Содержание
- 2. Energy Storage Renewable energy is often intermittent (like wind and sun), and storage allows use at
- 3. Renewable Energy: Energy source/fuel type that can regenerate and can replenish itself indefinitely. Biomass, Wind, Solar,
- 4. Hydroelectric power plants take advantage of the gravitational potential energy of water as it falls from
- 5. The joule (J) is a measure of energy, or the ability or capacity to do work.
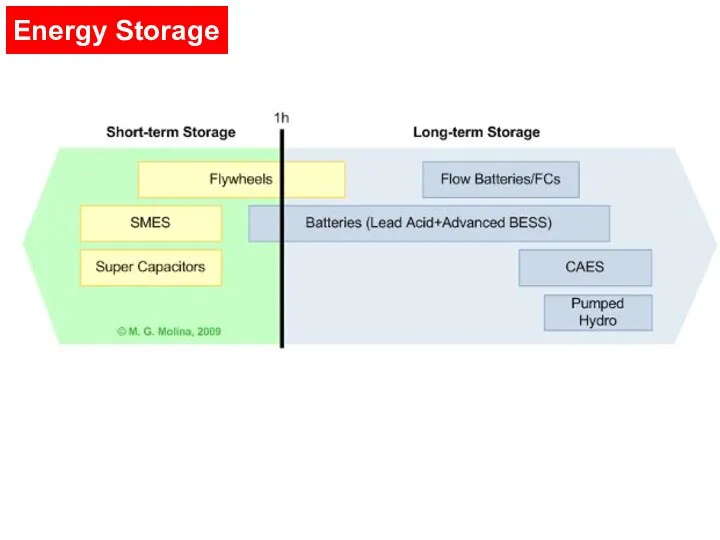
- 6. Types of Energy Storage Electricity can be stored by converting it into another form such as
