Содержание
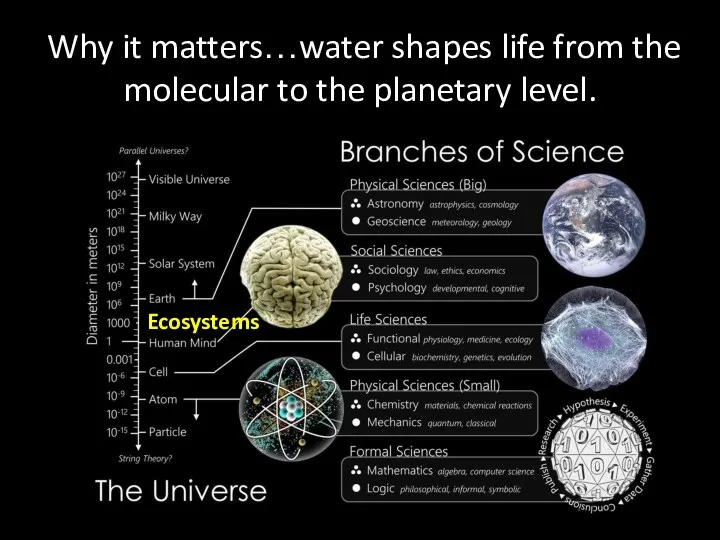
- 2. Why it matters…water shapes life from the molecular to the planetary level. Ecosystems
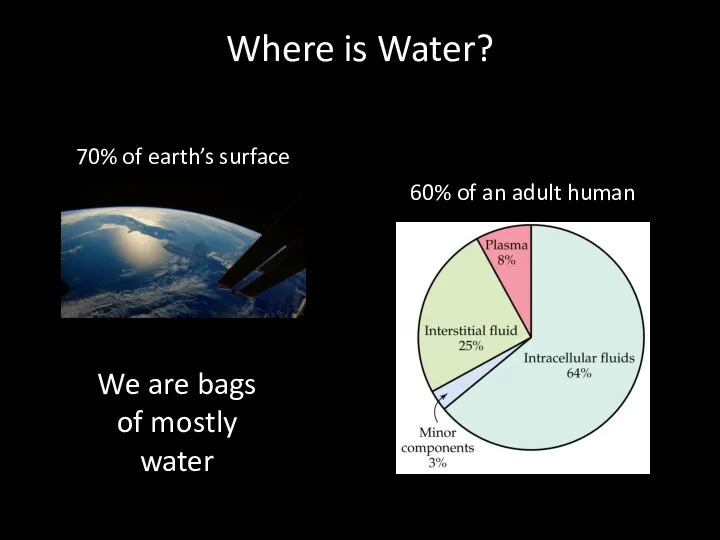
- 3. Where is Water? 70% of earth’s surface 60% of an adult human We are bags of
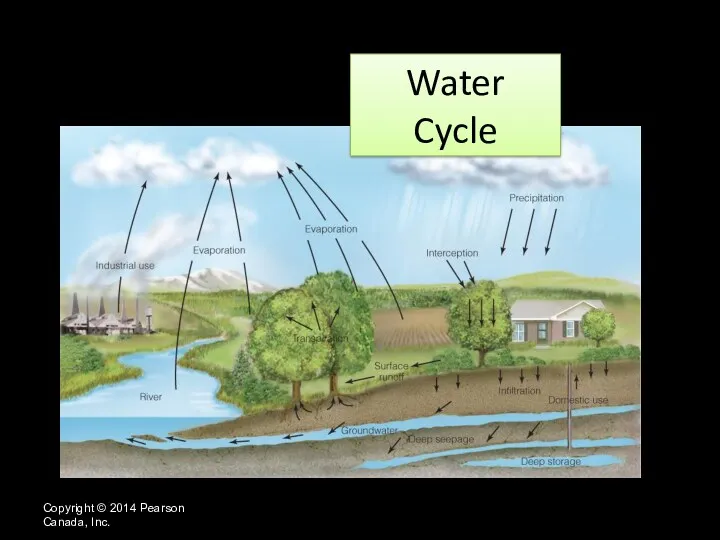
- 4. Water Cycle Copyright © 2014 Pearson Canada, Inc.
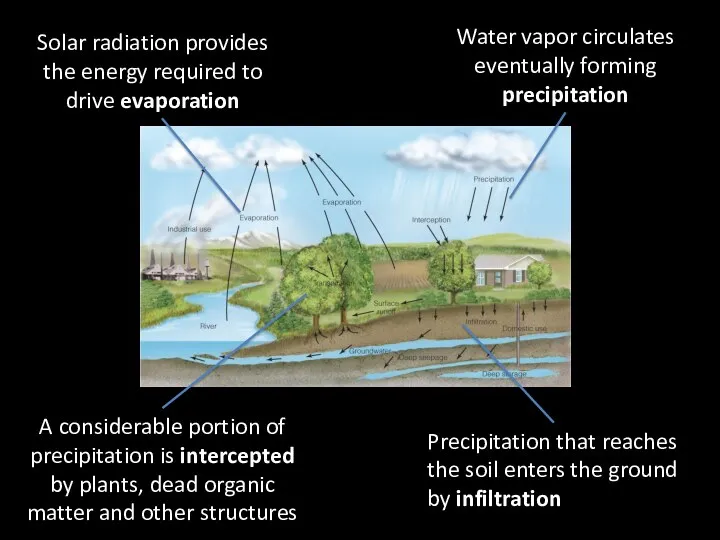
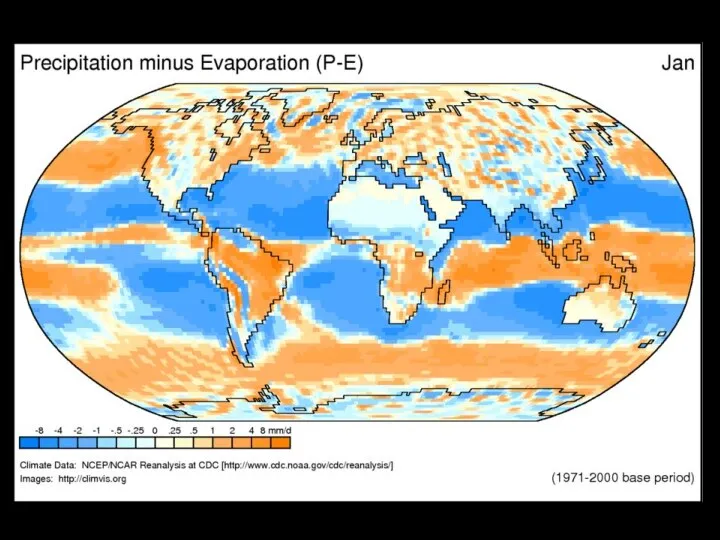
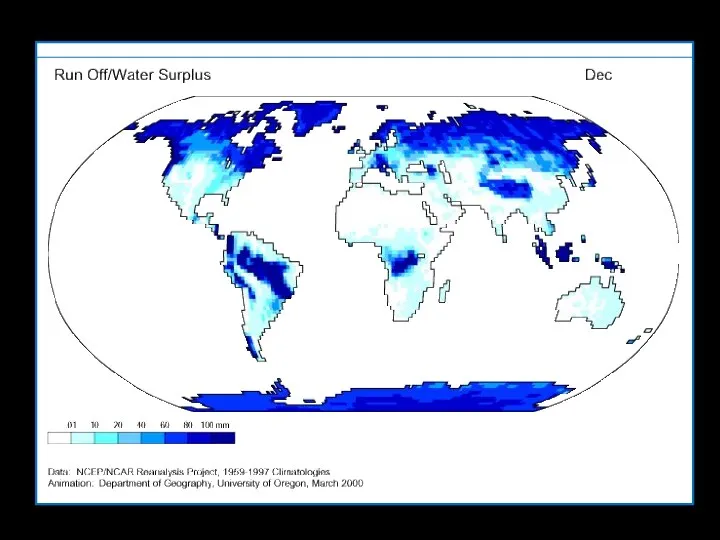
- 5. Solar radiation provides the energy required to drive evaporation Water vapor circulates eventually forming precipitation A
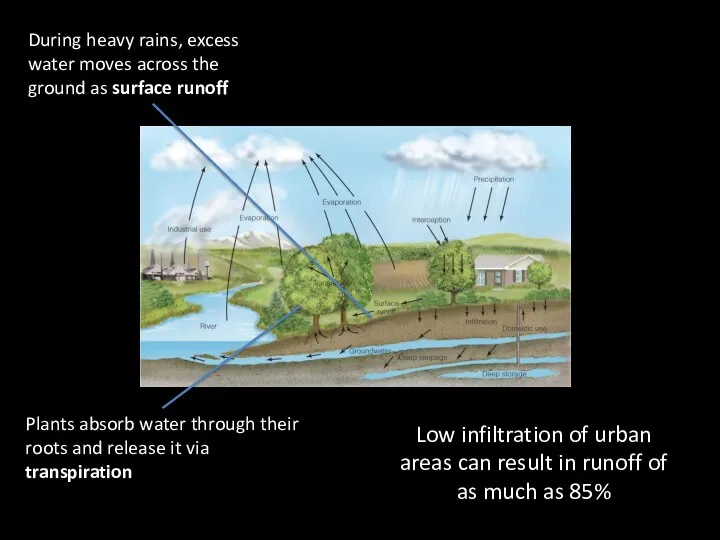
- 7. During heavy rains, excess water moves across the ground as surface runoff Low infiltration of urban
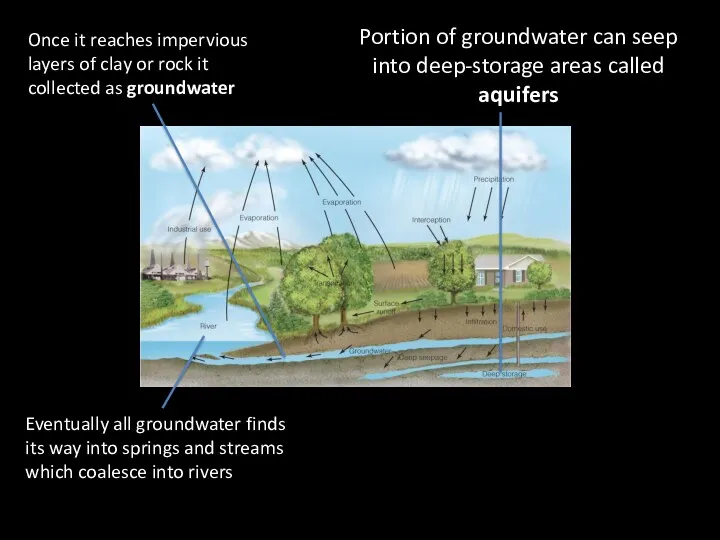
- 9. Once it reaches impervious layers of clay or rock it collected as groundwater Portion of groundwater
