Содержание
- 2. History of Earth’s Climate Life appeared ~3.8 billion years ago Photosynthesis began 3.5-2.5 billion years ago
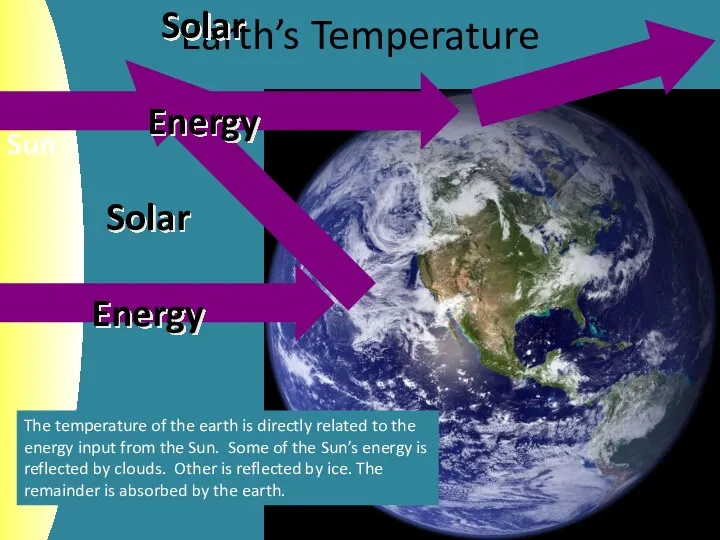
- 3. Earth’s Temperature The temperature of the earth is directly related to the energy input from the
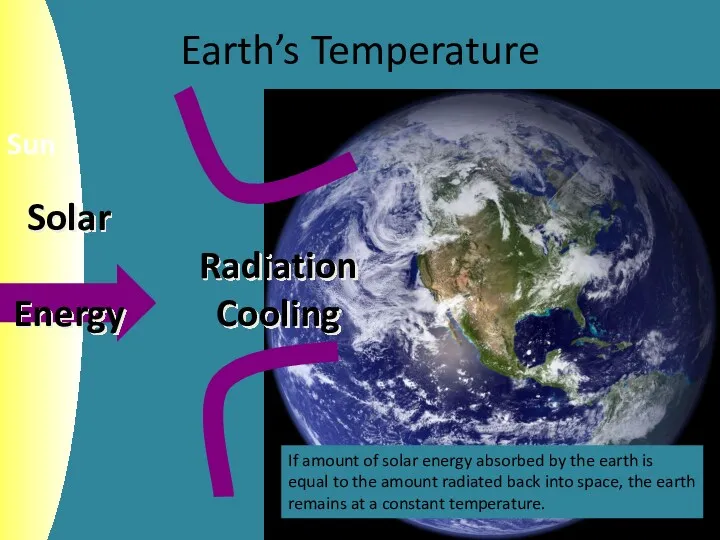
- 4. Earth’s Temperature If amount of solar energy absorbed by the earth is equal to the amount
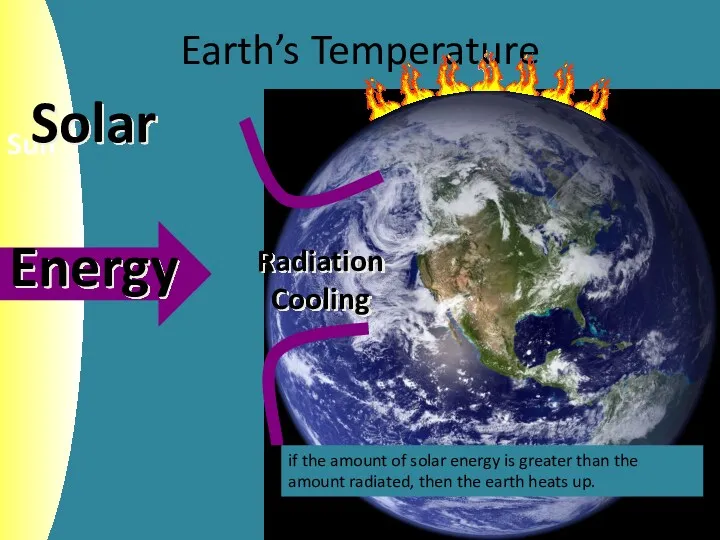
- 5. Earth’s Temperature if the amount of solar energy is greater than the amount radiated, then the
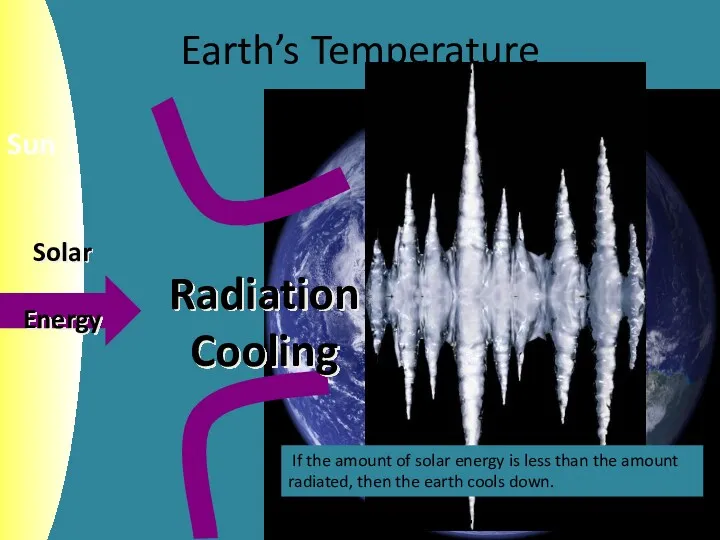
- 6. Earth’s Temperature If the amount of solar energy is less than the amount radiated, then the
- 7. Greenhouse Effect Sun To a certain degree, the earth acts like a greenhouse. Energy from the
