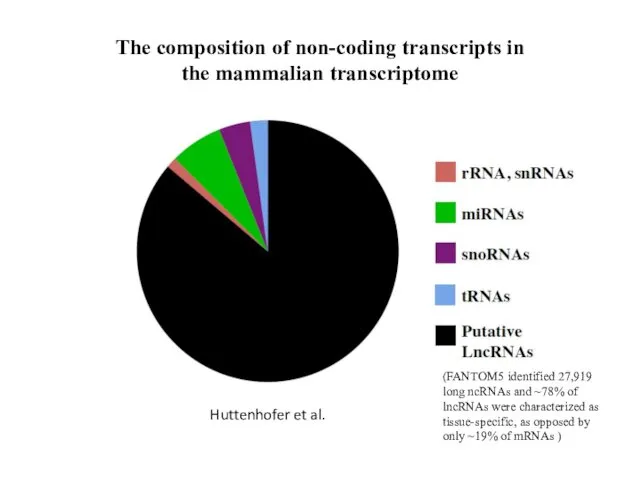
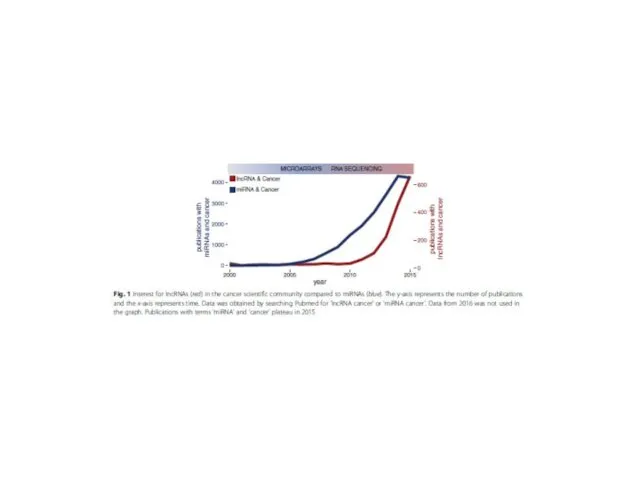
- 7. The composition of non-coding transcripts in the mammalian transcriptome Huttenhofer et al. (FANTOM5 identified 27,919 long
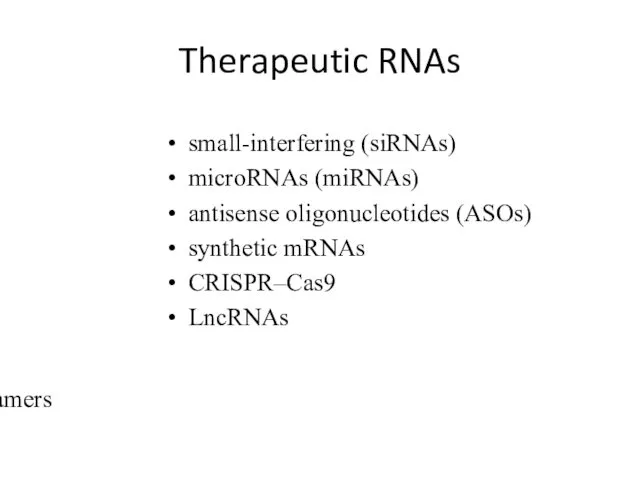
- 8. Therapeutic RNAs small-interfering (siRNAs) microRNAs (miRNAs) antisense oligonucleotides (ASOs) synthetic mRNAs CRISPR–Cas9 LncRNAs aptamers
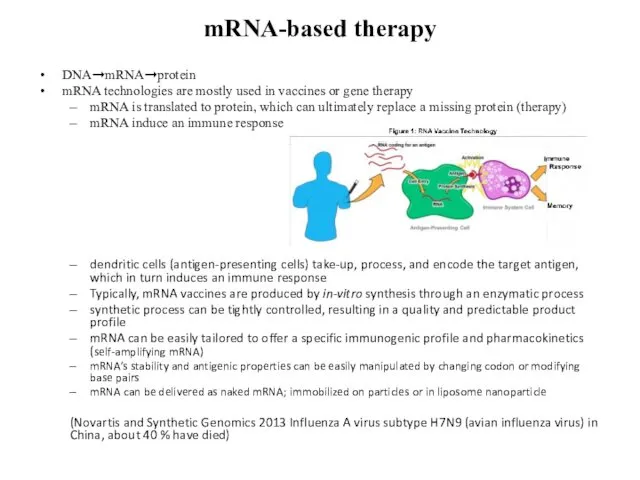
- 9. mRNA-based therapy DNA➞mRNA➞protein mRNA technologies are mostly used in vaccines or gene therapy mRNA is translated
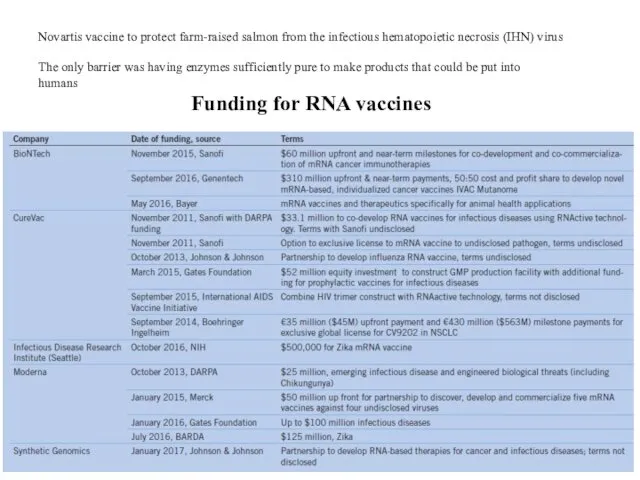
- 10. Novartis vaccine to protect farm-raised salmon from the infectious hematopoietic necrosis (IHN) virus The only barrier
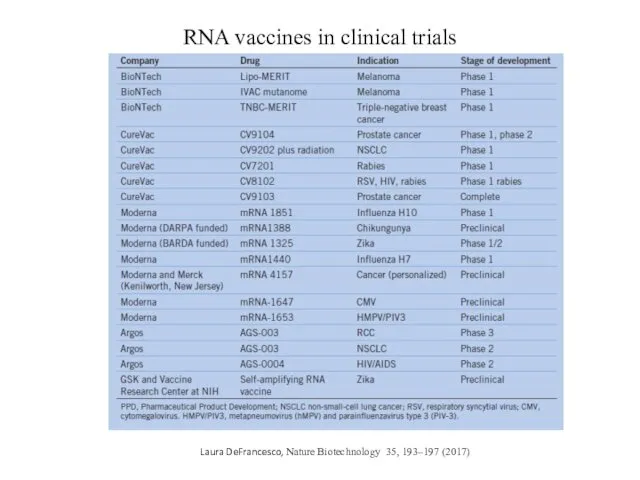
