Содержание
- 2. Paroxysmal Nocturnal Haemoglobinuria Clinical aspects of PNH New ICCS Guidelines EQA and PNH testing
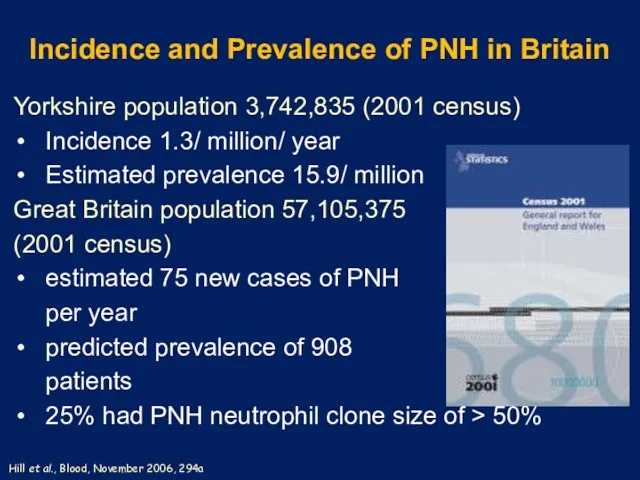
- 3. Incidence and Prevalence of PNH in Britain Yorkshire population 3,742,835 (2001 census) Incidence 1.3/ million/ year
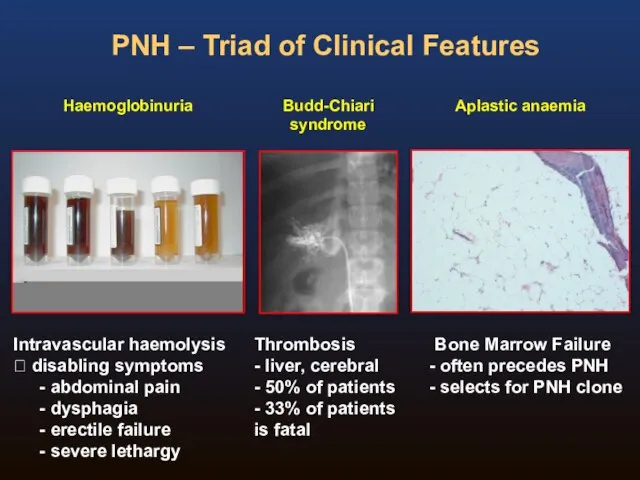
- 4. PNH – Triad of Clinical Features Haemoglobinuria Intravascular haemolysis ? disabling symptoms abdominal pain dysphagia erectile
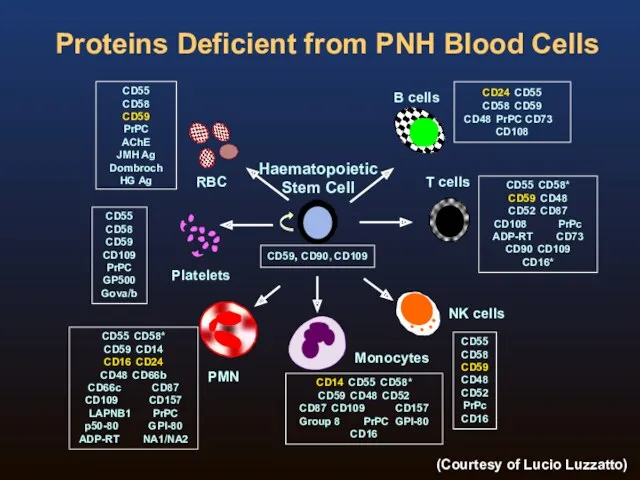
- 5. Proteins Deficient from PNH Blood Cells CD59, CD90, CD109 CD55 CD58 CD59 CD48 CD52 PrPc CD16
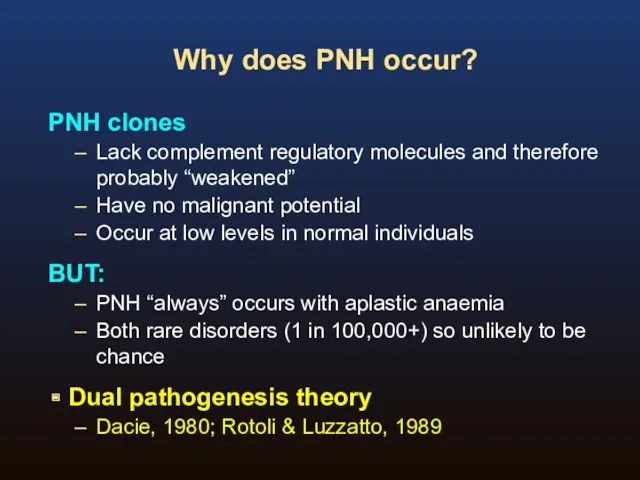