- 8. Earth’s Atmospheric Gases Non- Greenhouse Gases 99% Greenhouse Gases 1%
- 11. Recap and importance: The photochemical reactions produce ATP and NADH at sites in the stroma. The
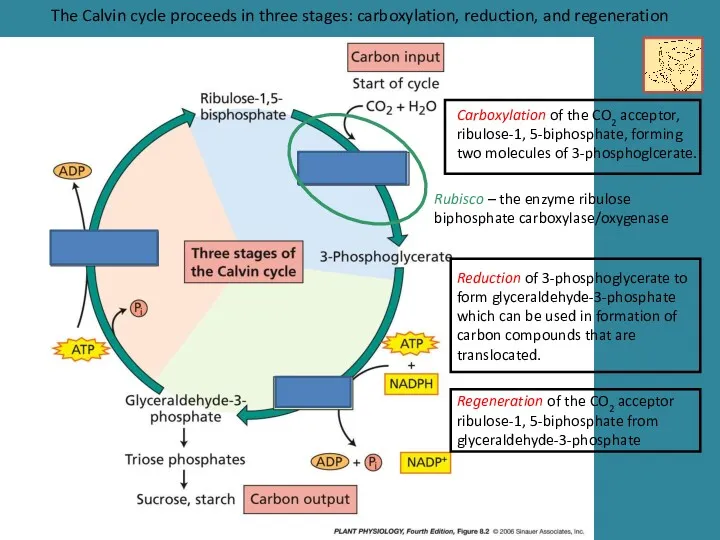
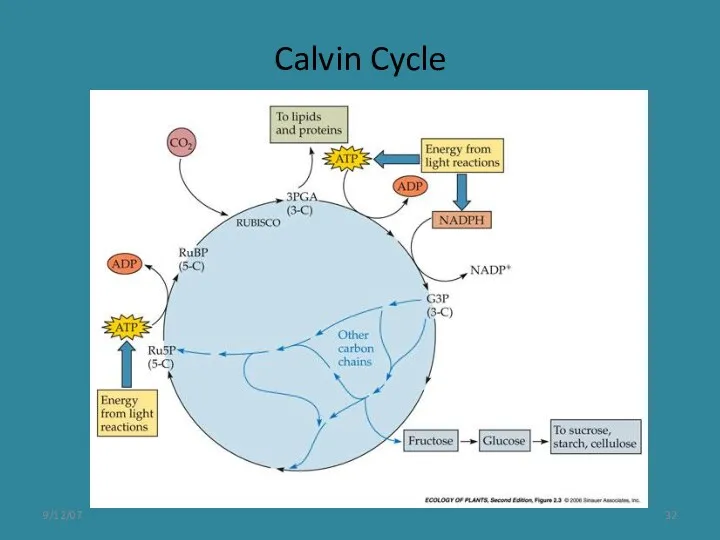
- 12. The Calvin cycle proceeds in three stages: carboxylation, reduction, and regeneration Carboxylation of the CO2 acceptor,
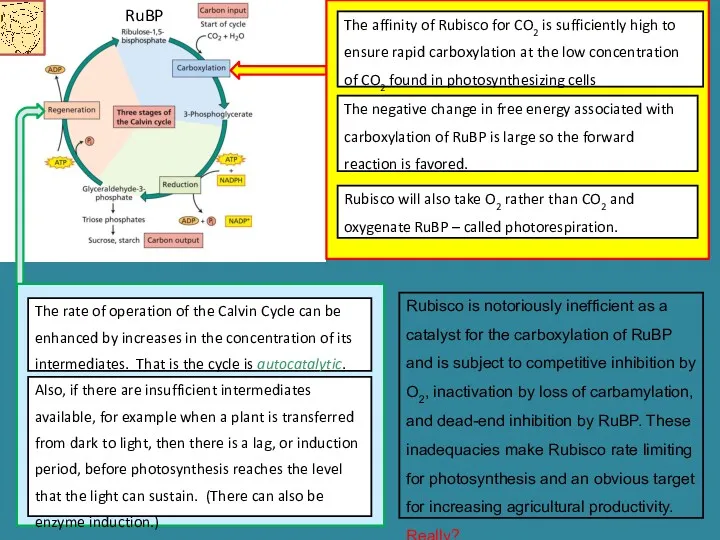
- 13. The affinity of Rubisco for CO2 is sufficiently high to ensure rapid carboxylation at the low
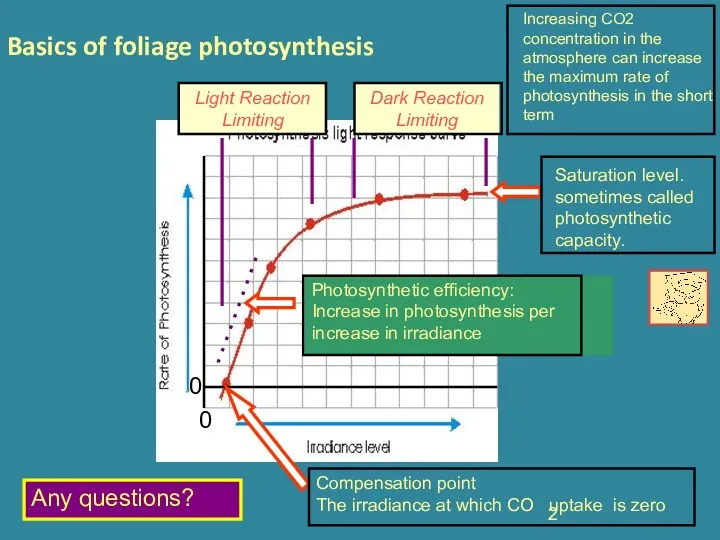
- 14. Basics of foliage photosynthesis Any questions? Increasing CO2 concentration in the atmosphere can increase the maximum