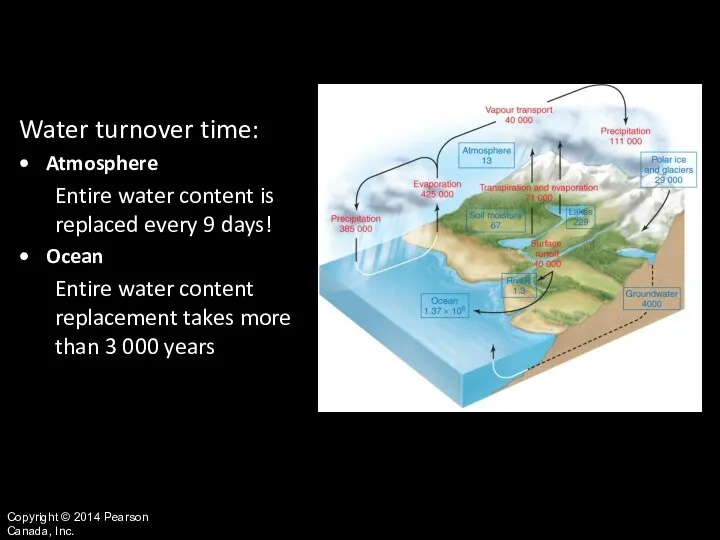
- 10. Copyright © 2014 Pearson Canada, Inc. Water turnover time: Atmosphere Entire water content is replaced every
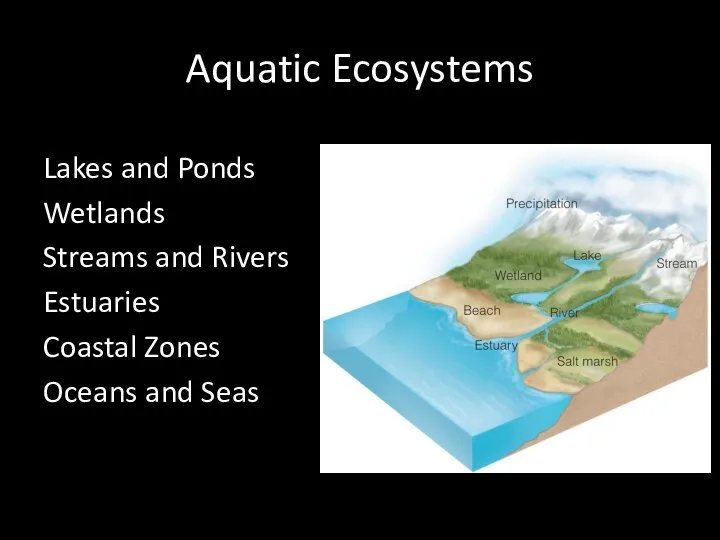
- 11. Aquatic Ecosystems Lakes and Ponds Wetlands Streams and Rivers Estuaries Coastal Zones Oceans and Seas
- 12. Copyright © 2014 Pearson Canada, Inc. Lake and ponds origins: Glacial erosion and deposition (kettle lakes
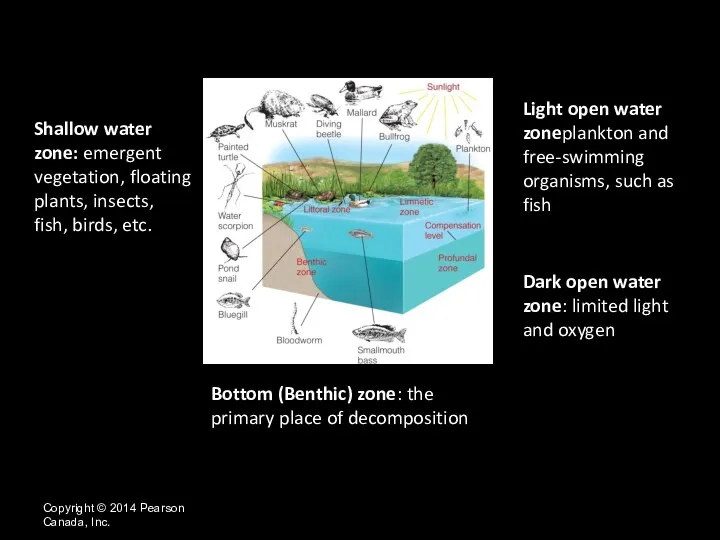
- 13. Bottom (Benthic) zone: the primary place of decomposition Copyright © 2014 Pearson Canada, Inc. Shallow water
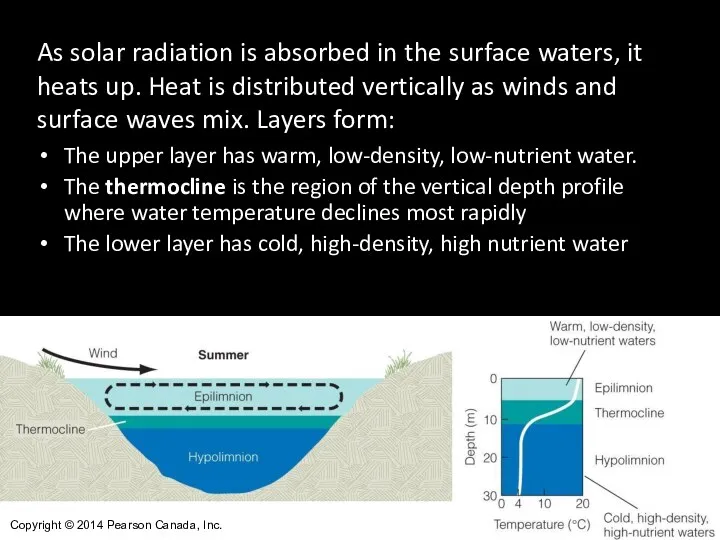
- 14. As solar radiation is absorbed in the surface waters, it heats up. Heat is distributed vertically
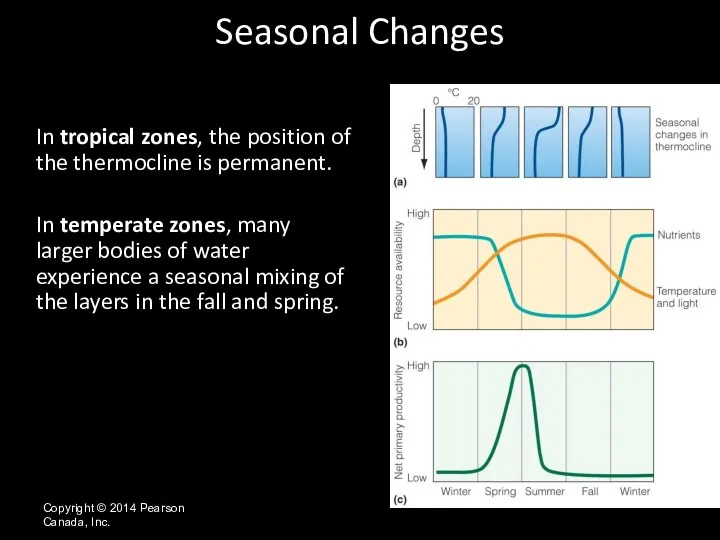
- 15. Copyright © 2014 Pearson Canada, Inc. In tropical zones, the position of the thermocline is permanent.