- 11. RNA vaccines in clinical trials Laura DeFrancesco, Nature Biotechnology 35, 193–197 (2017)
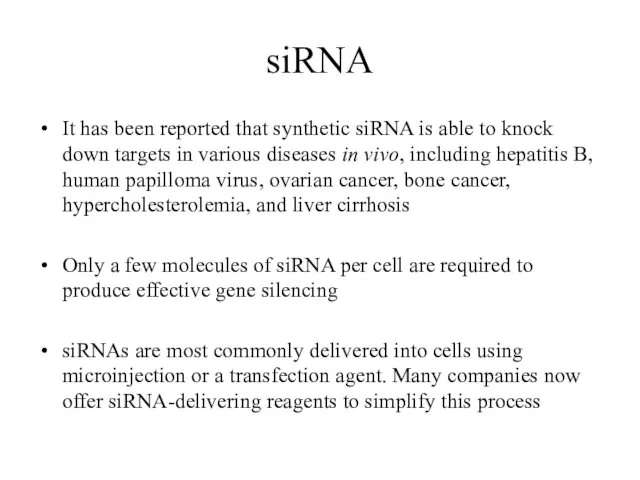
- 12. siRNA It has been reported that synthetic siRNA is able to knock down targets in various
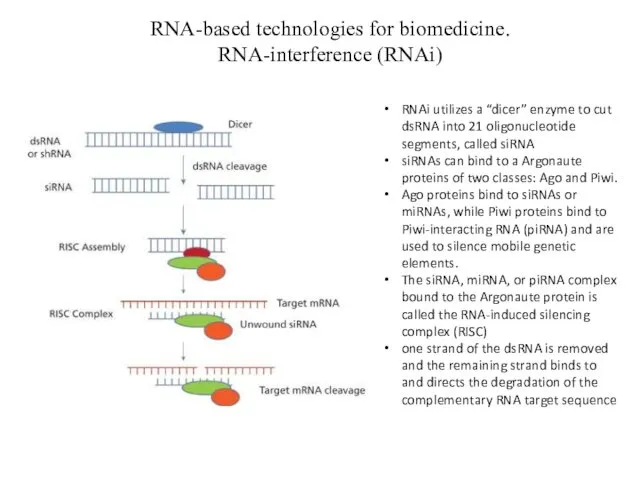
- 13. RNA-based technologies for biomedicine. RNA-interference (RNAi) RNAi utilizes a “dicer” enzyme to cut dsRNA into 21
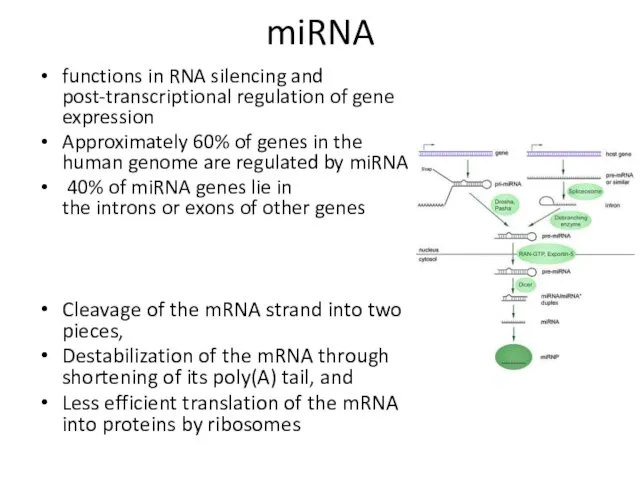
- 14. miRNA functions in RNA silencing and post-transcriptional regulation of gene expression Approximately 60% of genes in
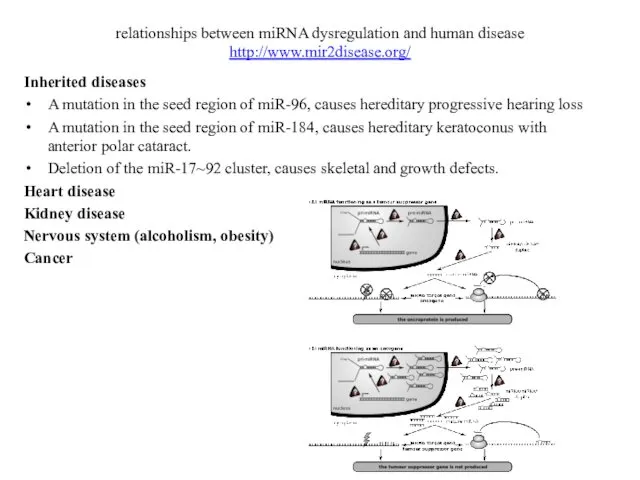
- 15. relationships between miRNA dysregulation and human disease http://www.mir2disease.org/ Inherited diseases A mutation in the seed region
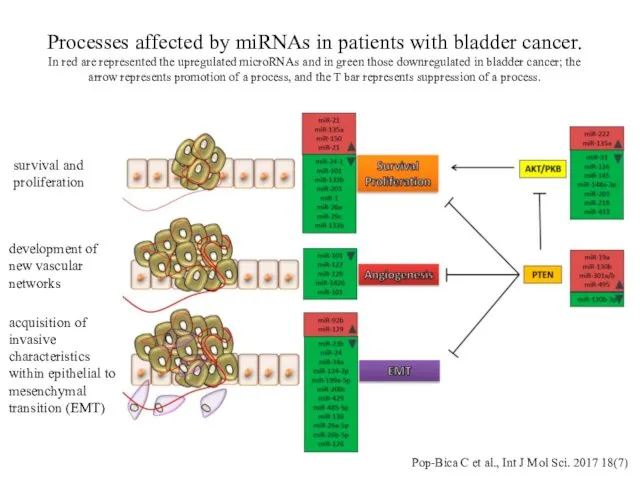
- 16. Processes affected by miRNAs in patients with bladder cancer. In red are represented the upregulated microRNAs