- 15. It is believed that photorespiration in plants has increased over geologic time due to increasing atmospheric
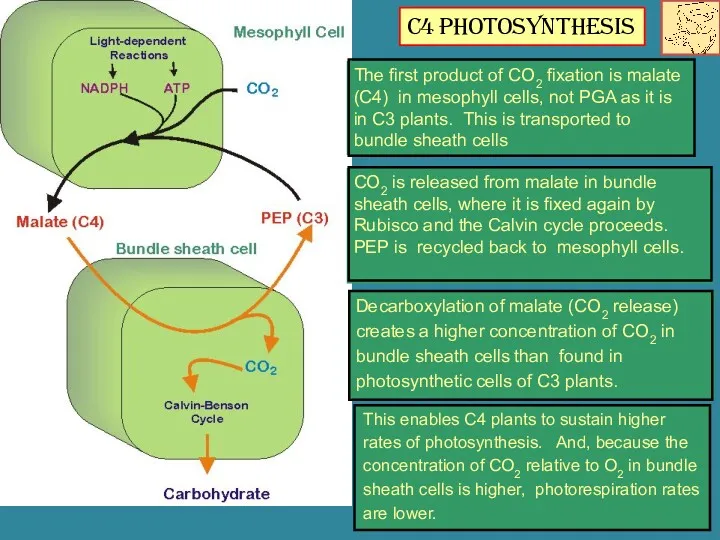
- 16. Decarboxylation of malate (CO2 release) creates a higher concentration of CO2 in bundle sheath cells than
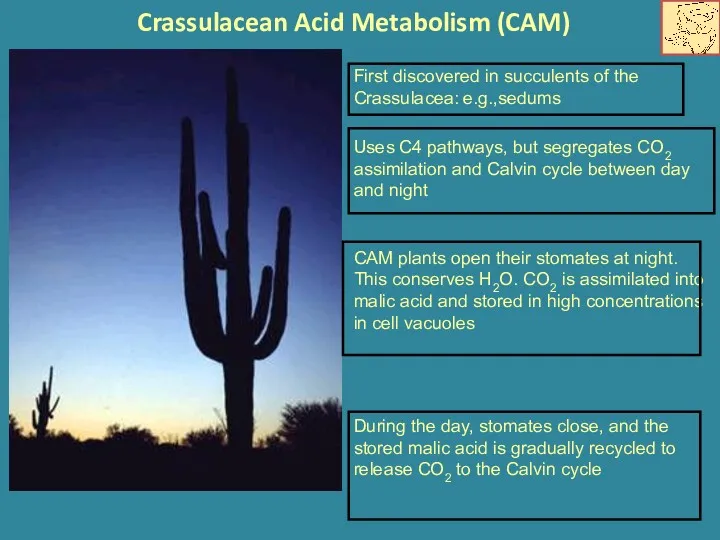
- 17. Crassulacean Acid Metabolism (CAM) Uses C4 pathways, but segregates CO2 assimilation and Calvin cycle between day
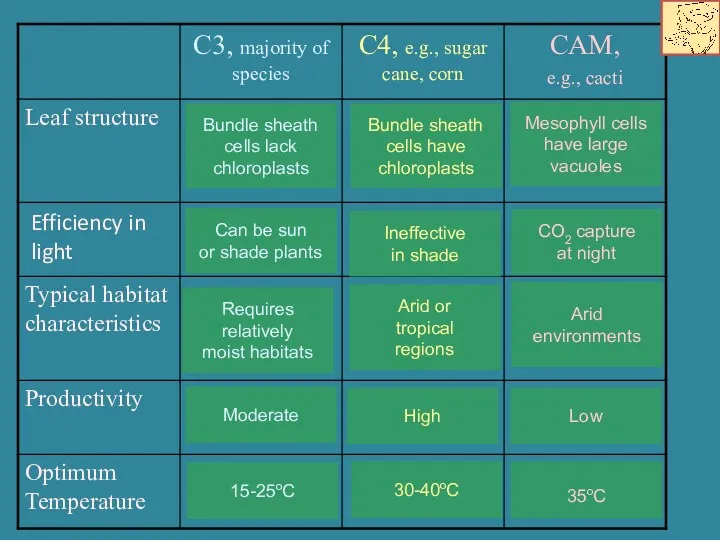
- 18. Efficiency in light
- 19. ISOTOPES AND LAND PLANT ECOLOGY C3 vs. C4 vs. CAM
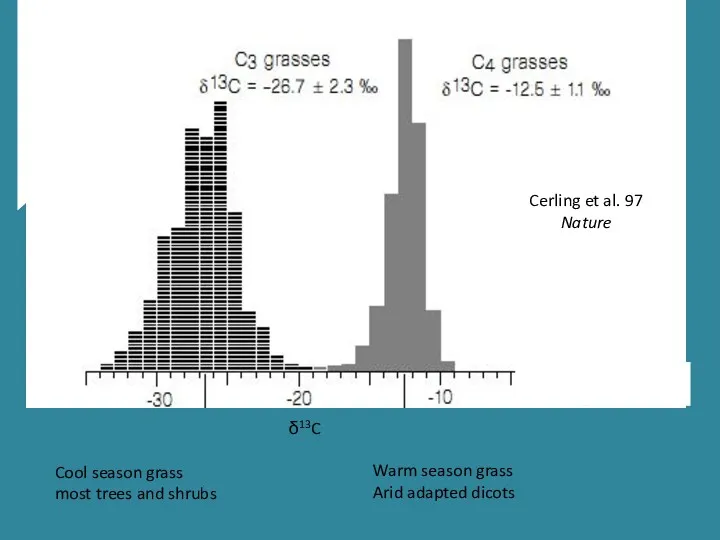
- 20. Cool season grass most trees and shrubs Warm season grass Arid adapted dicots Cerling et al.
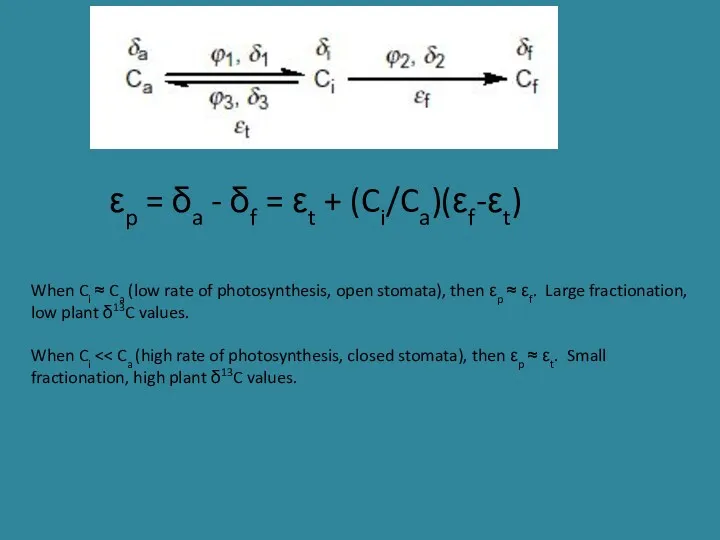
- 21. εp = δa - δf = εt + (Ci/Ca)(εf-εt) When Ci ≈ Ca (low rate of