- 7. Energy Storage
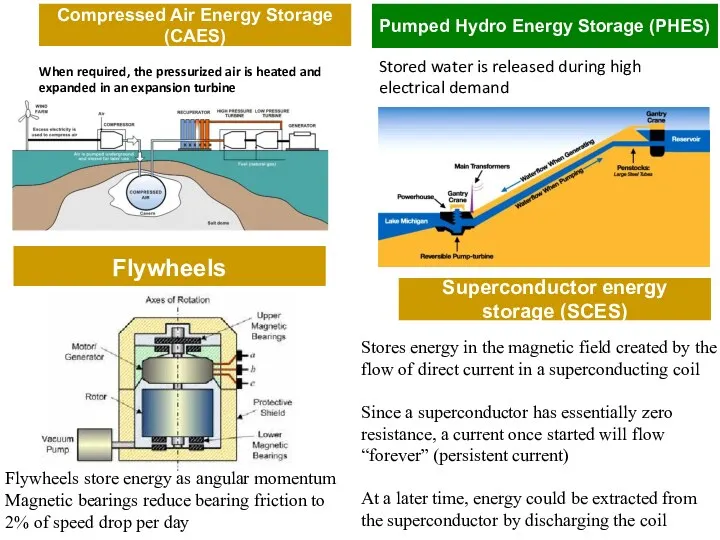
- 9. Flywheels When required, the pressurized air is heated and expanded in an expansion turbine Pumped Hydro
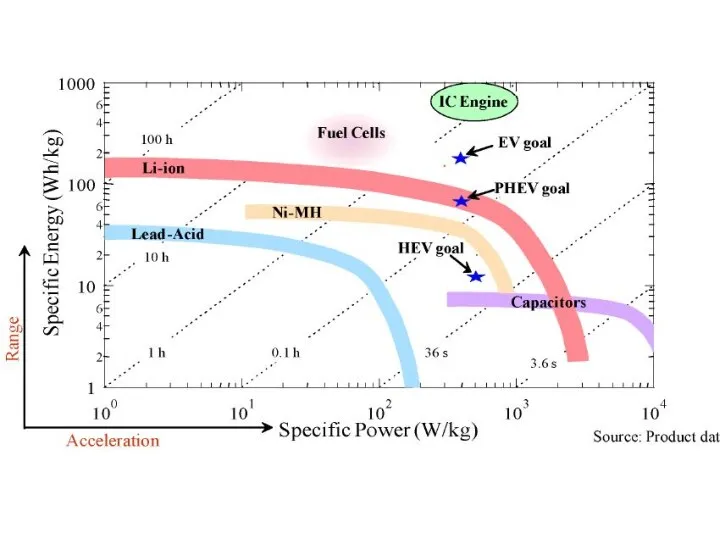
- 10. Battery Electric Storage System (BESS) have high energy densities technology is matured relatively easy to use
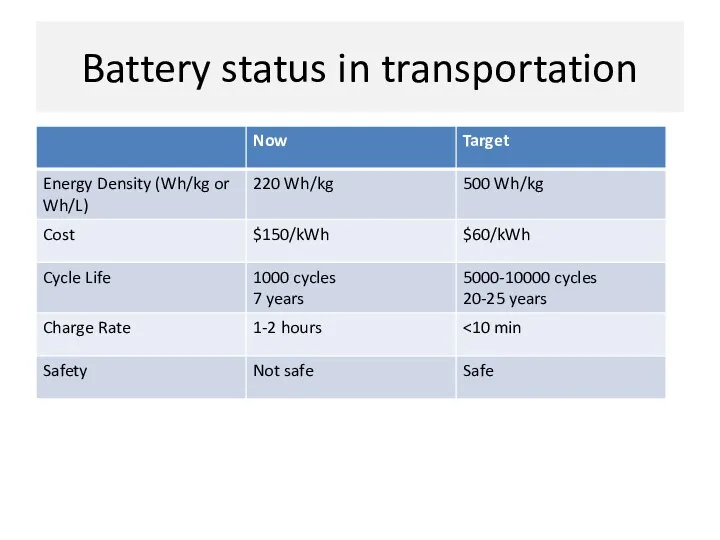
- 11. Battery status in transportation
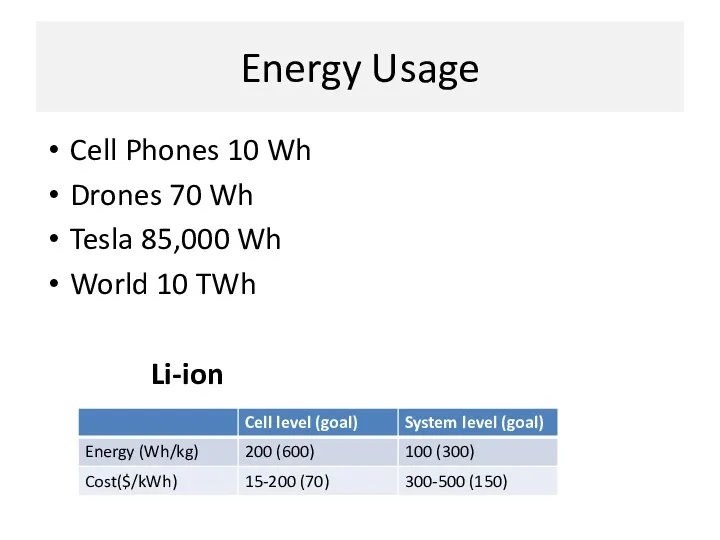
- 12. Energy Usage Cell Phones 10 Wh Drones 70 Wh Tesla 85,000 Wh World 10 TWh Li-ion