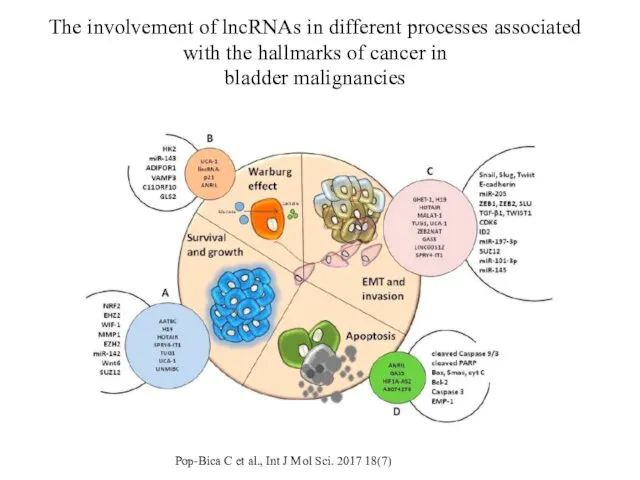
- 17. The involvement of lncRNAs in different processes associated with the hallmarks of cancer in bladder malignancies
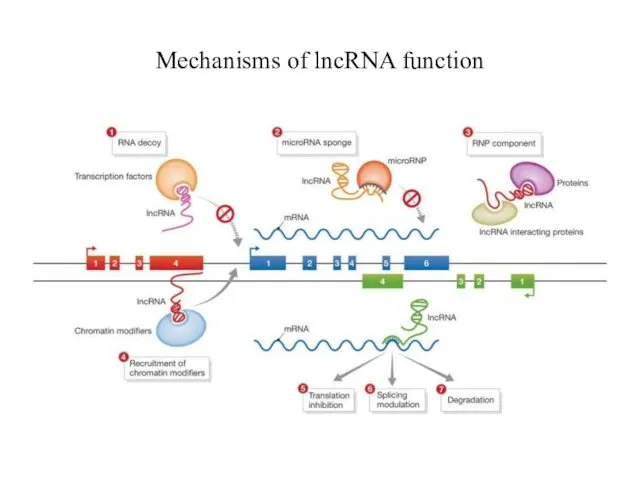
- 18. Mechanisms of lncRNA function
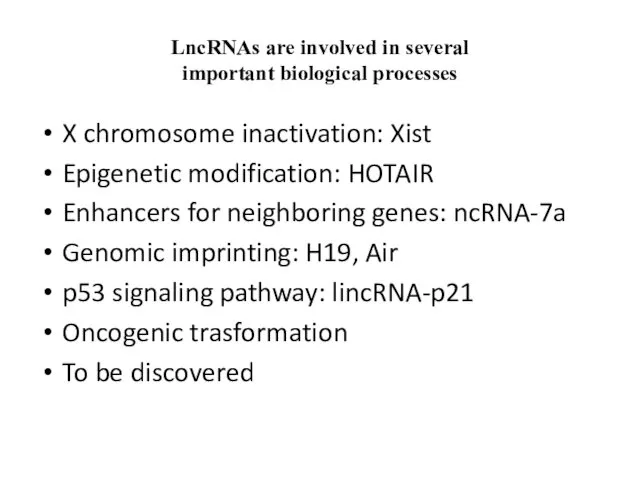
- 19. LncRNAs are involved in several important biological processes X chromosome inactivation: Xist Epigenetic modification: HOTAIR Enhancers
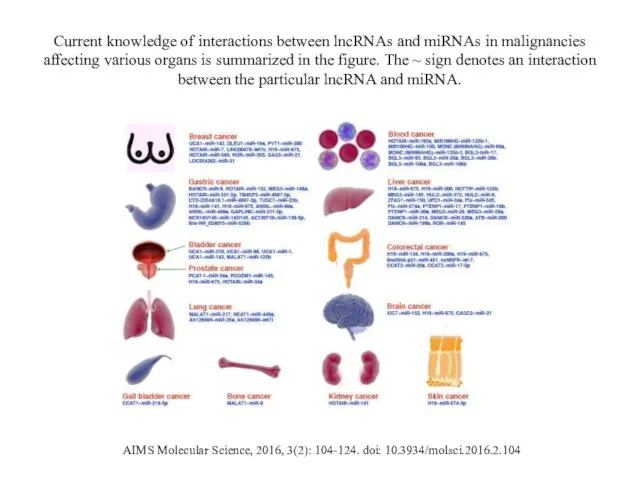
- 20. Current knowledge of interactions between lncRNAs and miRNAs in malignancies affecting various organs is summarized in
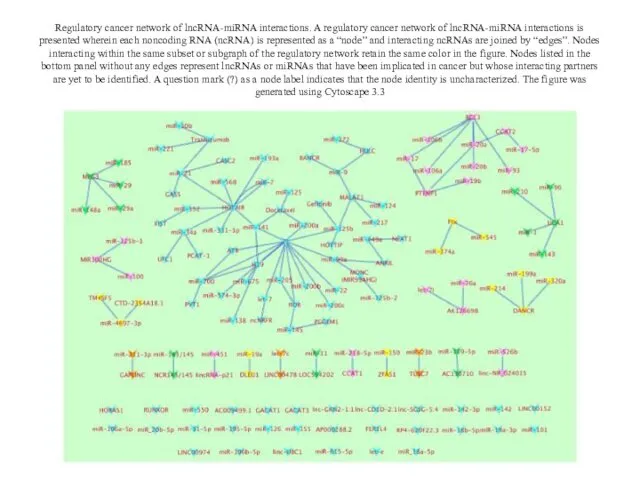
- 21. Regulatory cancer network of lncRNA-miRNA interactions. A regulatory cancer network of lncRNA-miRNA interactions is presented wherein