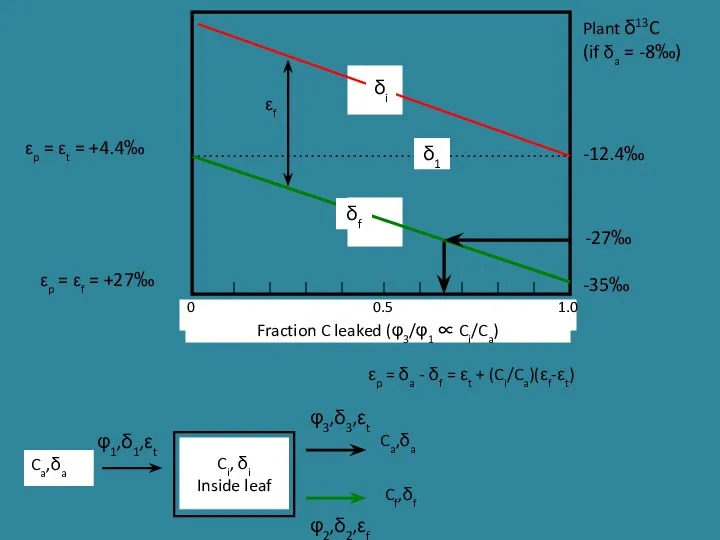
- 22. Ci, δi Inside leaf Ca,δa Ca,δa Cf,δf φ1,δ1,εt φ3,δ3,εt φ2,δ2,εf -12.4‰ -35‰ -27‰ Plant δ13C (if
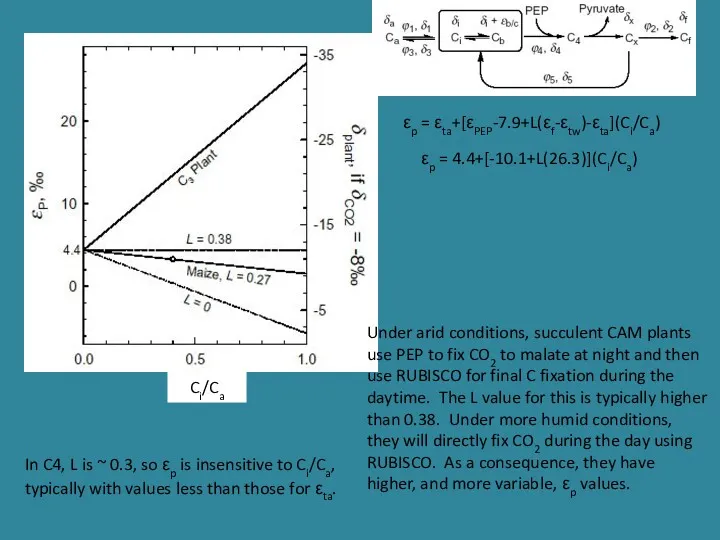
- 23. Ci/Ca In C4, L is ~ 0.3, so εp is insensitive to Ci/Ca, typically with values
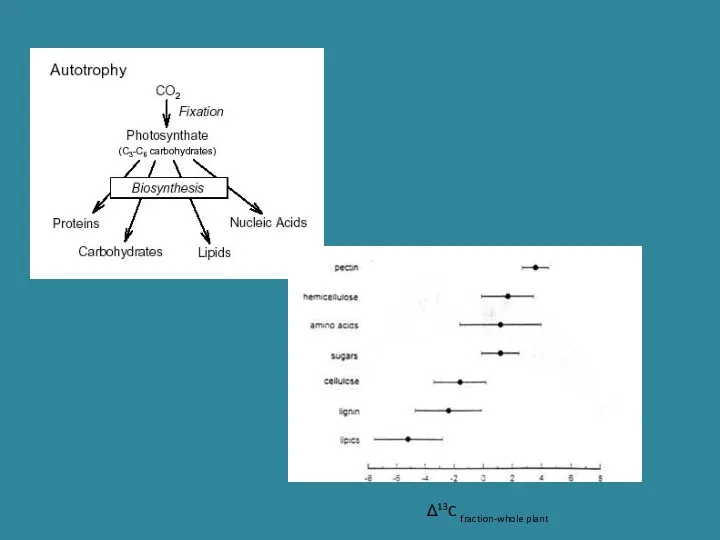
- 24. Δ13C fraction-whole plant
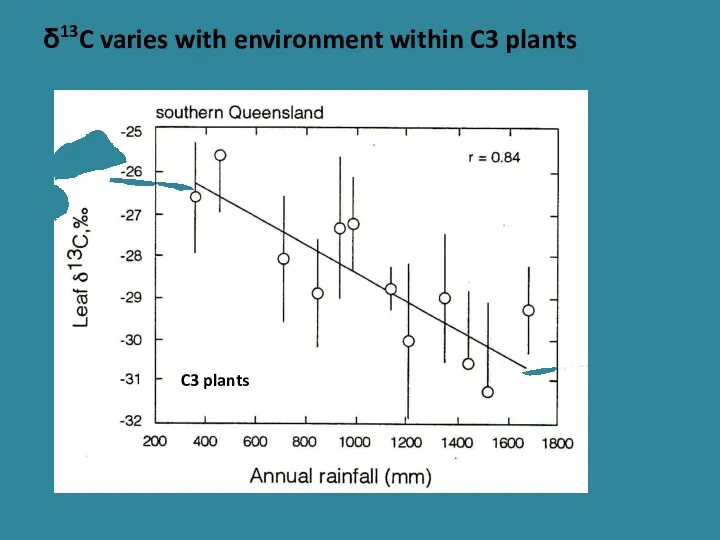
- 25. δ13C varies with environment within C3 plants C3 plants
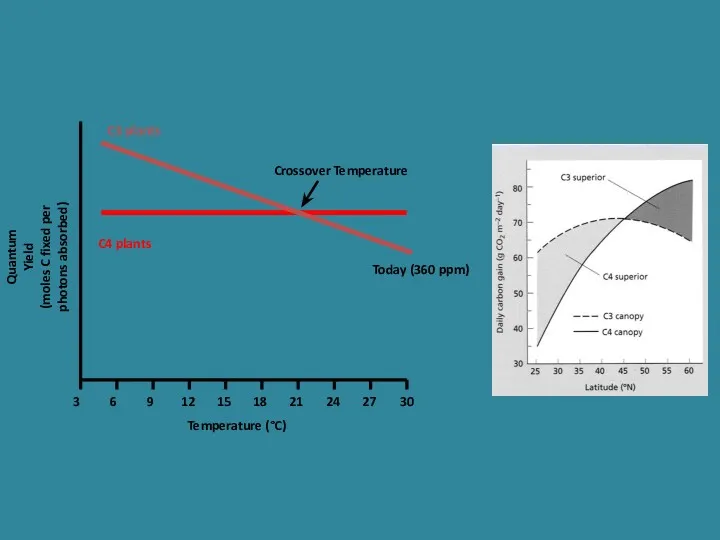
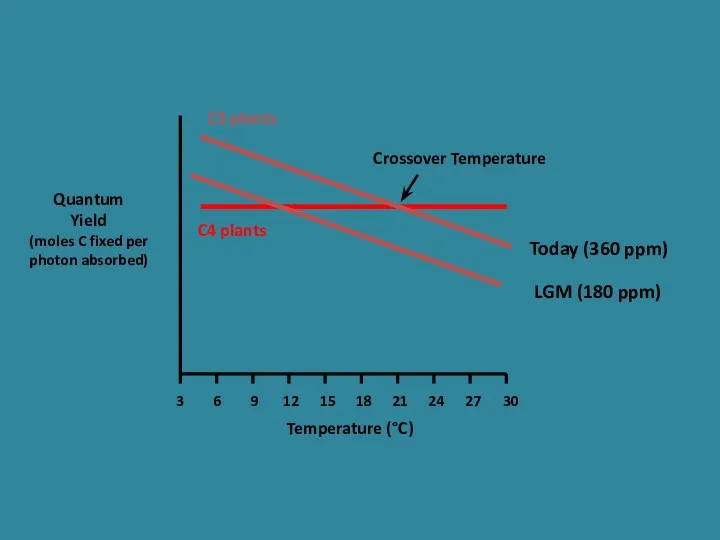
- 26. Quantum Yield (moles C fixed per photons absorbed) Temperature (°C) 3 6 9 12 15 18
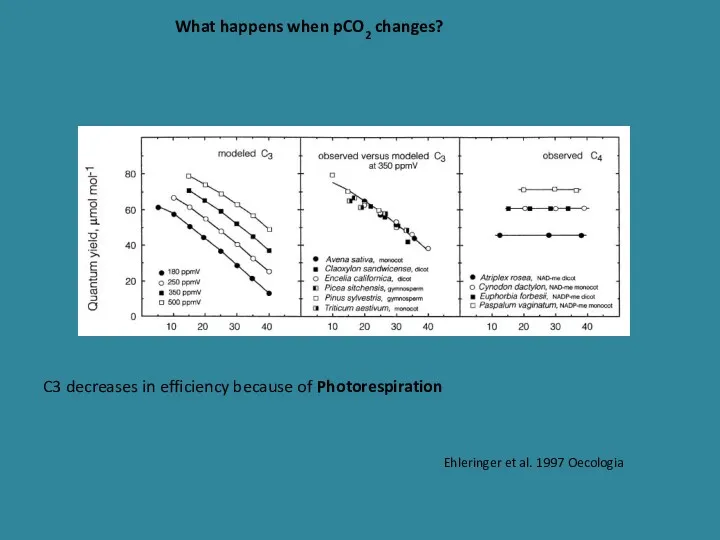
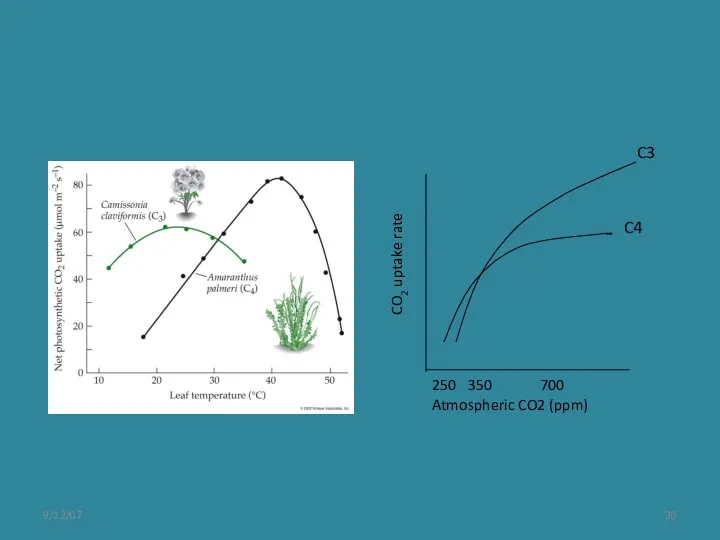
- 27. What happens when pCO2 changes? Ehleringer et al. 1997 Oecologia C3 decreases in efficiency because of