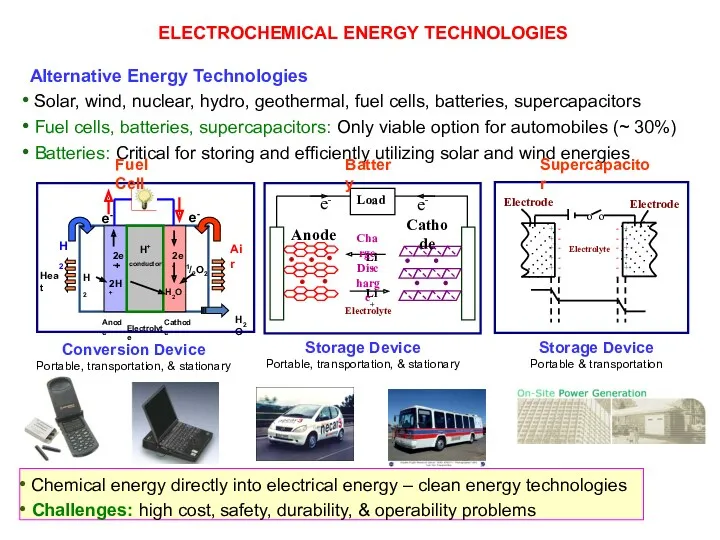
- 13. ELECTROCHEMICAL ENERGY TECHNOLOGIES Chemical energy directly into electrical energy – clean energy technologies Challenges: high cost,
- 14. HIGH ENERGY CATHODES FOR LITHIUM ION BATTERIES LiMn2O4 LiMn1.8Li0.1Ni0.1O4 LiMn1.8Li0.1Ni0.1O3.8F0.2 Li[Li0.2Mn0.54Co0.13Ni0.13]O2 Li[Li0.2Mn0.54Co0.13Ni0.13]O2 / Nano Al2O3 LiCoO2
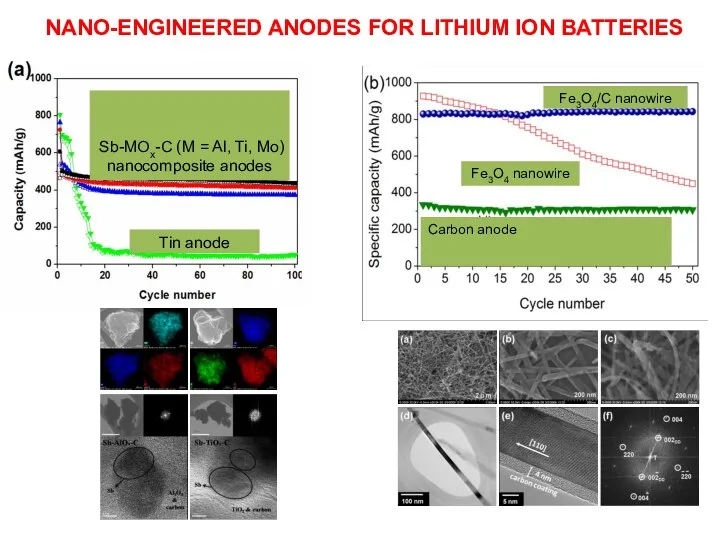
- 15. NANO-ENGINEERED ANODES FOR LITHIUM ION BATTERIES Fe3O4 nanowire
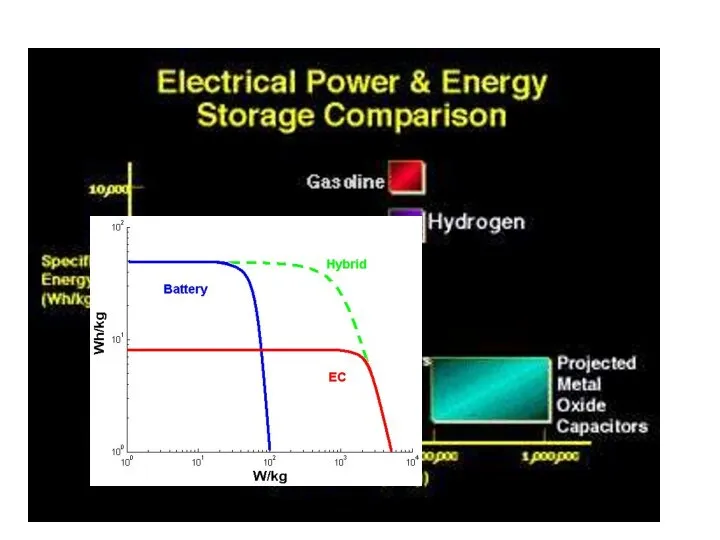
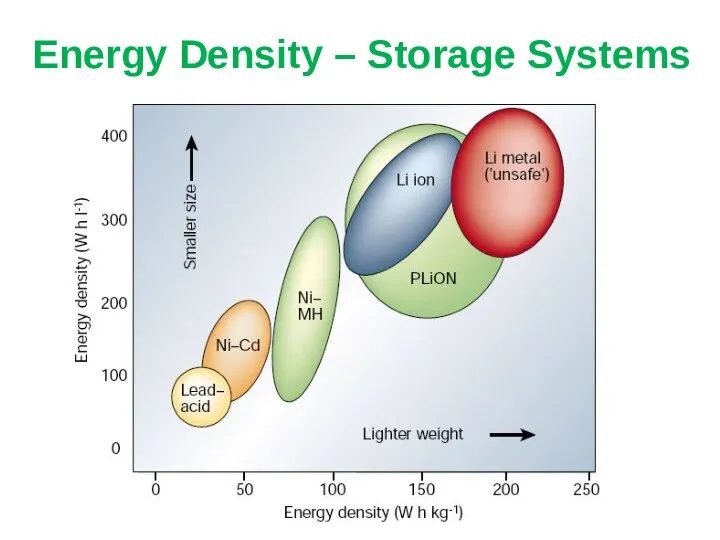