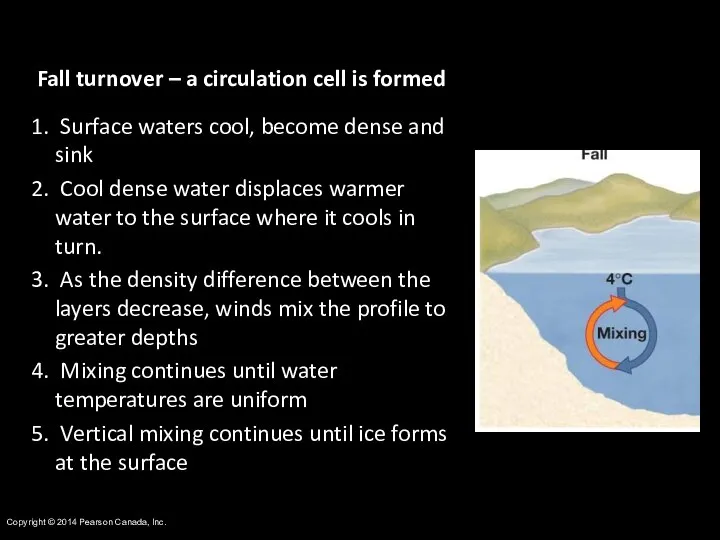
- 16. Copyright © 2014 Pearson Canada, Inc. Fall turnover – a circulation cell is formed Surface waters
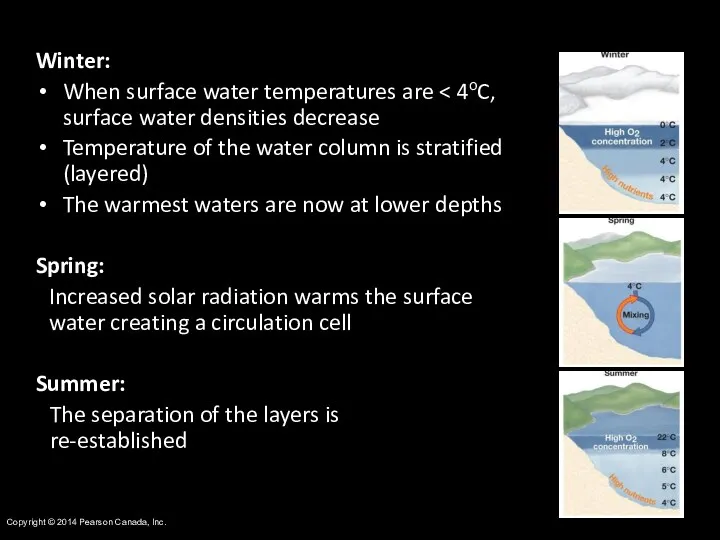
- 17. Copyright © 2014 Pearson Canada, Inc. Winter: When surface water temperatures are Temperature of the water
- 18. Copyright © 2014 Pearson Canada, Inc. Wetlands cover 6% of the Earth’s surface and are found
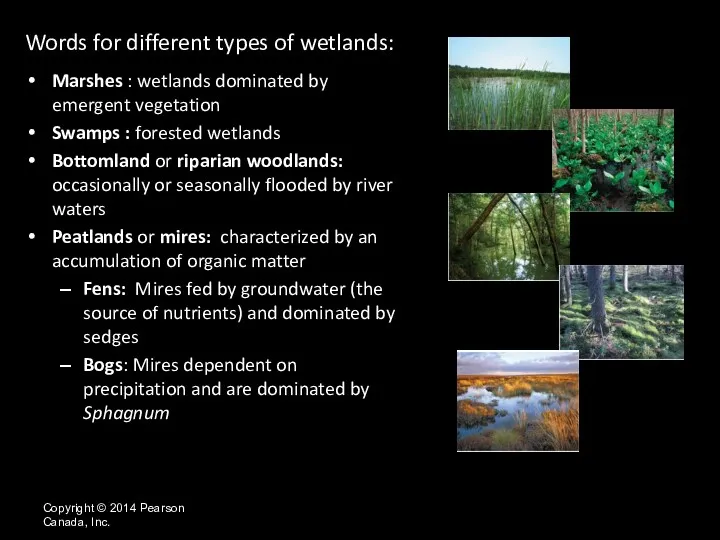
- 19. Copyright © 2014 Pearson Canada, Inc. Words for different types of wetlands: Marshes : wetlands dominated
- 20. Methane is produced in anaerobic conditions, organic decay: wetlands, rice fields, grazing animals intestines, termites, landfills,
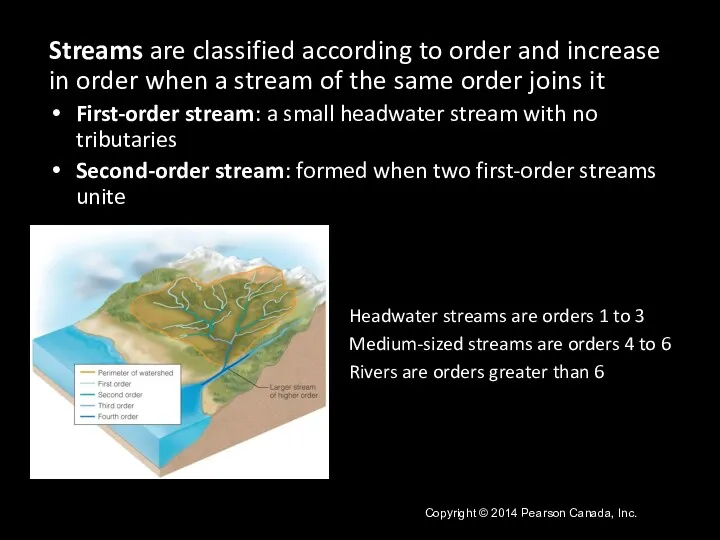
- 21. Streams are classified according to order and increase in order when a stream of the same
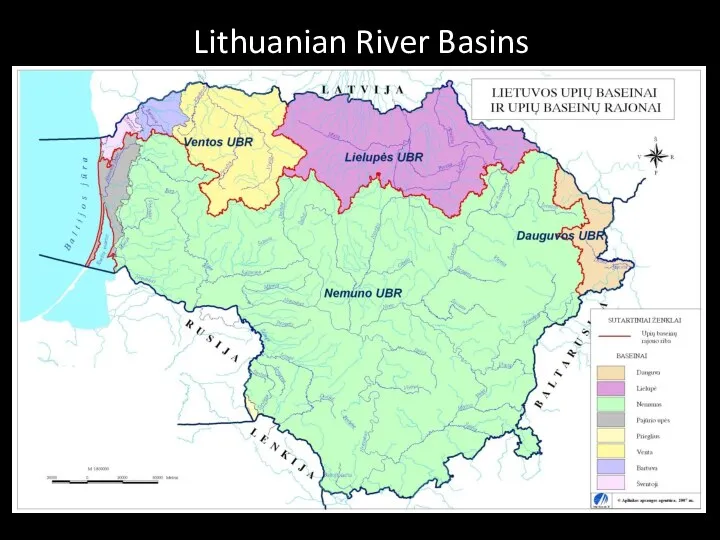
- 22. Lithuanian River Basins
- 23. Mixing waters of different salinities and temperatures Complex currents Nutrients are carried into the estuary by
- 24. Copyright © 2014 Pearson Canada, Inc. Wherever land and water meet, there is a transitional zone
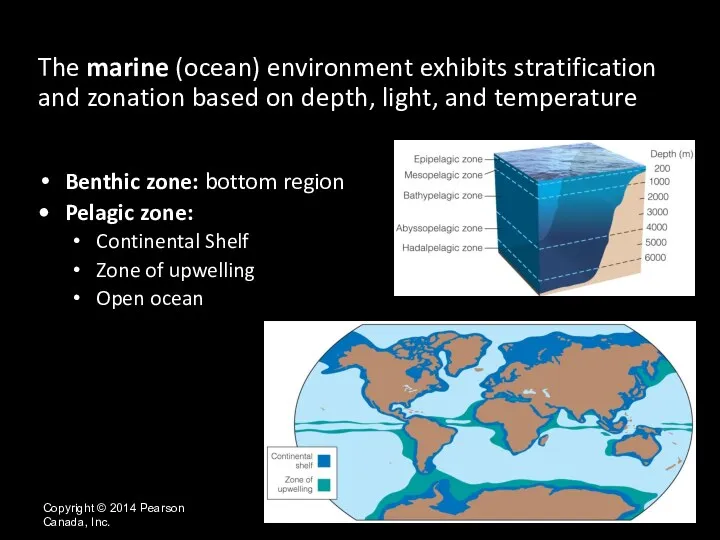
- 25. Copyright © 2014 Pearson Canada, Inc. The marine (ocean) environment exhibits stratification and zonation based on
- 26. Copyright © 2014 Pearson Canada, Inc. Ocean depth varies from a few hundred meters to 10,000
- 27. Why is water so amazing? Water exists in gas, liquid, and solid form on Earth Ice
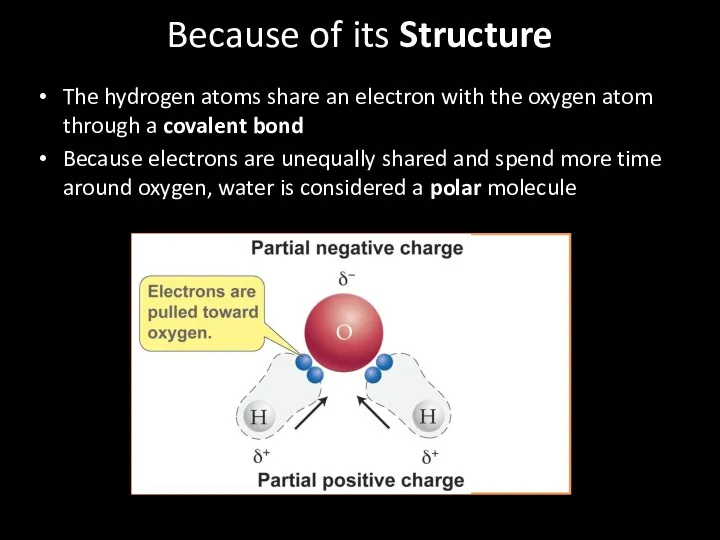
- 28. Because of its Structure The hydrogen atoms share an electron with the oxygen atom through a