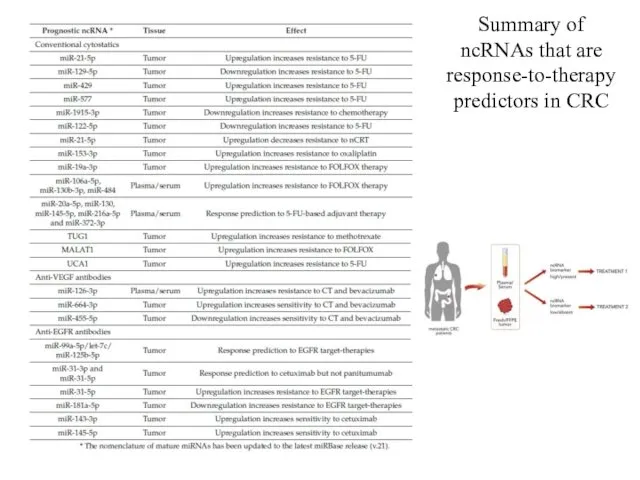
- 22. Summary of ncRNAs that are response-to-therapy predictors in CRC
- 23. RNA-interference (RNAi) steps into biomedicine 1998 Andrew Fire and Craig Mello first demonstrated RNAi in C.
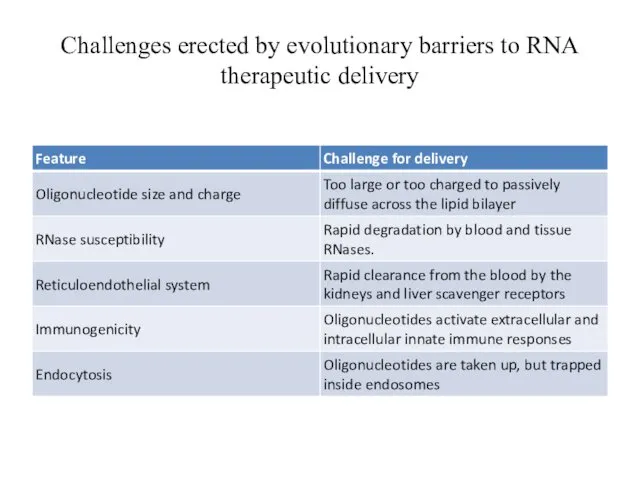
- 24. Challenges erected by evolutionary barriers to RNA therapeutic delivery
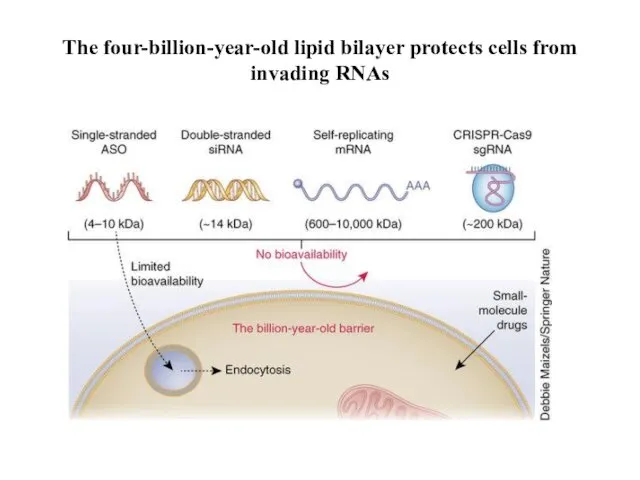
- 25. The four-billion-year-old lipid bilayer protects cells from invading RNAs
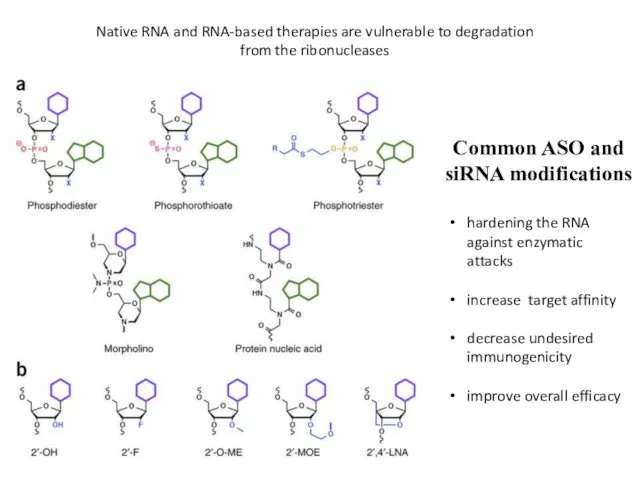
- 26. Common ASO and siRNA modifications hardening the RNA against enzymatic attacks increase target affinity decrease undesired
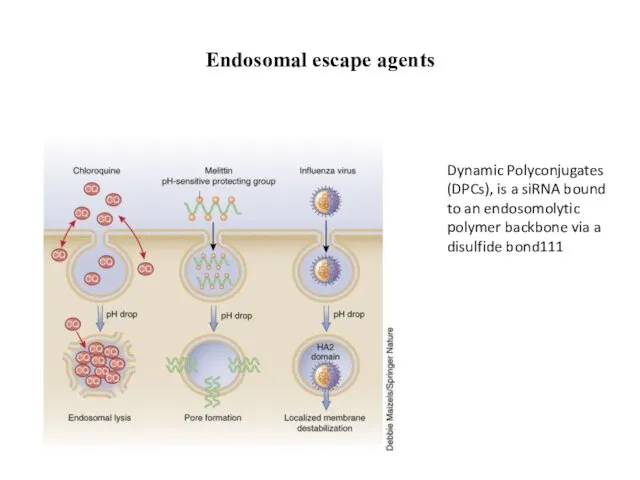
- 27. Endosomal escape agents Dynamic Polyconjugates (DPCs), is a siRNA bound to an endosomolytic polymer backbone via
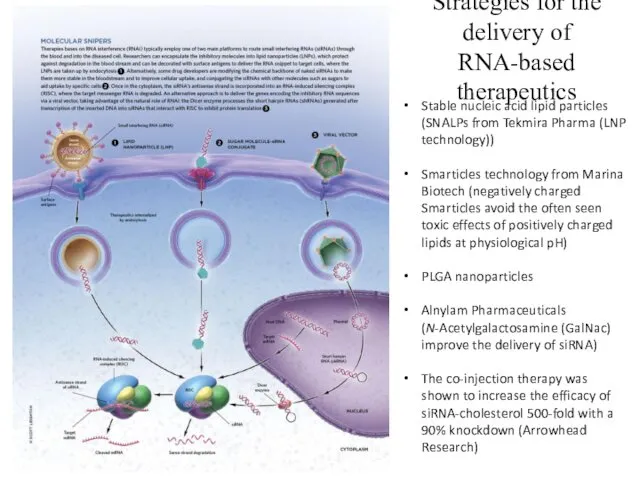