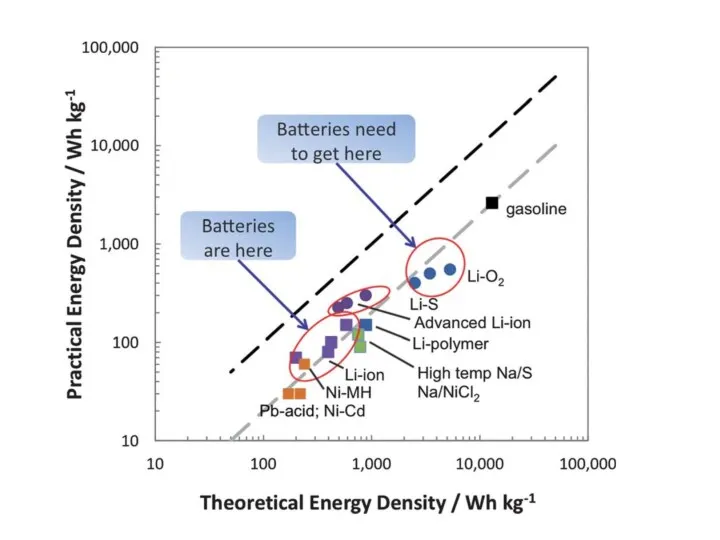
- 16. Energy Density – Storage Systems
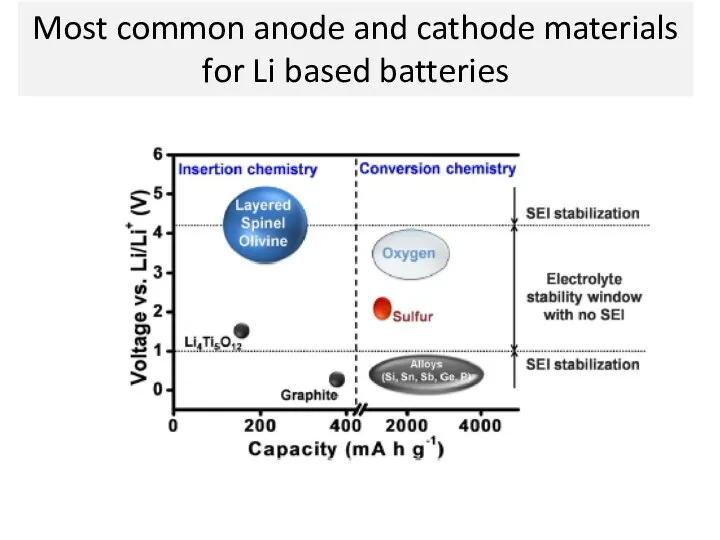
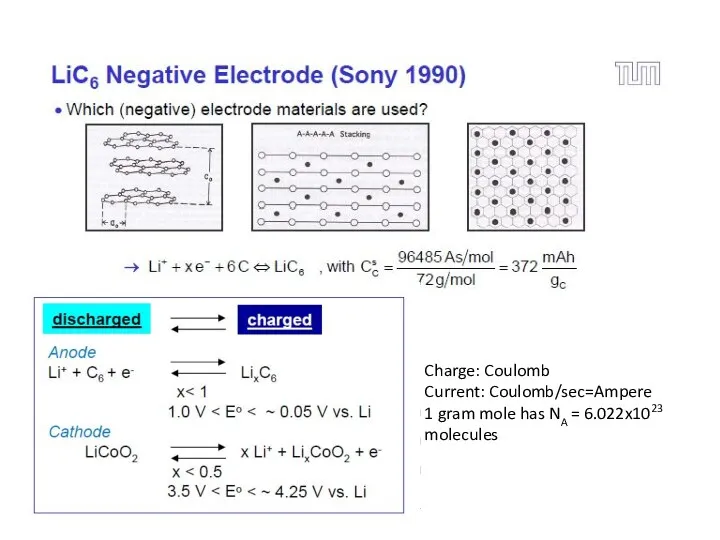
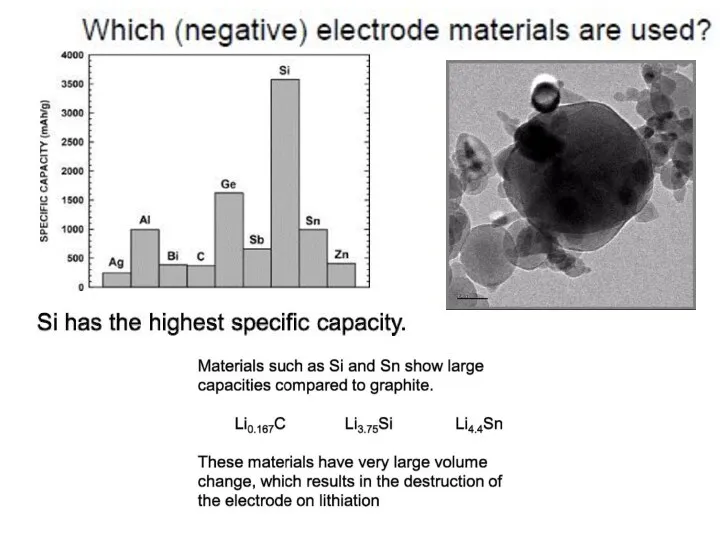
- 18. Most common anode and cathode materials for Li based batteries
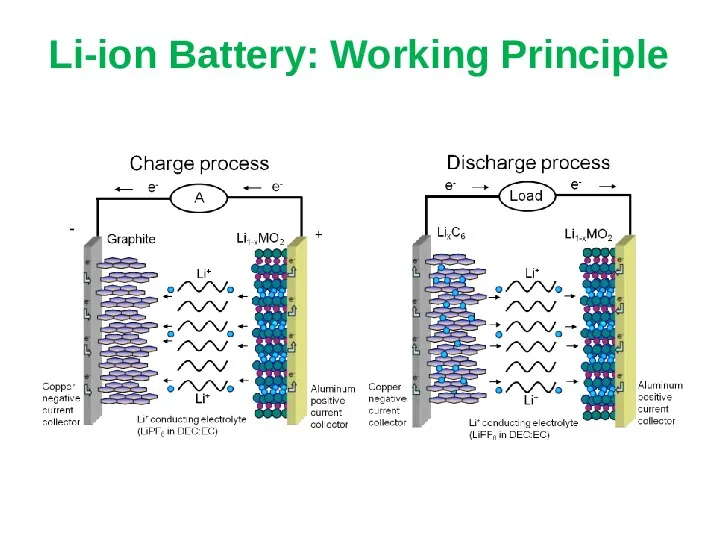
- 20. Li-ion Battery: Working Principle
- 21. Charge: Coulomb Current: Coulomb/sec=Ampere 1 gram mole has NA = 6.022x1023 molecules
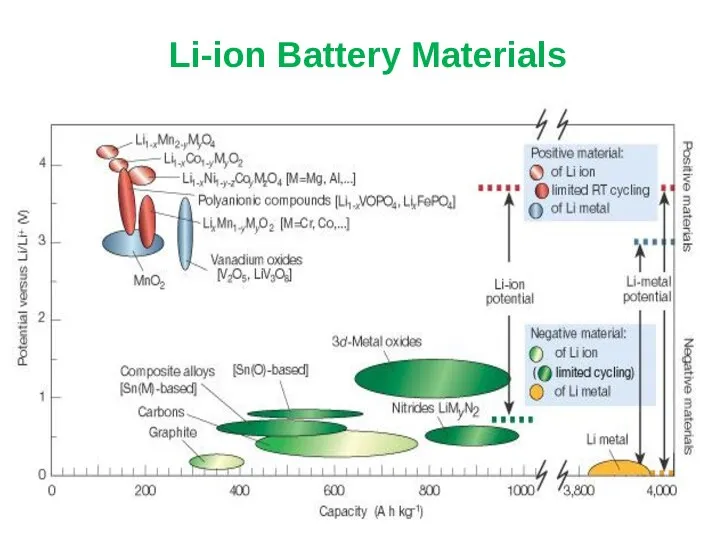
- 22. Li-ion Battery Materials
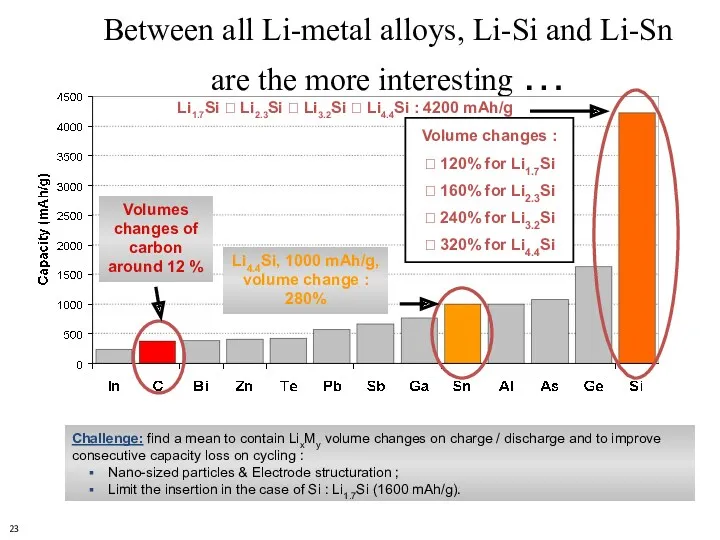