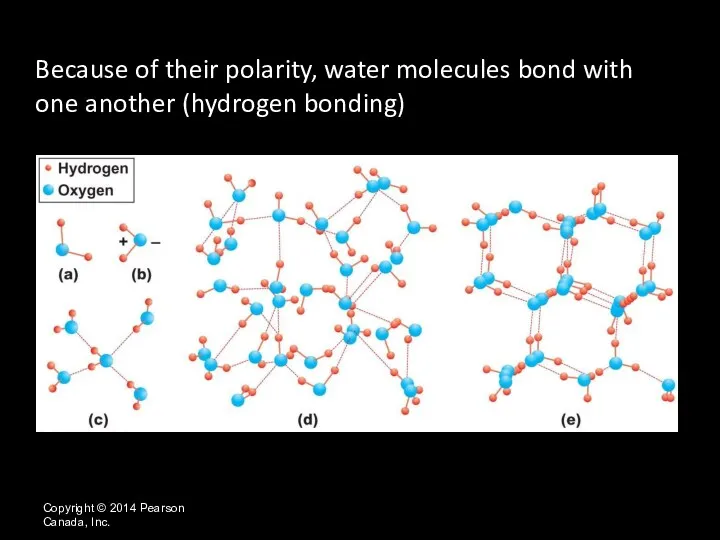
- 29. Copyright © 2014 Pearson Canada, Inc. Because of their polarity, water molecules bond with one another
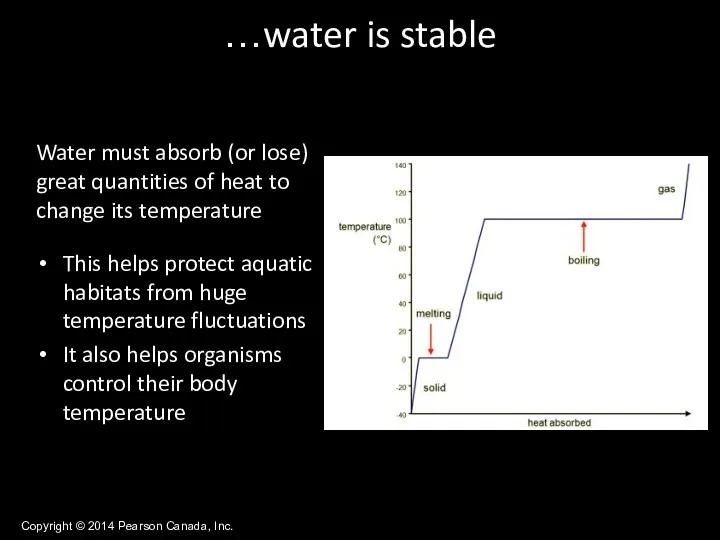
- 30. Copyright © 2014 Pearson Canada, Inc. Water must absorb (or lose) great quantities of heat to
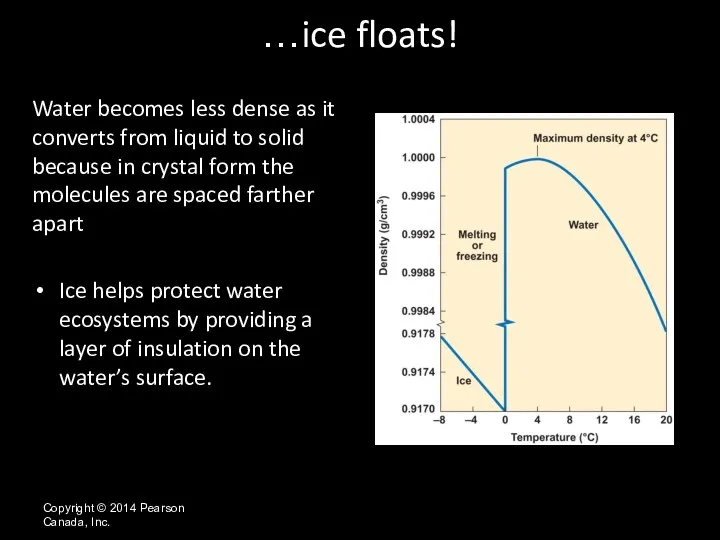
- 31. Copyright © 2014 Pearson Canada, Inc. Water becomes less dense as it converts from liquid to
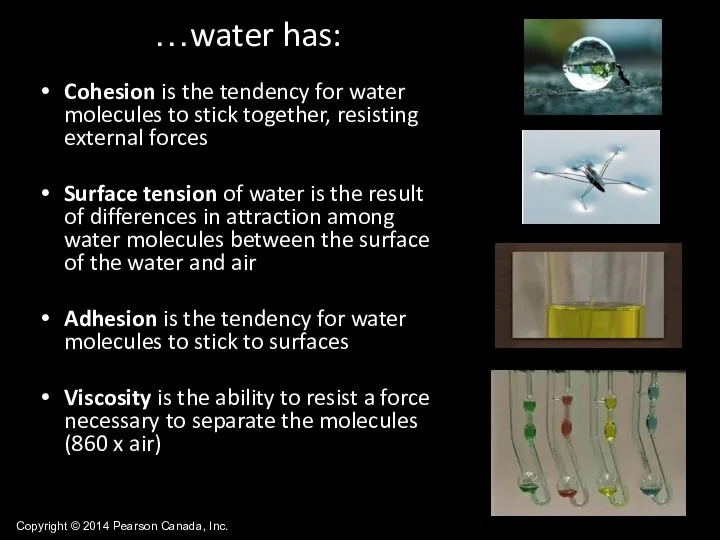
- 32. Copyright © 2014 Pearson Canada, Inc. Cohesion is the tendency for water molecules to stick together,
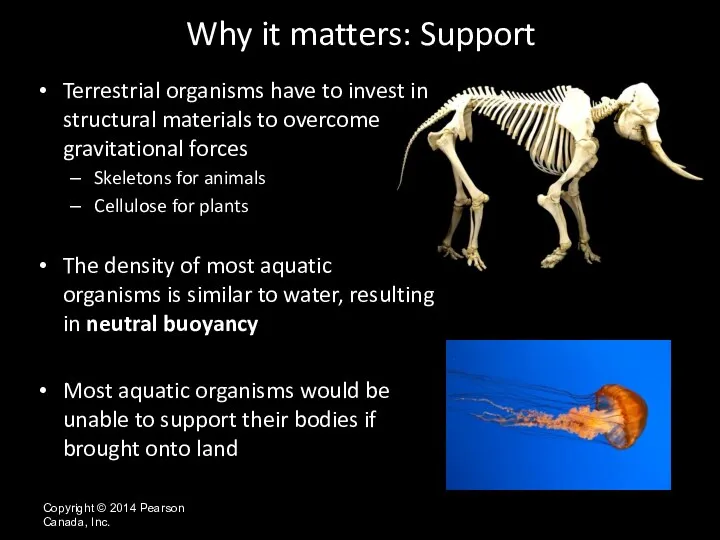
- 33. Copyright © 2014 Pearson Canada, Inc. Terrestrial organisms have to invest in structural materials to overcome
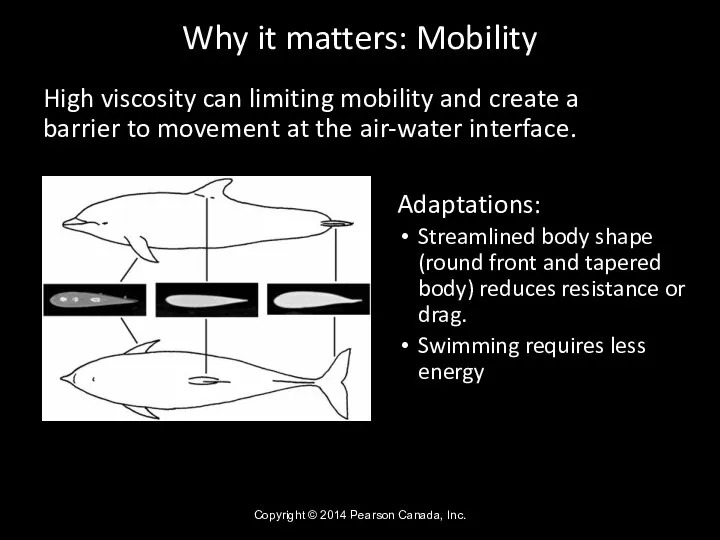
- 34. Why it matters: Mobility High viscosity can limiting mobility and create a barrier to movement at
- 35. Capillary action: When adhesion is stronger than cohesion, water will move up the surface This allows
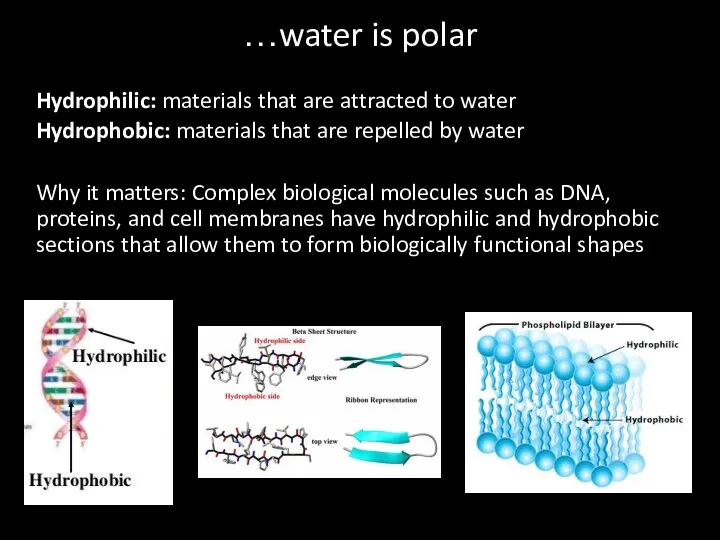
- 36. Hydrophilic: materials that are attracted to water Hydrophobic: materials that are repelled by water Why it
- 37. Water can dissolve more substances than any other liquid Ionic and polar molecules easily dissolve in
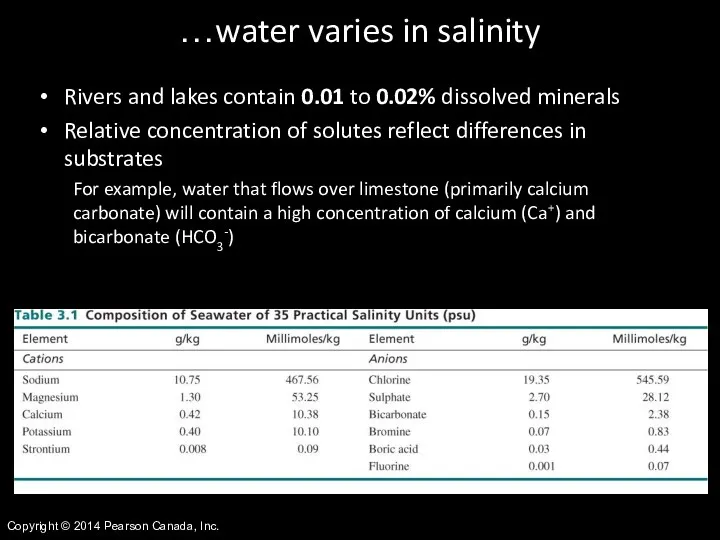
- 38. Copyright © 2014 Pearson Canada, Inc. …water varies in salinity Rivers and lakes contain 0.01 to
- 39. …water varies in pH Water reacts even with itself! H2O ⇆ OH– + H3O+ Pure water
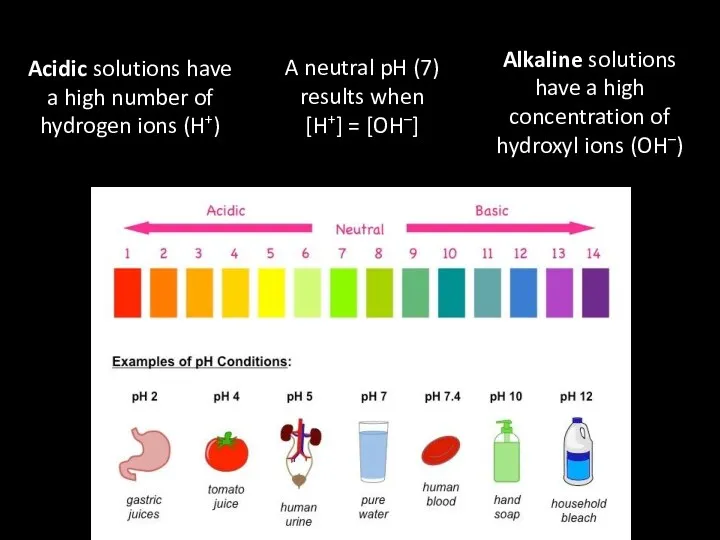