- 28. Quantum Yield (moles C fixed per photon absorbed) Temperature (°C) 3 6 9 12 15 18
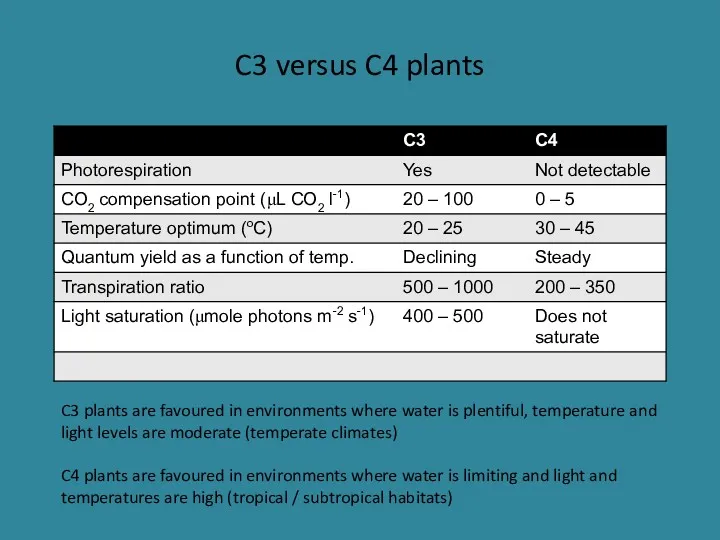
- 29. C3 versus C4 plants C3 plants are favoured in environments where water is plentiful, temperature and
- 30. 9/12/07
- 31. 9/12/07 Three modes of photosynthesis C3 pathway, aka Calvin cycle, most common. Ribulose bisphosphate (RuBP, Rubisco)
- 32. 9/12/07 Calvin Cycle
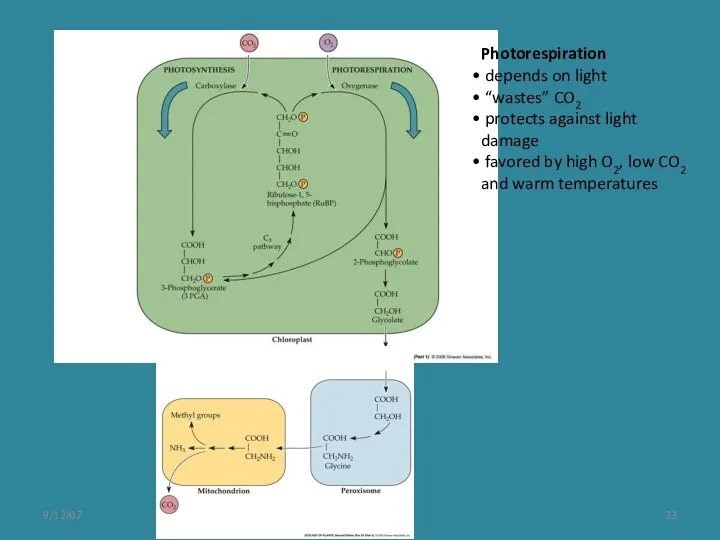
- 33. 9/12/07 Photorespiration depends on light “wastes” CO2 protects against light damage favored by high O2, low
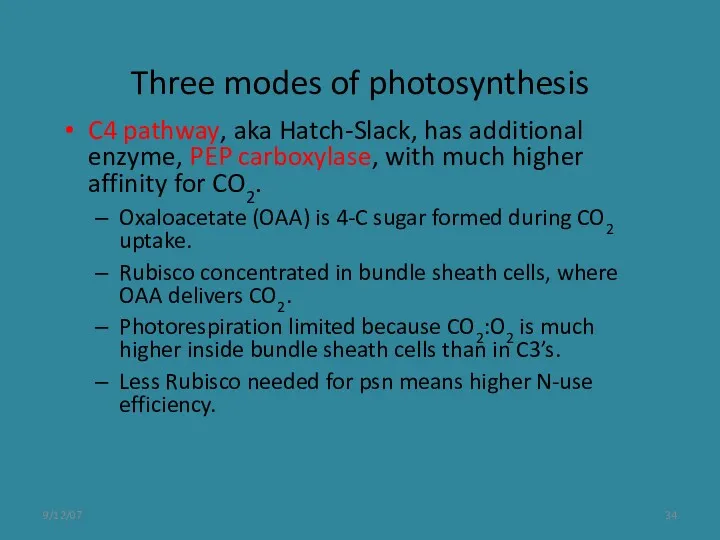
- 34. 9/12/07 Three modes of photosynthesis C4 pathway, aka Hatch-Slack, has additional enzyme, PEP carboxylase, with much