- 23. Challenge: find a mean to contain LixMy volume changes on charge / discharge and to improve
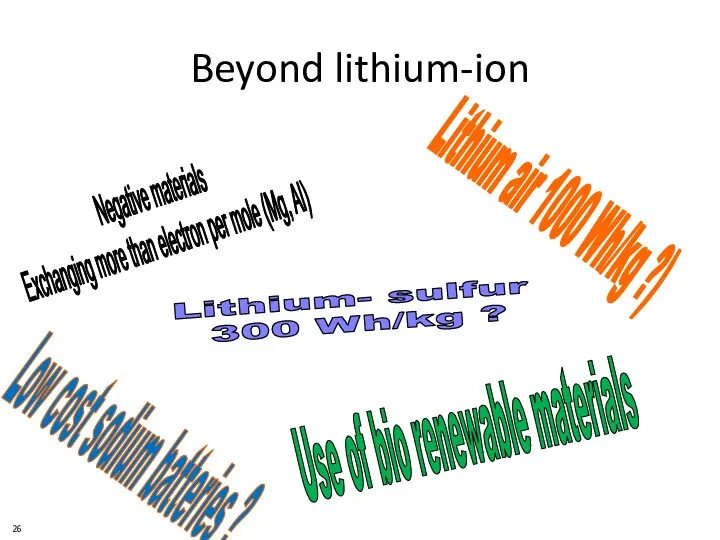
- 26. Beyond lithium-ion Negative materials Exchanging more than electron per mole (Mg, Al) Lithium air 1000 Wh/kg
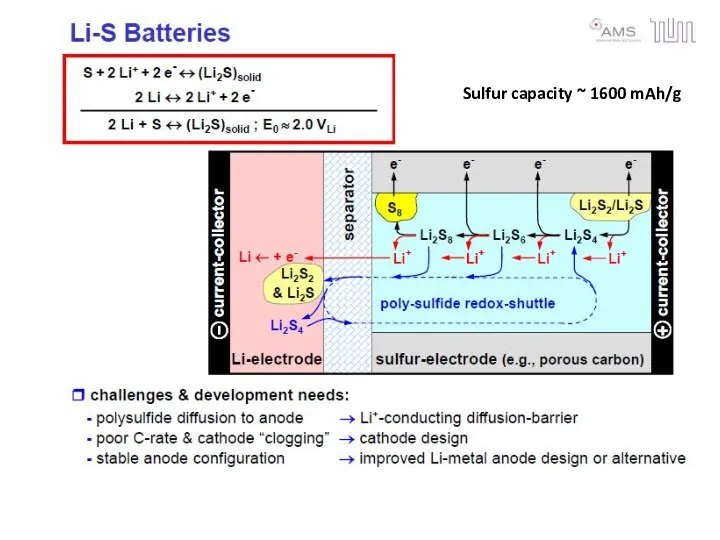
- 27. Sulfur capacity ~ 1600 mAh/g
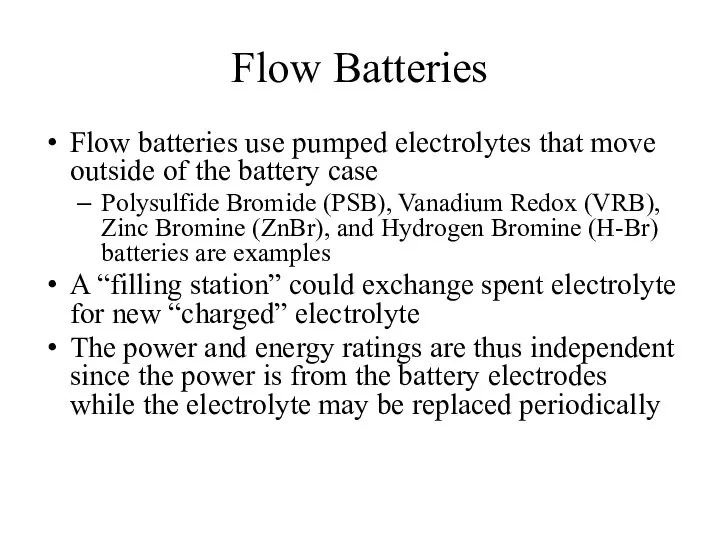
- 28. Flow Batteries Flow batteries use pumped electrolytes that move outside of the battery case Polysulfide Bromide
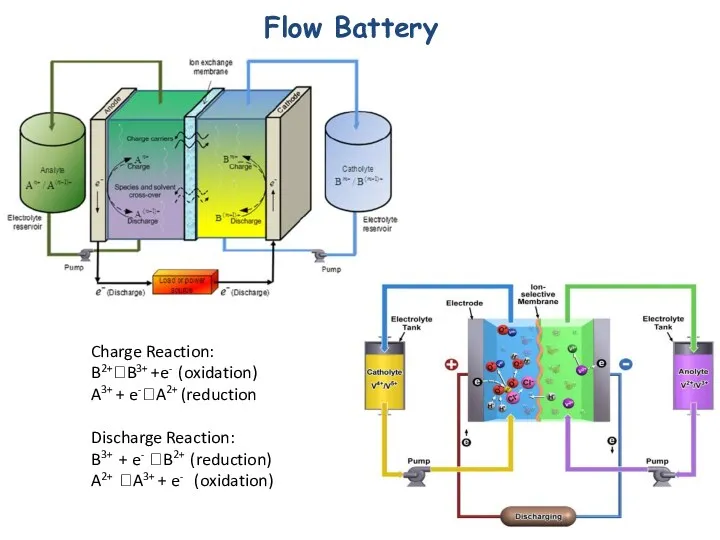