- 40. Acidic solutions have a high number of hydrogen ions (H+) A neutral pH (7) results when
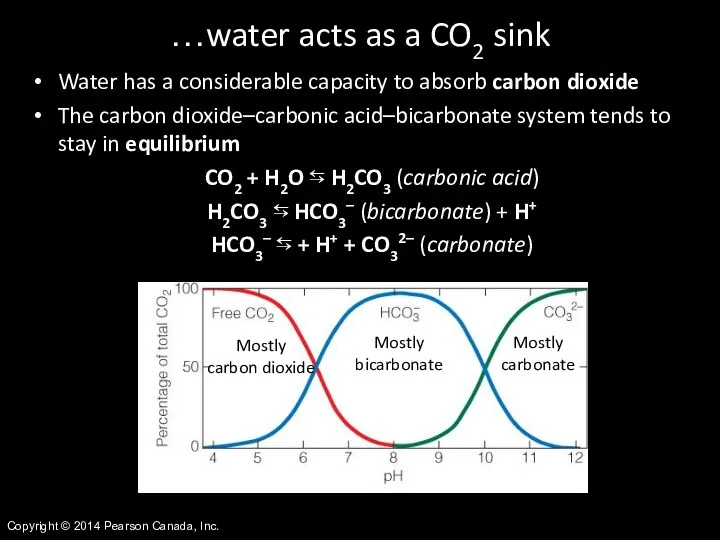
- 41. Copyright © 2014 Pearson Canada, Inc. Water has a considerable capacity to absorb carbon dioxide The
- 42. Copyright © 2014 Pearson Canada, Inc. The carbon system directly affects the pH of aquatic ecosystems,
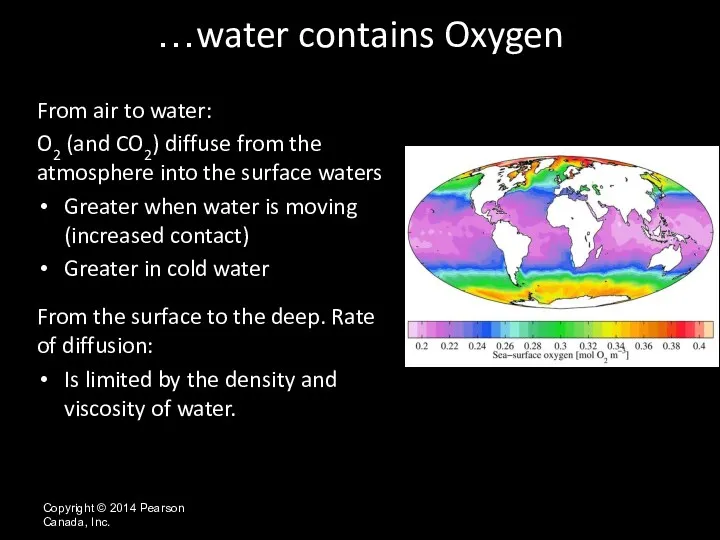
- 43. Copyright © 2014 Pearson Canada, Inc. From air to water: O2 (and CO2) diffuse from the
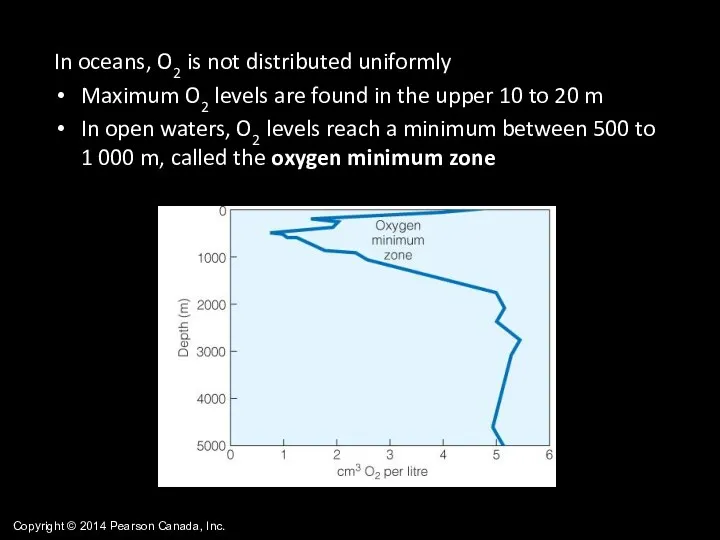
- 44. Copyright © 2014 Pearson Canada, Inc. In oceans, O2 is not distributed uniformly Maximum O2 levels
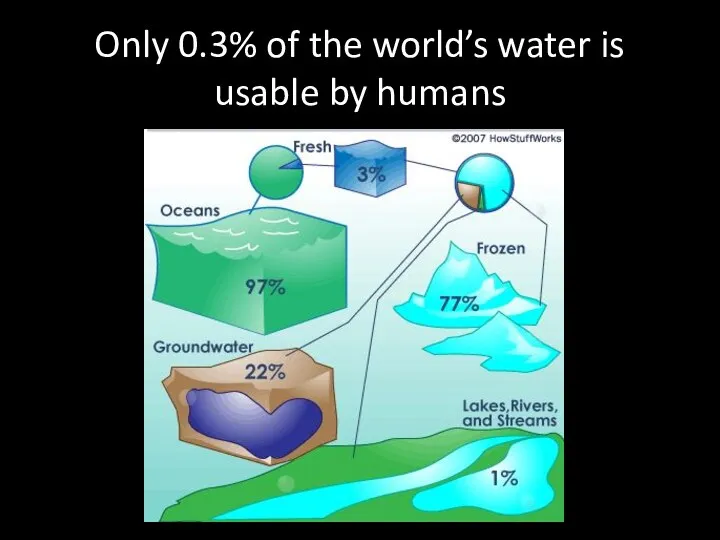
- 45. Only 0.3% of the world’s water is usable by humans
- 46. Copyright © 2014 Pearson Canada, Inc. Dessication, or the loss of water, is probably the greatest
- 47. Why it matters…water shapes societies. The first known treaty in human history was between two Sumerian
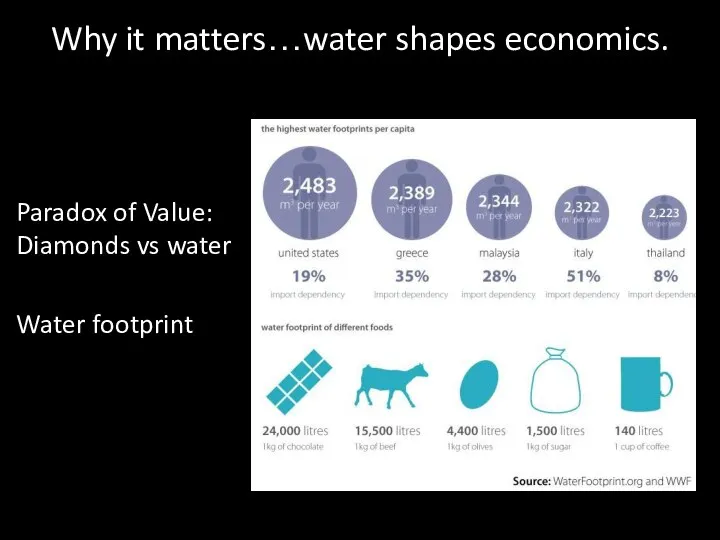
- 48. Why it matters…water shapes economics. Paradox of Value: Diamonds vs water Water footprint
- 49. Aral Sea Colorado River
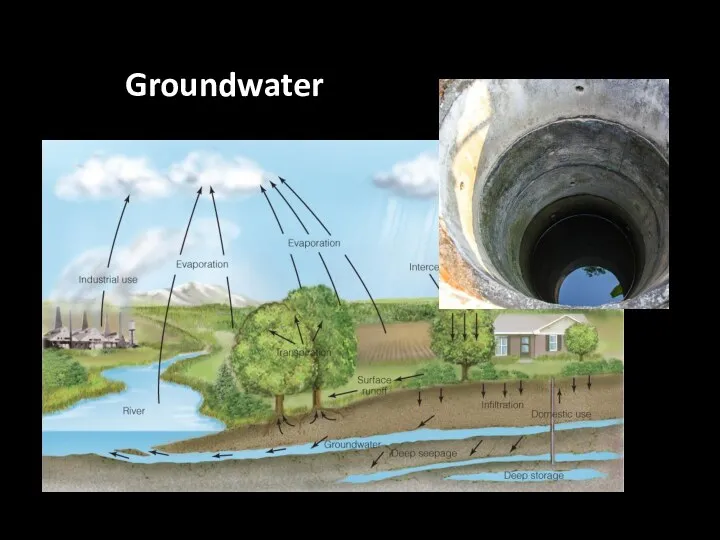
- 50. Groundwater
- 51. Porosity – the percentage of open space within sediment or rock Primary: space between grains Secondary:
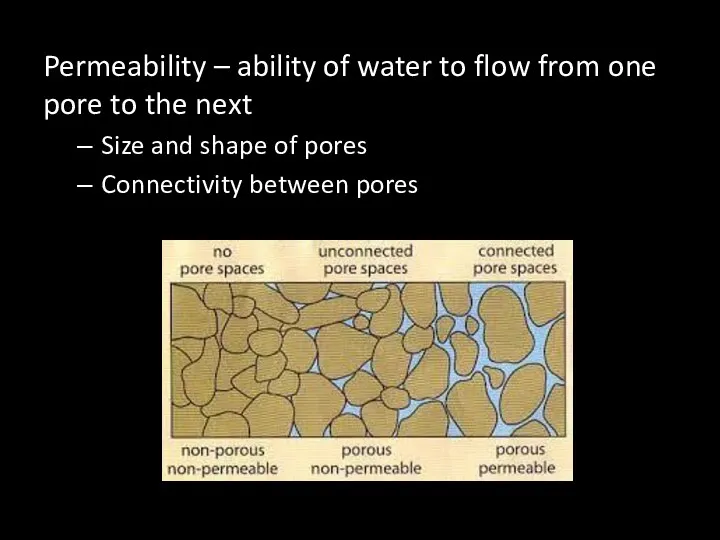
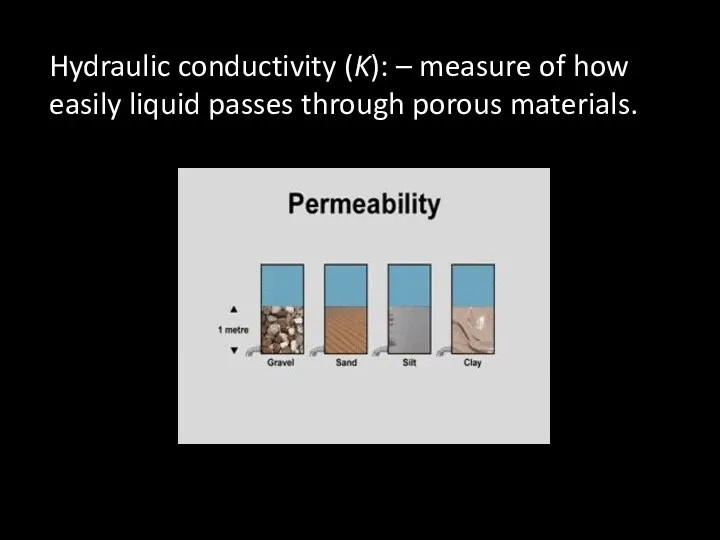
- 52. Permeability – ability of water to flow from one pore to the next Size and shape
- 53. Hydraulic conductivity (K): – measure of how easily liquid passes through porous materials.