- 6. Why does PNH occur? PNH clones Lack complement regulatory molecules and therefore probably “weakened” Have no
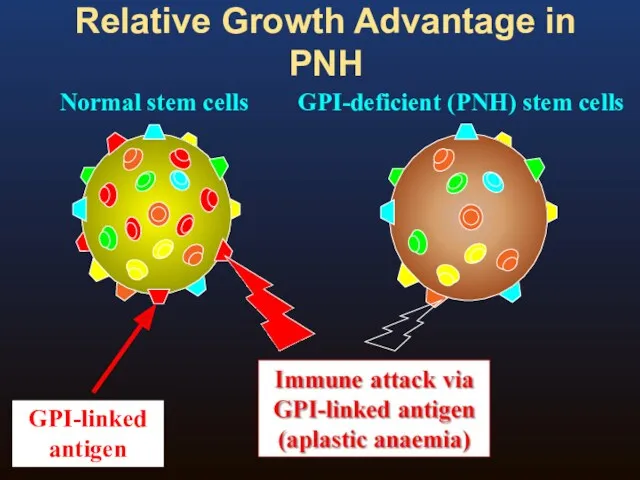
- 7. Relative Growth Advantage in PNH Normal stem cells GPI-deficient (PNH) stem cells
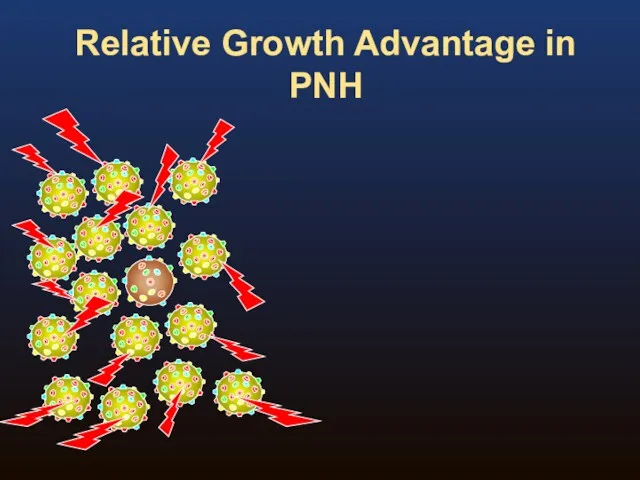
- 8. Relative Growth Advantage in PNH
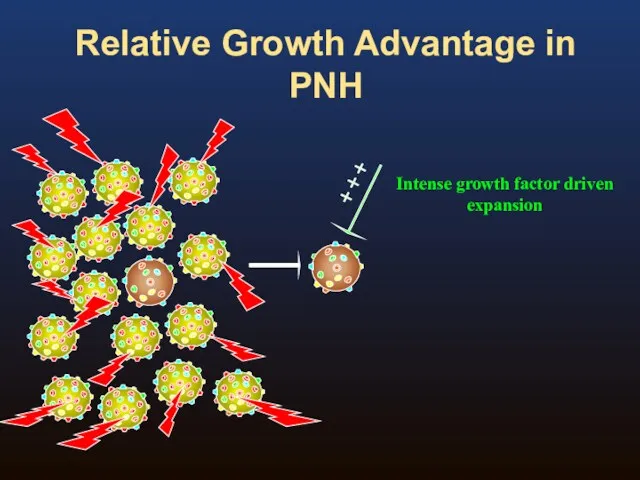
- 9. Relative Growth Advantage in PNH
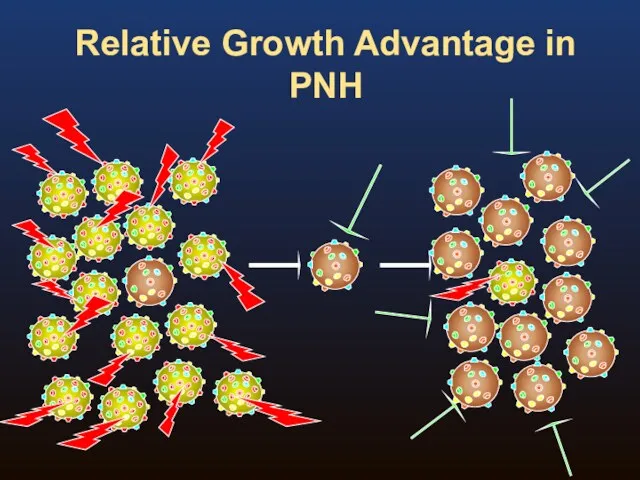
- 10. Relative Growth Advantage in PNH
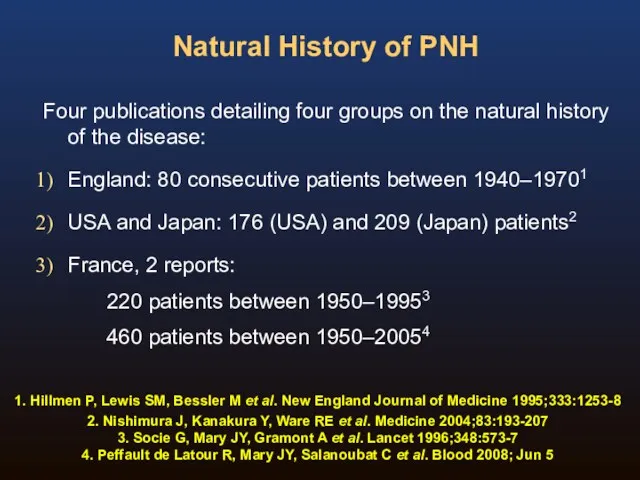
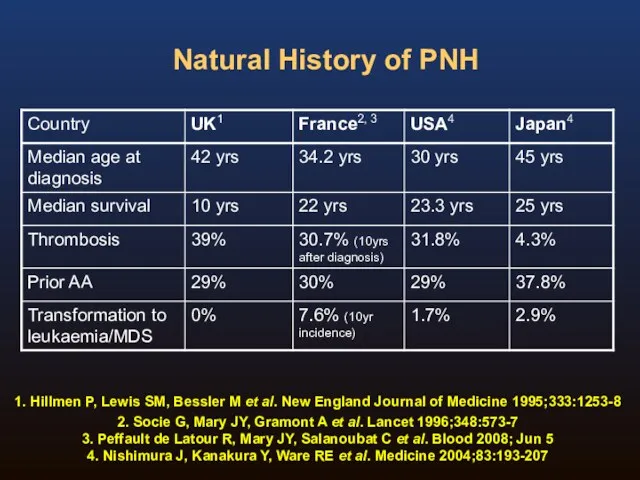
- 11. Natural History of PNH Four publications detailing four groups on the natural history of the disease:
- 12. Natural History of PNH 1. Hillmen P, Lewis SM, Bessler M et al. New England Journal
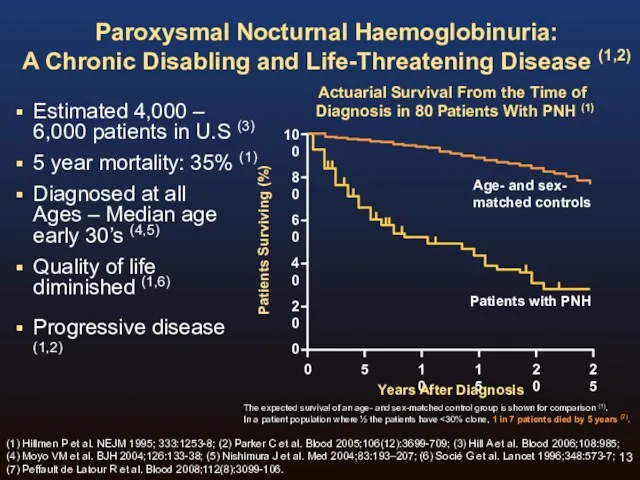
- 13. Paroxysmal Nocturnal Haemoglobinuria: A Chronic Disabling and Life-Threatening Disease (1,2) Estimated 4,000 – 6,000 patients in
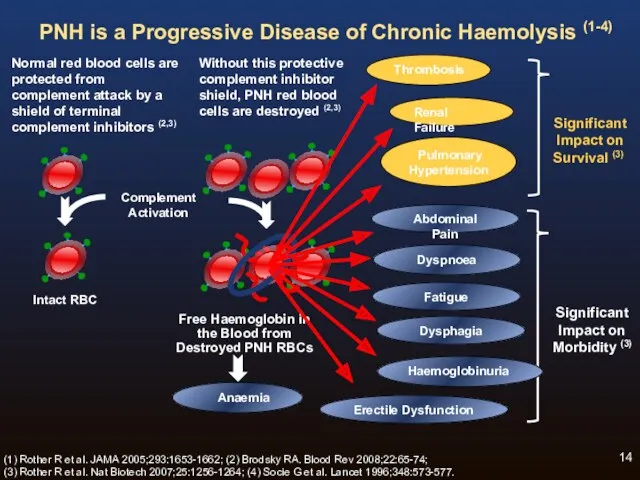
- 14. Normal red blood cells are protected from complement attack by a shield of terminal complement inhibitors
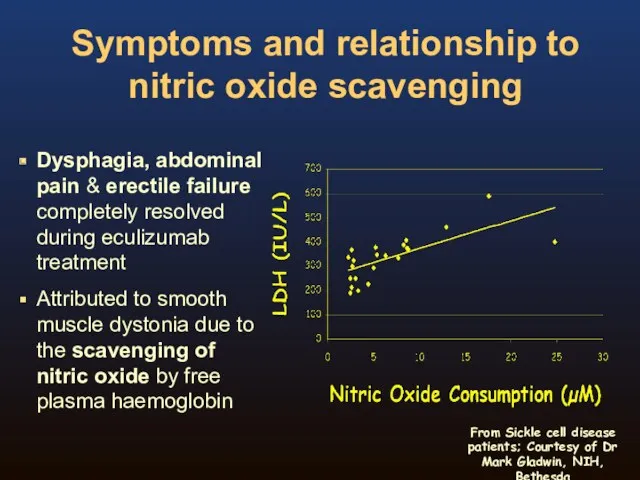
- 15. Symptoms and relationship to nitric oxide scavenging Dysphagia, abdominal pain & erectile failure completely resolved during
- 16. Haemolysis and Nitric Oxide Red blood cell destruction during haemolysis releases cell-free haemoglobin (1) Cell-free haemoglobin