- 29. Flow Battery Charge Reaction: B2+?B3+ +e- (oxidation) A3+ + e-?A2+ (reduction Discharge Reaction: B3+ + e-
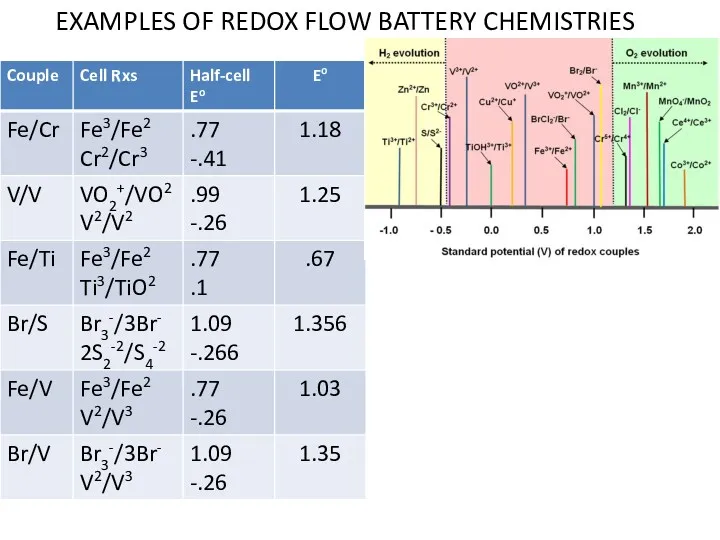
- 30. EXAMPLES OF REDOX FLOW BATTERY CHEMISTRIES
- 32. Скачать презентацию




![HIGH ENERGY CATHODES FOR LITHIUM ION BATTERIES LiMn2O4 LiMn1.8Li0.1Ni0.1O4 LiMn1.8Li0.1Ni0.1O3.8F0.2 Li[Li0.2Mn0.54Co0.13Ni0.13]O2 Li[Li0.2Mn0.54Co0.13Ni0.13]O2 / Nano Al2O3 LiCoO2](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/320337/slide-13.jpg)
 Презентация к уроку по физике для 8 класса по теме Влажность воздуха