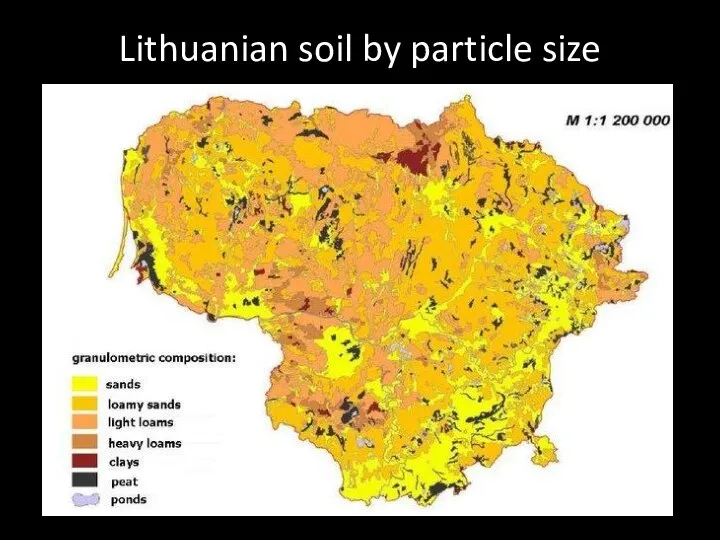
- 54. Lithuanian soil by particle size
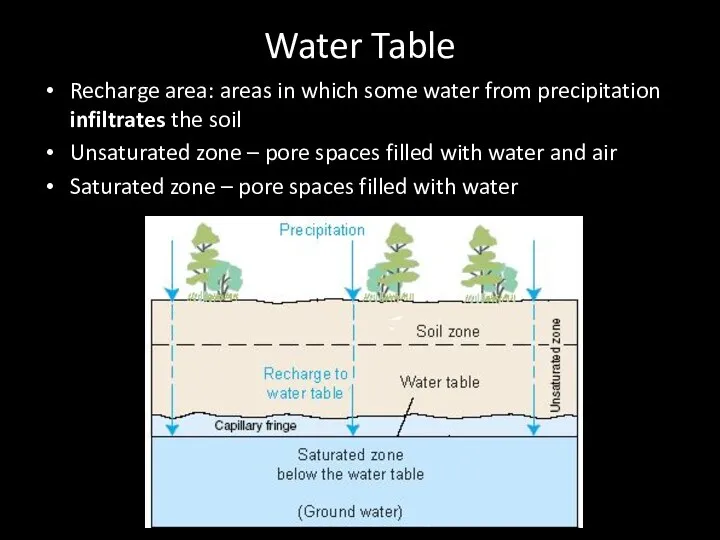
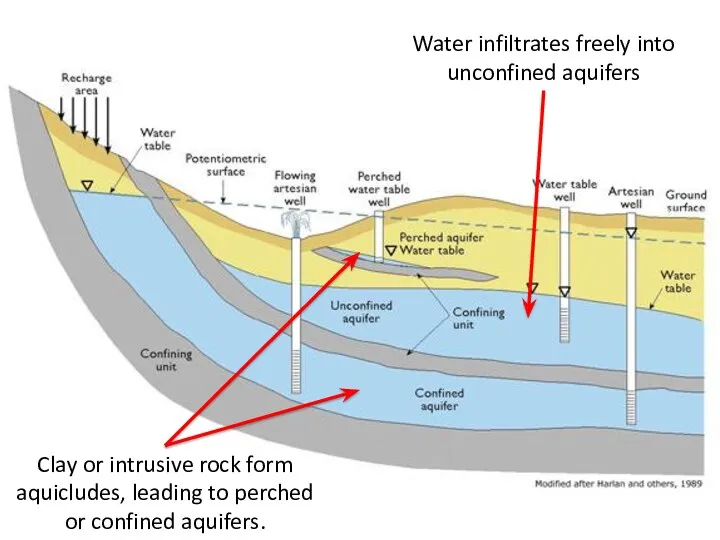
- 55. Water Table Recharge area: areas in which some water from precipitation infiltrates the soil Unsaturated zone
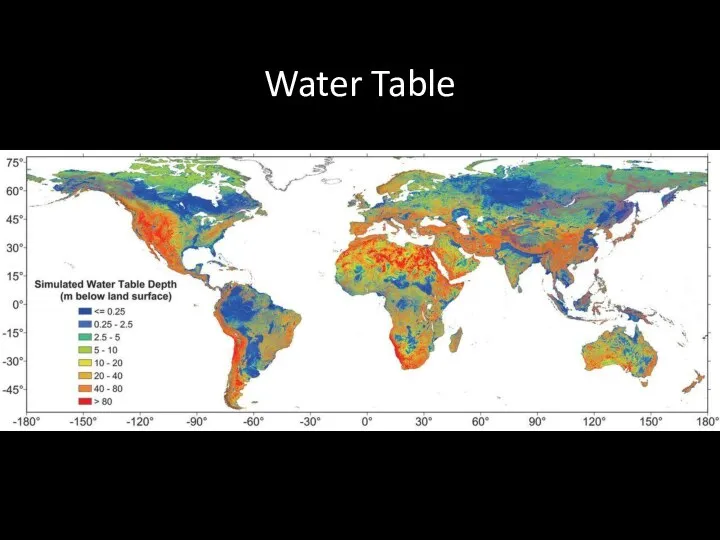
- 56. Water Table
- 58. Clay or intrusive rock form aquicludes, leading to perched or confined aquifers. Water infiltrates freely into
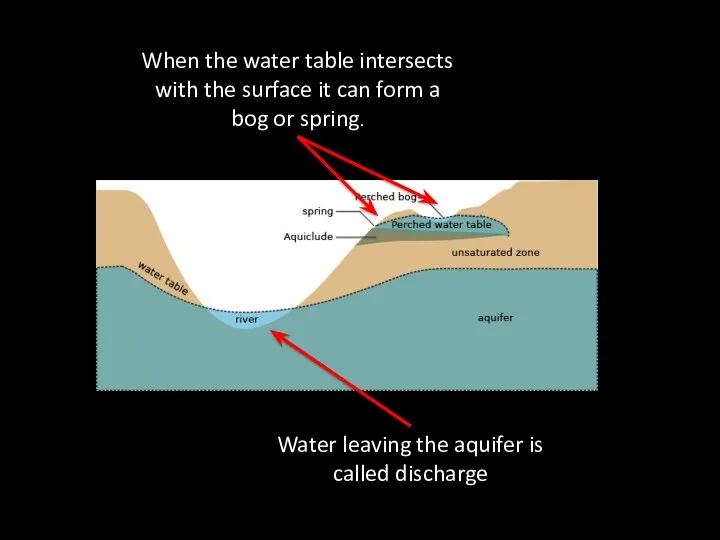
- 59. When the water table intersects with the surface it can form a bog or spring. Water
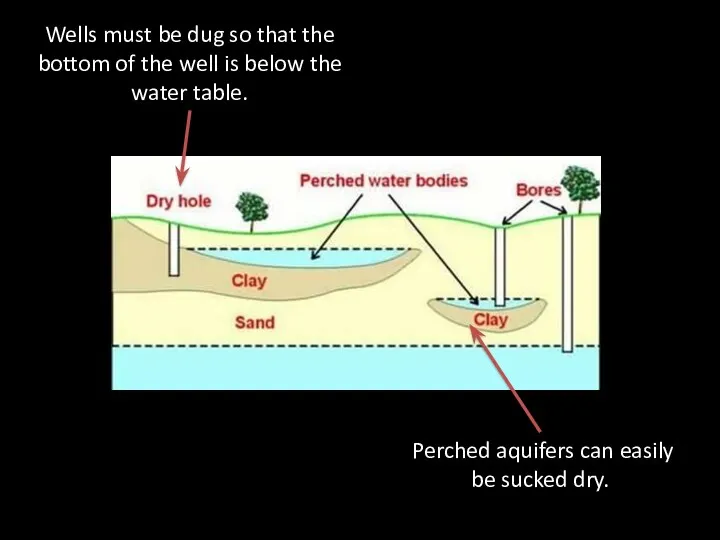
- 60. Wells must be dug so that the bottom of the well is below the water table.