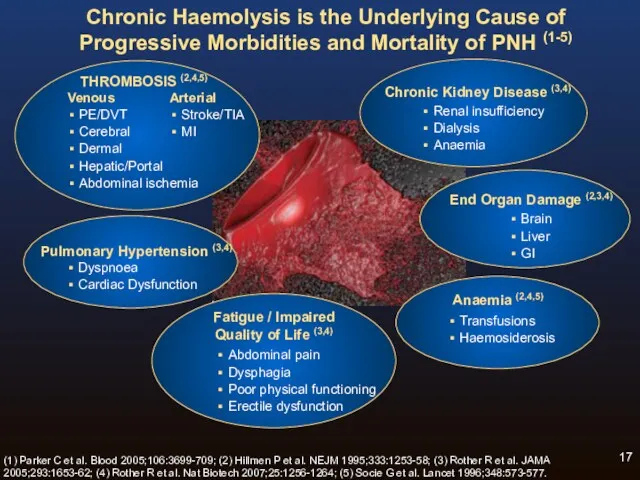
- 17. Chronic Haemolysis is the Underlying Cause of Progressive Morbidities and Mortality of PNH (1-5) Fatigue /
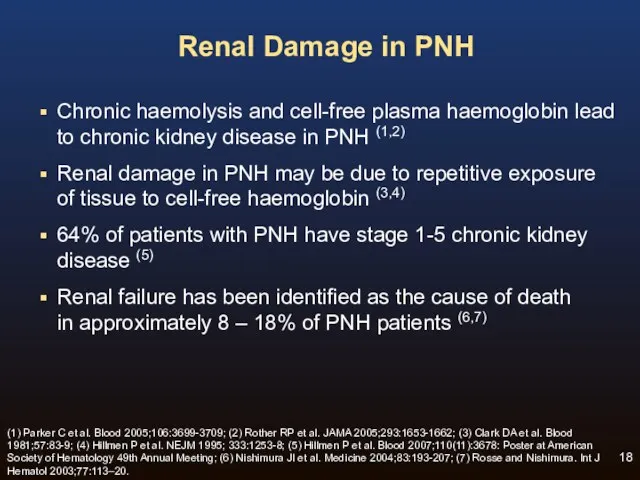
- 18. Renal Damage in PNH Chronic haemolysis and cell-free plasma haemoglobin lead to chronic kidney disease in
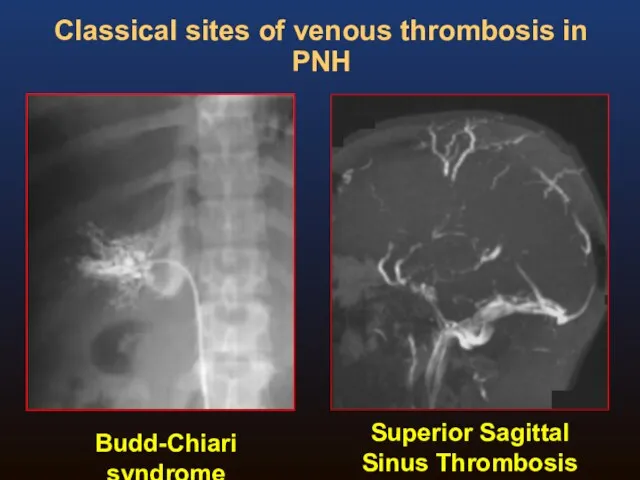
- 19. Budd-Chiari syndrome Superior Sagittal Sinus Thrombosis Classical sites of venous thrombosis in PNH
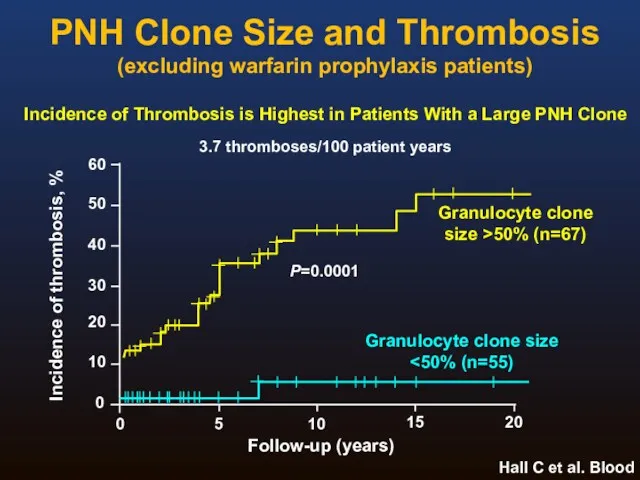
- 20. PNH Clone Size and Thrombosis (excluding warfarin prophylaxis patients) Hall C et al. Blood 2003;102(10):3587-3591. 0