- 28. Strategies for the delivery of RNA-based therapeutics Stable nucleic acid lipid particles (SNALPs from Tekmira Pharma
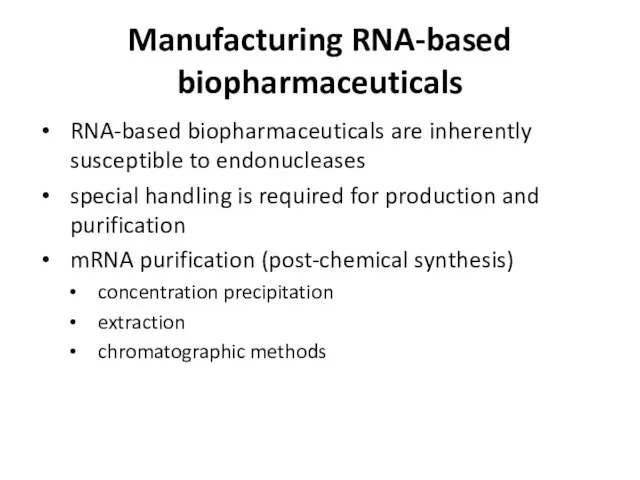
- 29. Manufacturing RNA-based biopharmaceuticals RNA-based biopharmaceuticals are inherently susceptible to endonucleases special handling is required for production
- 30. First ever RNA-based gene-silencing drug approved by FDA hereditary transthyretin-mediated amyloidosis (hATTR) US$450,000 per year for
- 32. Какие разделы добавить: (конкретные примеры) https://nplus1.ru/news/2017/10/05/gold-crispr https://nplus1.ru/news/2017/10/06/Cas13-vs-interference
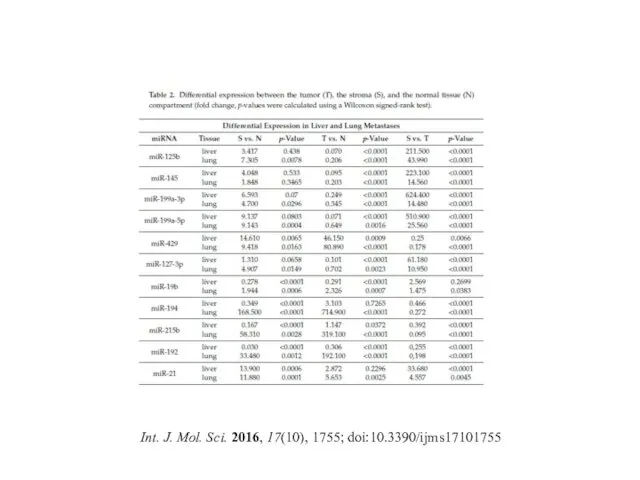
- 33. Int. J. Mol. Sci. 2016, 17(10), 1755; doi:10.3390/ijms17101755
- 36. Скачать презентацию




 Введение в анатомию