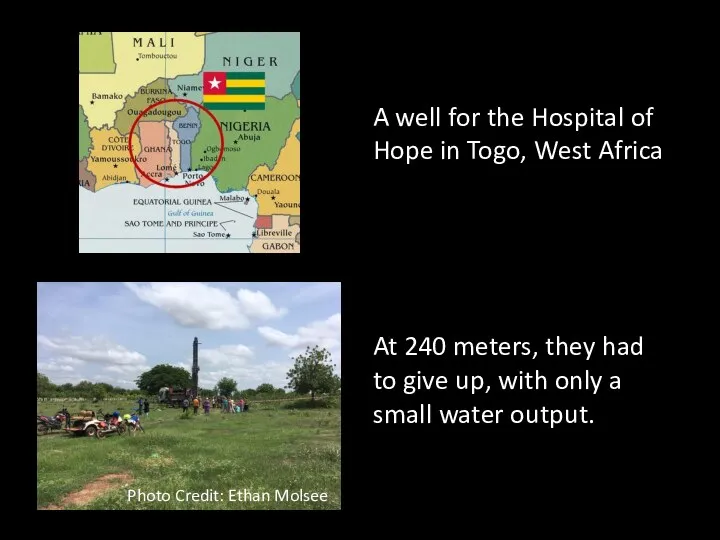
- 61. A well for the Hospital of Hope in Togo, West Africa At 240 meters, they had
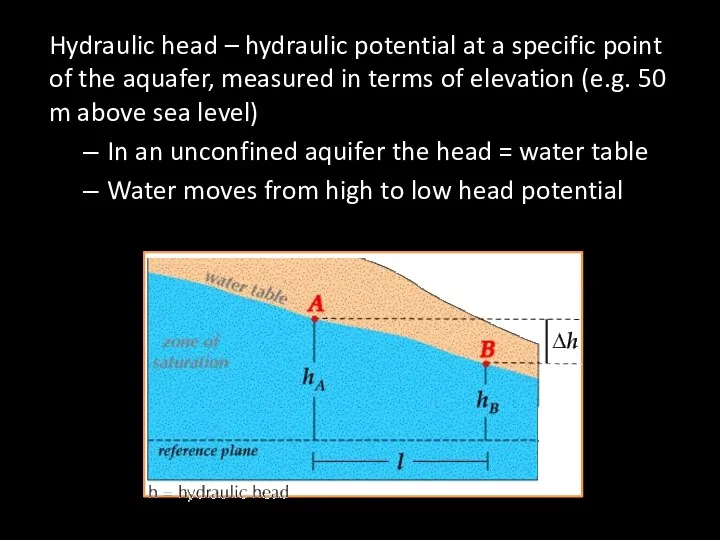
- 62. Hydraulic head – hydraulic potential at a specific point of the aquafer, measured in terms of
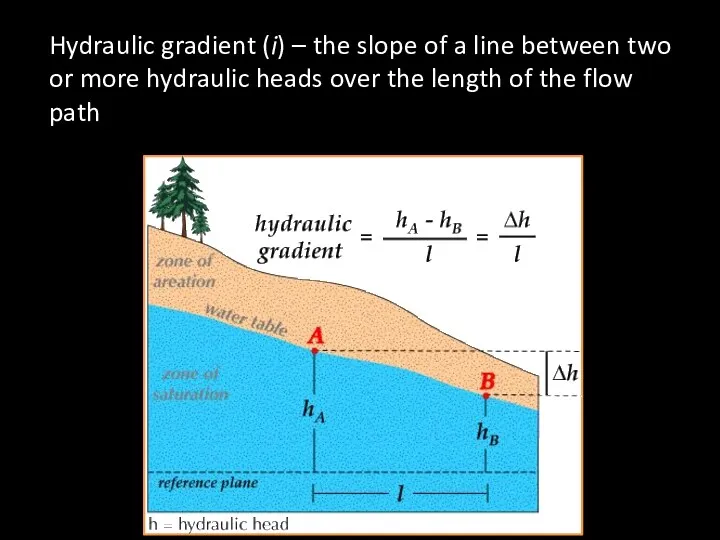
- 63. Hydraulic gradient (i) – the slope of a line between two or more hydraulic heads over
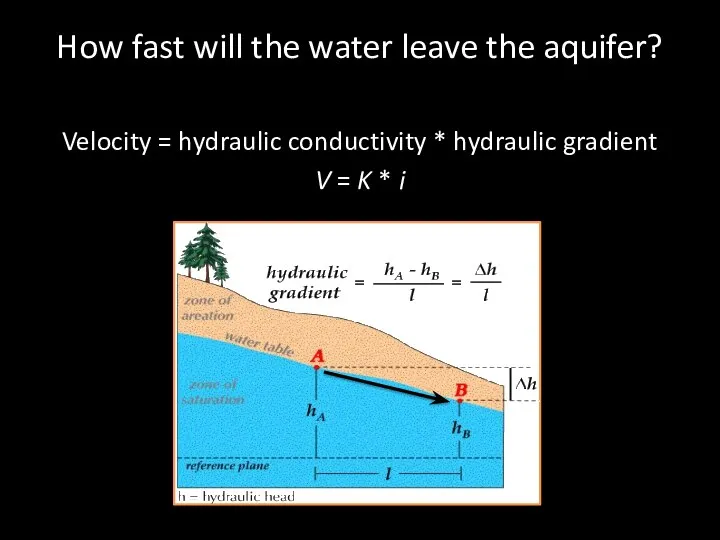
- 64. How fast will the water leave the aquifer? Velocity = hydraulic conductivity * hydraulic gradient V
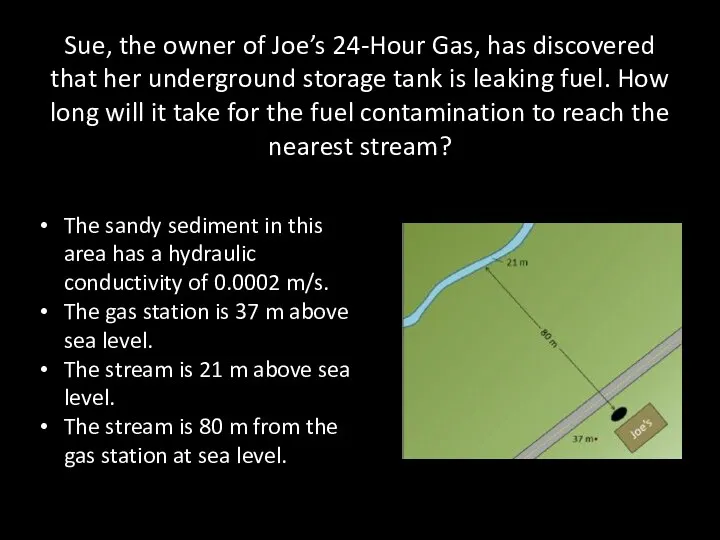
- 65. Sue, the owner of Joe’s 24-Hour Gas, has discovered that her underground storage tank is leaking
- 66. How fast will the water leave the aquifer? Velocity = hydraulic conductivity * hydraulic gradient V
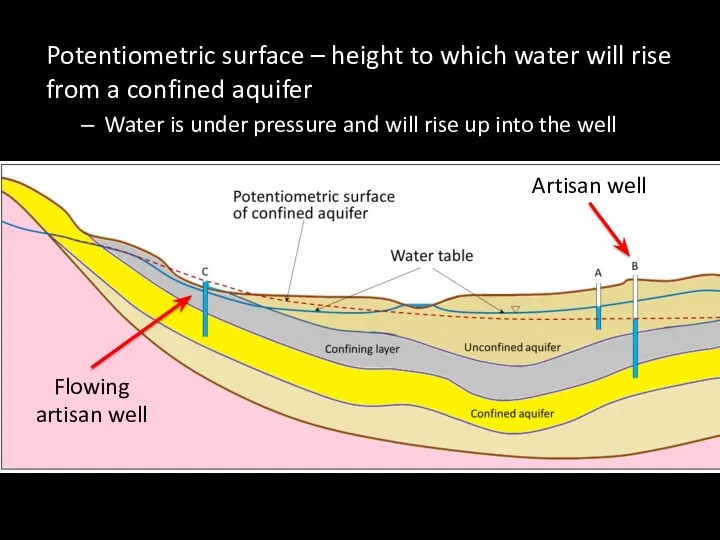
- 67. Potentiometric surface – height to which water will rise from a confined aquifer Water is under
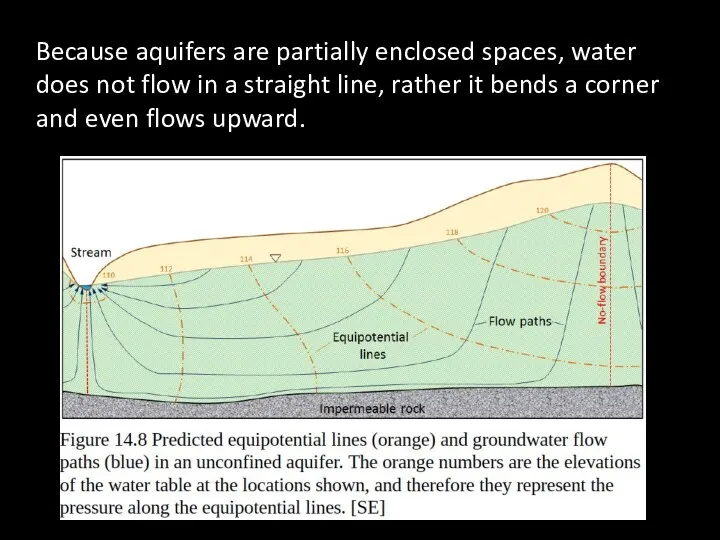
- 68. Because aquifers are partially enclosed spaces, water does not flow in a straight line, rather it
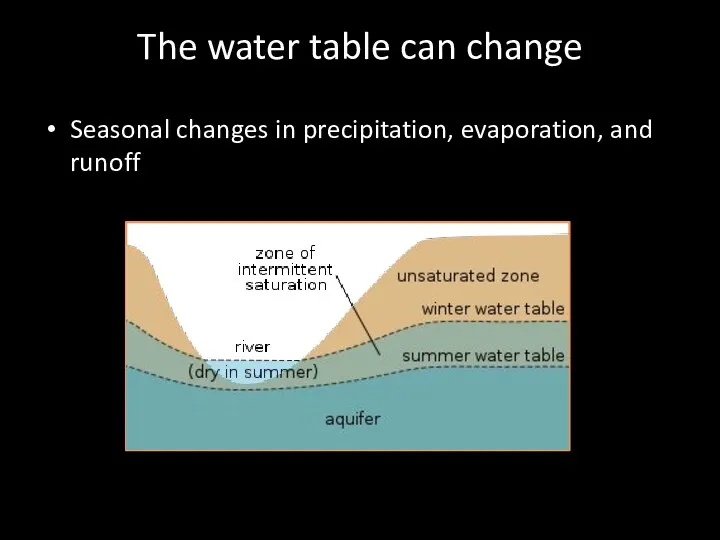
- 69. The water table can change Seasonal changes in precipitation, evaporation, and runoff
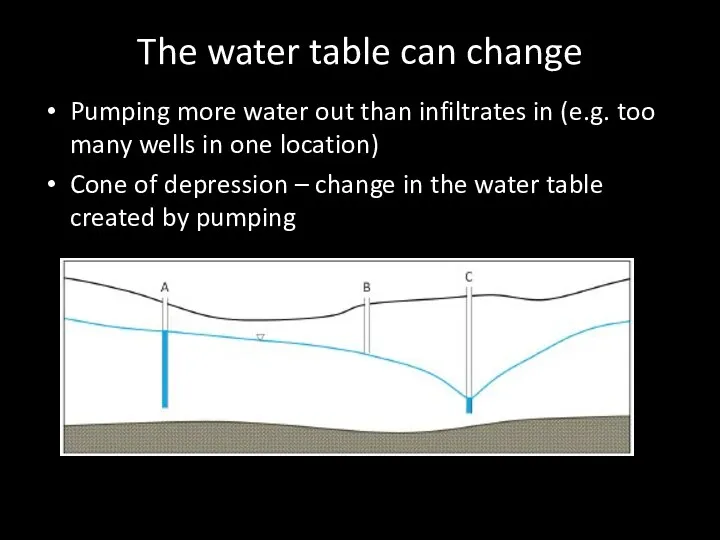
- 70. The water table can change Pumping more water out than infiltrates in (e.g. too many wells
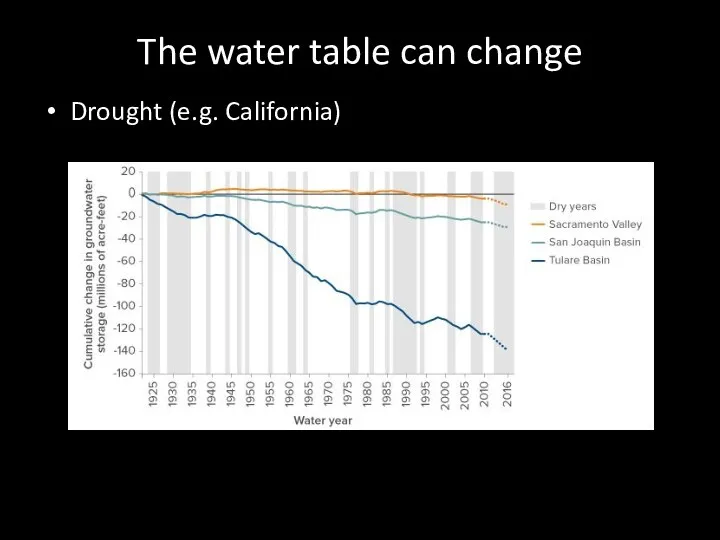
- 71. The water table can change Drought (e.g. California)
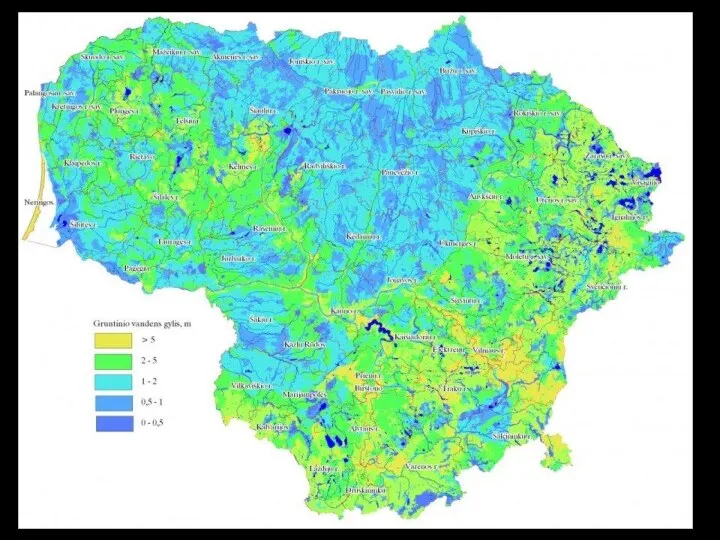
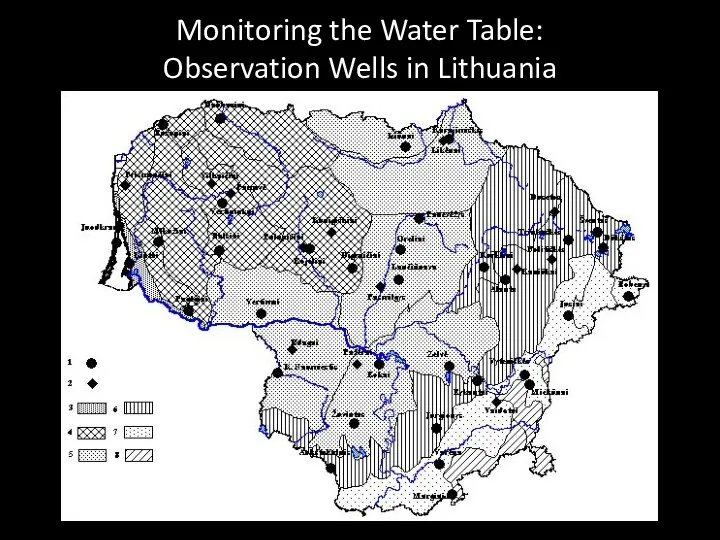
- 72. Monitoring the Water Table: Observation Wells in Lithuania
- 73. Hard Water Groundwater absorbs minerals from the surrounding rocks/sediment Calcium Magnesium carbonate Some of which can
- 75. Скачать презентацию

















 Город Партизанск. Проект
Город Партизанск. Проект Особо охраняемые природные территории России
Особо охраняемые природные территории России Менеджмент экологической безопасности. Раздел 3
Менеджмент экологической безопасности. Раздел 3 Экологические требования при использовании недр
Экологические требования при использовании недр Здоровый образ жизни