- 21. Laboratory Investigation of PNH Flow cytometry immunophenotyping is the method of choice for PNH testing Diagnosis
- 22. Background In 2008 the Clinical Cytometry Society sponsored a workshop on PNH testing Approximately 100 attendees
- 23. The disease is rare and most labs have limited experience in PNH testing Clinical documents have
- 24. Consensus Committee Michael J Borowitz, MD, PhD Johns Hopkins Fiona E Craig, MD University of Pittsburgh
- 25. ICCS PNH Testing Guidelines Borowitz M, Craig F, DiGiuseppe J, Illingworth A, Rosse W, Sutherland R,
- 26. Recommendations in the ICCS PNH Testing Guidelines Document Recommendations tried to strike a balance between the
- 27. Contents Of The Document Rationale and History Clinical Indications Methodology Routine testing High sensitivity testing RBC
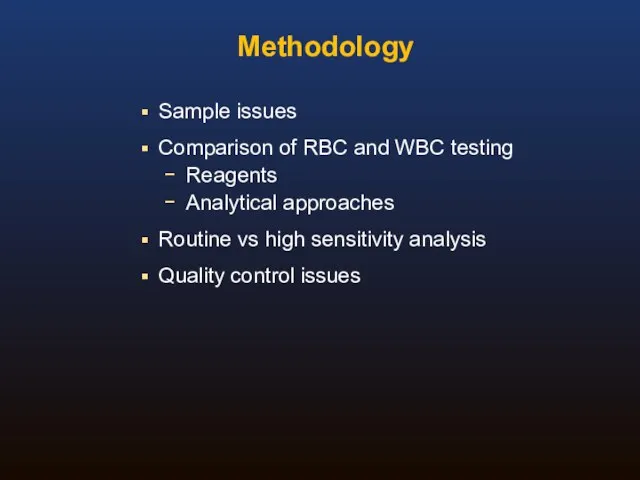
- 28. Methodology Sample issues Comparison of RBC and WBC testing Reagents Analytical approaches Routine vs high sensitivity
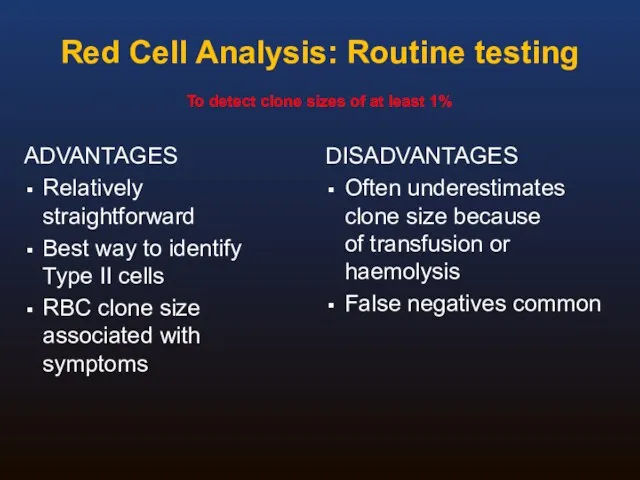
- 29. Red Cell Analysis: Routine testing ADVANTAGES Relatively straightforward Best way to identify Type II cells RBC
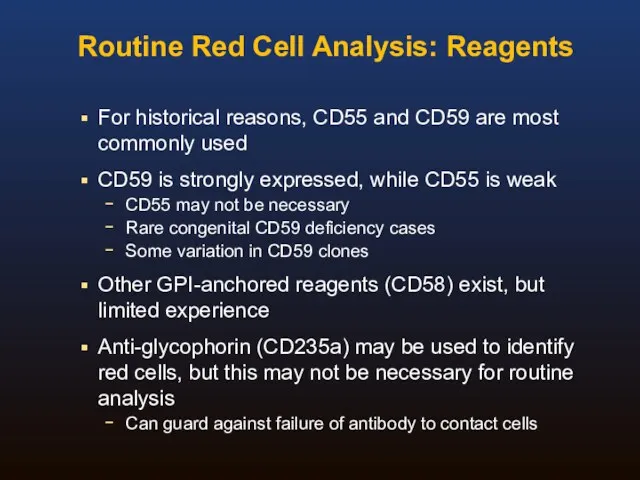
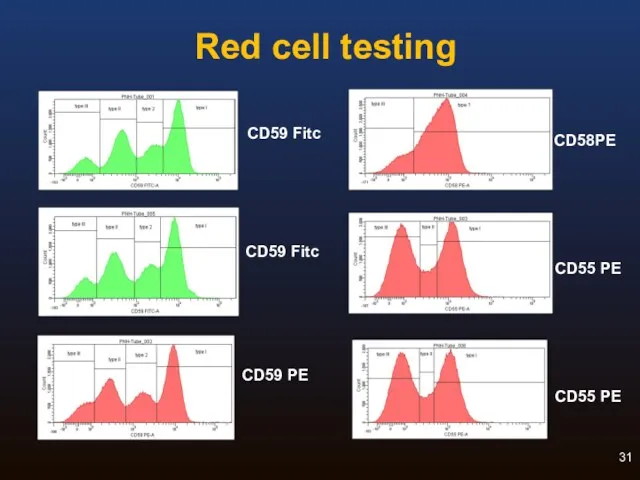
- 30. Routine Red Cell Analysis: Reagents For historical reasons, CD55 and CD59 are most commonly used CD59
- 31. Red cell testing CD58PE CD55 PE CD55 PE CD59 Fitc CD59 PE CD59 Fitc