Введение в анатомию Головач гигантский
Головач гигантский Прогулки с динозаврами
Прогулки с динозаврами Изолирующие механизмы. 9 класс
Изолирующие механизмы. 9 класс Голосеменные растения
Голосеменные растения Презентация Основные типы экологических взаимодействий
Презентация Основные типы экологических взаимодействий Наши домашние питомцы (1 класс)
Наши домашние питомцы (1 класс) Викторина для знатоков природы
Викторина для знатоков природы Аққауданды капуста сорттарын бағалау
Аққауданды капуста сорттарын бағалау Практика по общей биологии
Практика по общей биологии Вид. Критерии вида
Вид. Критерии вида Мышцы туловища
Мышцы туловища Плоды
Плоды презентация к уроку биологии 7 класс
презентация к уроку биологии 7 класс Жасыл балдырлар бөлімі – Chlorophyta
Жасыл балдырлар бөлімі – Chlorophyta Цікаві факти про гриби
Цікаві факти про гриби Физиология обмена веществ и энергии
Физиология обмена веществ и энергии Розмноження птахів
Розмноження птахів Вены большого и малого круга кровообращения
Вены большого и малого круга кровообращения Класс Пресмыкающиеся. Особенности внешнего и внутреннего строения. Многообразие. Происхождение
Класс Пресмыкающиеся. Особенности внешнего и внутреннего строения. Многообразие. Происхождение Класс Насекомые
Класс Насекомые Фотосинтез и дыхание. Презентация 6 класс
Фотосинтез и дыхание. Презентация 6 класс Відозміна листка
Відозміна листка Основные направления эволюции
Основные направления эволюции Тонкий кишечник
Тонкий кишечник Семейство растений капустные
Семейство растений капустные Одомашнивание, как начальный этап селекции
Одомашнивание, как начальный этап селекции Анализаторы
Анализаторы