Презентация к уроку по физике для 8 класса по теме Влажность воздуха Атмосферное давление и человек
Атмосферное давление и человек Жылулық сәулелену. Абсолют қара дене
Жылулық сәулелену. Абсолют қара дене Способы смесеобразования в бензиновых двигателях
Способы смесеобразования в бензиновых двигателях Типы подвесок
Типы подвесок Історія розвитку авіації
Історія розвитку авіації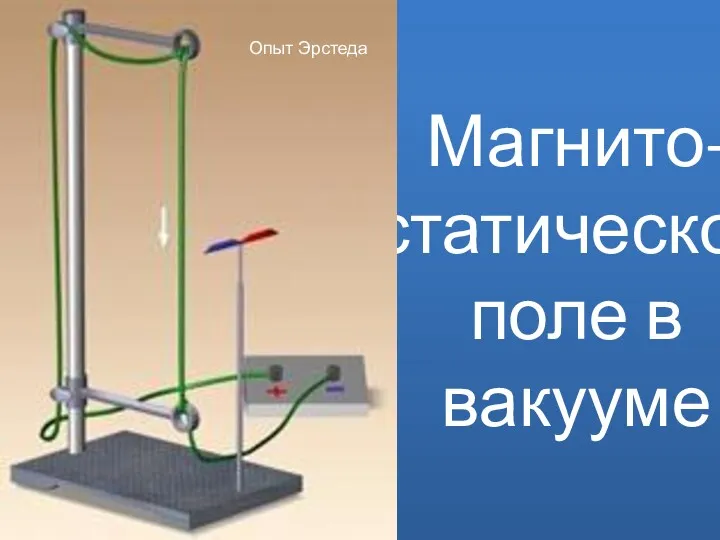 Магнито-статическое поле в вакууме
Магнито-статическое поле в вакууме Расчёт ферм
Расчёт ферм Закон Ома. Сопротивление. Что такое электрический ток?