- 35. 9/12/07
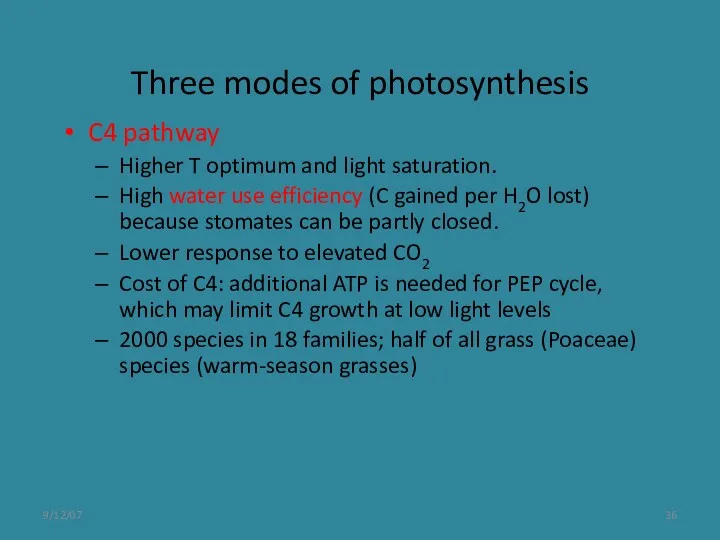
- 36. 9/12/07 Three modes of photosynthesis C4 pathway Higher T optimum and light saturation. High water use
- 39. • There is a clear correlation between the amount of anthropogenic CO2 released to the atmosphere
- 45. Скачать презентацию











 Почвы России (8 класс)
Почвы России (8 класс) Кіріспе. Теңіз кен орындарын меңгерудің қазіргі жағдайы
Кіріспе. Теңіз кен орындарын меңгерудің қазіргі жағдайы Расселение населения. Урбанизация
Расселение населения. Урбанизация Населення та політична карта Африки
Населення та політична карта Африки Государственный строй стран мира
Государственный строй стран мира Поход на Алтай
Поход на Алтай Анализ и обобщение данных о водном балансе речных бассейнов