Здоровый образ жизни Профессия сити-фермер, повышение престижа высококвалифицированных кадров по средством проведения чемпионатов World Skills
Профессия сити-фермер, повышение престижа высококвалифицированных кадров по средством проведения чемпионатов World Skills Эко-карта Кировского района города Самара
Эко-карта Кировского района города Самара Красная книга
Красная книга Техногенное загрязнение окружающей среды
Техногенное загрязнение окружающей среды Экологический мониторинг
Экологический мониторинг Платежи за негативное воздействие на окружающую природную среду
Платежи за негативное воздействие на окружающую природную среду Екологiчнi проблеми людства
Екологiчнi проблеми людства Пластиковое загрязнение планеты. Есть ли жизнь без пластика
Пластиковое загрязнение планеты. Есть ли жизнь без пластика Переработка отходов бурения при нефте- и газодобыче
Переработка отходов бурения при нефте- и газодобыче Межрегиональный эколого-просветительский проект Письма животным
Межрегиональный эколого-просветительский проект Письма животным Переработка пластиковой тары
Переработка пластиковой тары Биогеоценозы. Экосистемы. Строение и свойства
Биогеоценозы. Экосистемы. Строение и свойства Молодёжь в охране дикой природы
Молодёжь в охране дикой природы Негативні наслідки видобутку нафти (розливання нафти океанах, морях)
Негативні наслідки видобутку нафти (розливання нафти океанах, морях) Экология сообществ и экосистем
Экология сообществ и экосистем Биосфера как глобальная экосистема
Биосфера как глобальная экосистема Охрана биосферы от загрязнения выбросами хозяйственной деятельности. Лекция 103
Охрана биосферы от загрязнения выбросами хозяйственной деятельности. Лекция 103 Загрязнение воды и последствия
Загрязнение воды и последствия Автомобиль и окружающая среда
Автомобиль и окружающая среда Теплове забруднення, як екологічна проблема
Теплове забруднення, як екологічна проблема Международная организация Гринпис
Международная организация Гринпис Экологический проект Исследование свойств воды из разных источников Крыма
Экологический проект Исследование свойств воды из разных источников Крыма Величественный парк графа Лесли
Величественный парк графа Лесли