Закон Ома. Сопротивление. Что такое электрический ток?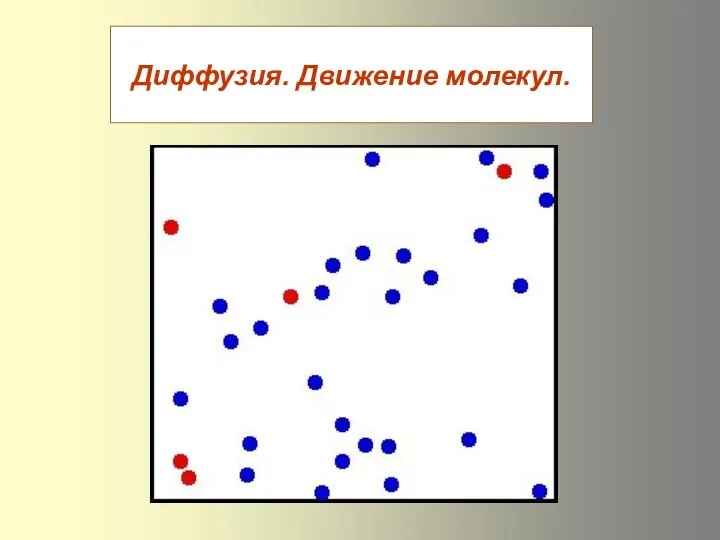 Презентация к уроку Диффузия в газах, жидкостях и твёрдых телах. Движение молекул
Презентация к уроку Диффузия в газах, жидкостях и твёрдых телах. Движение молекул Физика – наука о природе
Физика – наука о природе Законы Ньютона
Законы Ньютона 20231011_elektrizatsiya1
20231011_elektrizatsiya1 Презентация по теме Механическая работа
Презентация по теме Механическая работа Двигатели Стирлинга
Двигатели Стирлинга Backhoe Loader H200 Level 2 Pt 3 05-2008
Backhoe Loader H200 Level 2 Pt 3 05-2008 Термодинамика. Термодинамиканың бірінші бастамасы
Термодинамика. Термодинамиканың бірінші бастамасы Урок- игра по теме Масса вещества.Плотность
Урок- игра по теме Масса вещества.Плотность Презентация, урок изучения нового материала Закон Всемирного тяготения.
Презентация, урок изучения нового материала Закон Всемирного тяготения. Разработка урока-практикума Измерение объема тел.
Разработка урока-практикума Измерение объема тел. Ядерно-магнитный резонанс. Лекция 4
Ядерно-магнитный резонанс. Лекция 4 Коробка автомат элисон. Урок № 118
Коробка автомат элисон. Урок № 118 Явление электромагнитной индукции
Явление электромагнитной индукции Электростатика. Основные понятия
Электростатика. Основные понятия Специальная теория относительности. Лекция 6
Специальная теория относительности. Лекция 6 Методы атомной спектроскопии
Методы атомной спектроскопии Электропроводность биологических тканей на постоянном и переменном токах. Импеданс тканей. Физические основы реографии
Электропроводность биологических тканей на постоянном и переменном токах. Импеданс тканей. Физические основы реографии Где живет электричество?
Где живет электричество?