Содержание
- 2. Introduction We have to remember that this is for Pharmaceutical Manufacturers of Sterile Product – a
- 3. Revisions This is the first comprehensive update to the document, previous updates have been specific changes
- 4. Changes There is more reference to RISK and RECOMMEND/RECOMMENDATION – this is in line with a
- 5. Update as of 21 February 2020 It was communicated that an updated CONSULTATION document was being
- 6. Update as of 21 February 2020 The title has now been changed from Manufacture of Sterile
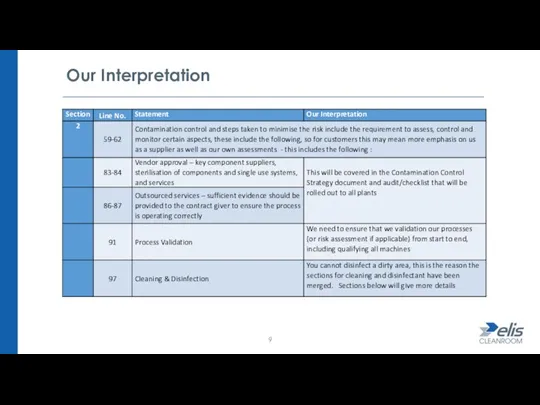
- 7. Contamination Control Strategy The requirement is to provide a detailed list of elements that we will
- 8. Contamination Control Strategy We have created a document that will form the basis of an audit
- 9. Our Interpretation
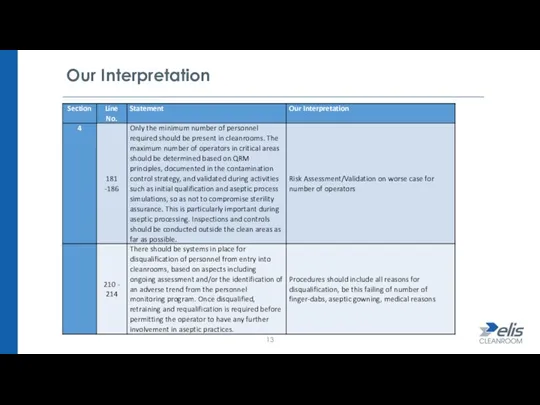
- 10. Gowning (Personnel) There is a recommendation for dedicated socks to be worn prior to entry into
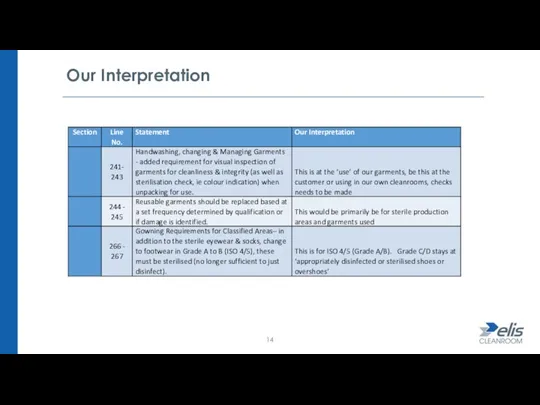
- 11. Gowning (Personnel) Added requirement for visual inspection of garments for cleanliness & integrity (as well as
- 12. Gowning (Personnel) Change to footwear such (as overboots) in Grade A/B (ISO 4), these must be
- 13. Our Interpretation
- 14. Our Interpretation
- 15. Our Interpretation
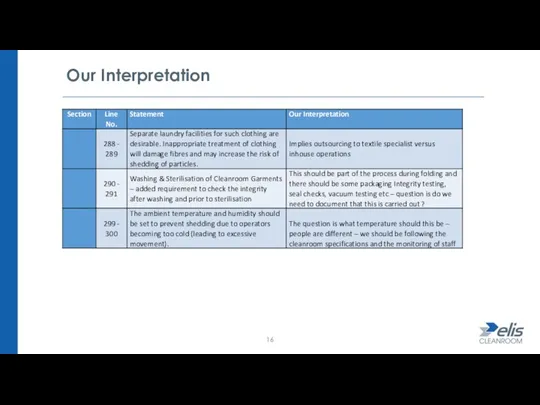
- 16. Our Interpretation
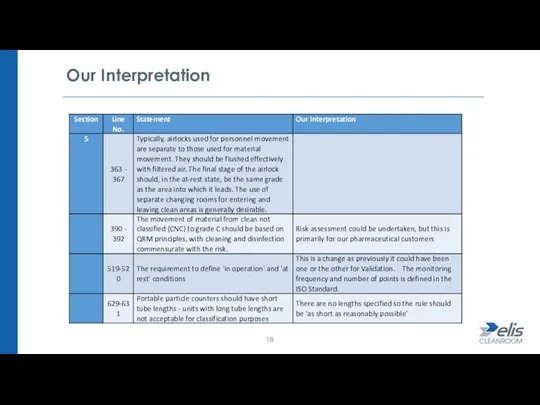
- 17. Cleanroom Design / Classification The requirement to define 'in operation' and 'at rest' conditions. Portable particle
- 18. Our Interpretation
- 19. Cleaning and Disinfection Cleaning & Disinfectant in previous versions they were treated separately these have now
- 20. Our Interpretation
- 22. Скачать презентацию














 Особливості паперу та фарби
Особливості паперу та фарби Секреты успешного резюме
Секреты успешного резюме Маркетинговый план. Месторасположение
Маркетинговый план. Месторасположение Основные результаты развития рынка розничной торговли в России
Основные результаты развития рынка розничной торговли в России 창업 타이밍 분석