Анализ и обобщение данных о водном балансе речных бассейнов Круговорот воды в природе
Круговорот воды в природе Материки и океаны на поверхности Земли. Части света
Материки и океаны на поверхности Земли. Части света Тайга Средней Сибири
Тайга Средней Сибири Федерати́вна Респу́бліка Брази́лія
Федерати́вна Респу́бліка Брази́лія Исследование туристского потенциала Ивановской области
Исследование туристского потенциала Ивановской области Гидрография, разнообразие природы и население Северной Америки
Гидрография, разнообразие природы и население Северной Америки Королевство Испания
Королевство Испания Водные богатства Земли
Водные богатства Земли Халық санағы. Ұдайы өсудің түрлері
Халық санағы. Ұдайы өсудің түрлері Schleswig-Holstein
Schleswig-Holstein Осетинский народ
Осетинский народ Республика Украина
Республика Украина Лекция 2. Внутреннее строение и химический состав Земли
Лекция 2. Внутреннее строение и химический состав Земли Карта России. Природные зоны. Зона арктических пустынь. Зона тундры
Карта России. Природные зоны. Зона арктических пустынь. Зона тундры Жер бетінің сумен шаюлуы – су эрозиясы деп аталады
Жер бетінің сумен шаюлуы – су эрозиясы деп аталады Ростов на Дону
Ростов на Дону Наши ближайшие соседи
Наши ближайшие соседи Погода. Элементы погоды
Погода. Элементы погоды Страны Северной Европы
Страны Северной Европы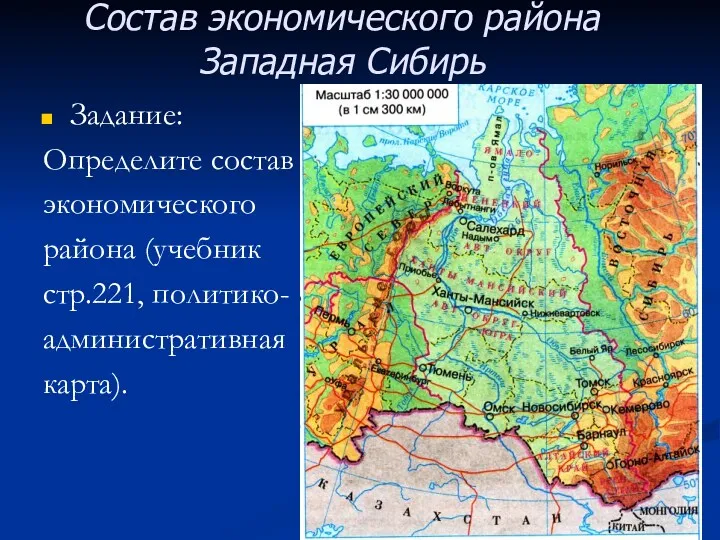 Состав экономического района Западная Сибирь
Состав экономического района Западная Сибирь Происхождение рас
Происхождение рас