창업 타이밍 분석 Презентация бренда MORIZO. Профессиональная косметика для тела
Презентация бренда MORIZO. Профессиональная косметика для тела Презентация ТЦ в г.Кольчугино
Презентация ТЦ в г.Кольчугино Продукты категории волосы. 2018
Продукты категории волосы. 2018 Kinder – брэнд N 1 в сознании мам по результатам маркетинговых исследований
Kinder – брэнд N 1 в сознании мам по результатам маркетинговых исследований С чего начать продавать себя и свой продукт
С чего начать продавать себя и свой продукт Автоматизированные информационные системы в промышленности. Электронные системы КОДЕКС
Автоматизированные информационные системы в промышленности. Электронные системы КОДЕКС Swot-анализ
Swot-анализ Квартал Красный кирпичник в Санкт-Петербурге для агентств
Квартал Красный кирпичник в Санкт-Петербурге для агентств End-Probation Presentation
End-Probation Presentation Introducing. The new world trade center. Brenda
Introducing. The new world trade center. Brenda Бостонская матрица: рост удельный вес в обороте рынка
Бостонская матрица: рост удельный вес в обороте рынка Мужской журнал MEN’s LIFE
Мужской журнал MEN’s LIFE Wildberries. Пошаговая инструкция
Wildberries. Пошаговая инструкция Виды планировок магазина
Виды планировок магазина Сплит-системы leran
Сплит-системы leran PR-коммуникации в условиях кризисной ситуации: на примере компании Volkswagen
PR-коммуникации в условиях кризисной ситуации: на примере компании Volkswagen Коммерциялық емес ұйымдардағы маркетинг
Коммерциялық емес ұйымдардағы маркетинг Яндекс Маркет для вашего бизнеса
Яндекс Маркет для вашего бизнеса Бонусная программа ProZaPass 2.0
Бонусная программа ProZaPass 2.0 Акция. Аренда торговых помещений
Акция. Аренда торговых помещений www.detstvo.info - интернет-ресурс для родителей
www.detstvo.info - интернет-ресурс для родителей Стандарты обслуживания сотрудника компании ДНС
Стандарты обслуживания сотрудника компании ДНС SMM – популяризация продукта среди целевой аудитории, проводимая в социальных сетях
SMM – популяризация продукта среди целевой аудитории, проводимая в социальных сетях