- 32. Leucocyte Analysis: Routine testing Granulocyte PNH clone probably gives most accurate estimate of PNH clone size
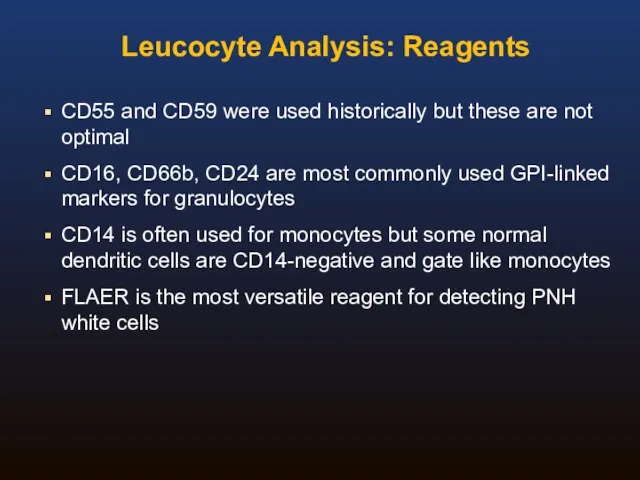
- 33. Leucocyte Analysis: Reagents CD55 and CD59 were used historically but these are not optimal CD16, CD66b,
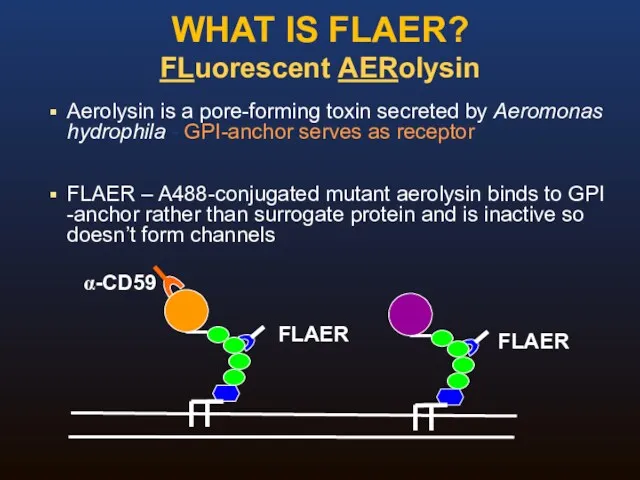
- 34. WHAT IS FLAER? FLuorescent AERolysin Aerolysin is a pore-forming toxin secreted by Aeromonas hydrophila - GPI-anchor
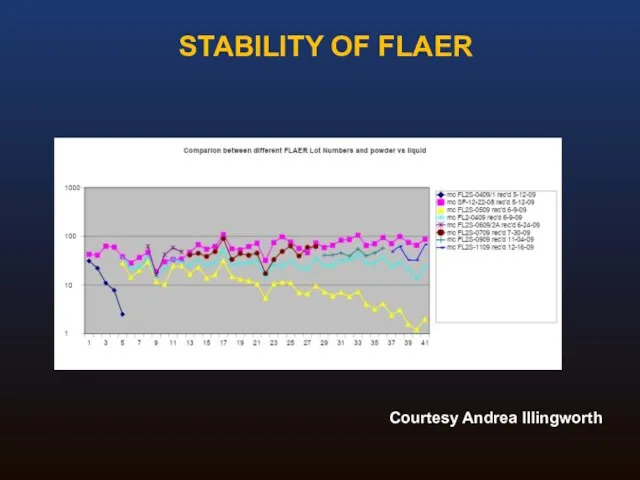
- 35. Original formulation was lyophilized, requiring aliquoting and freezing Reconstituted FLAER was unstable Stability problems better with
- 36. STABILITY OF FLAER Courtesy Andrea Illingworth
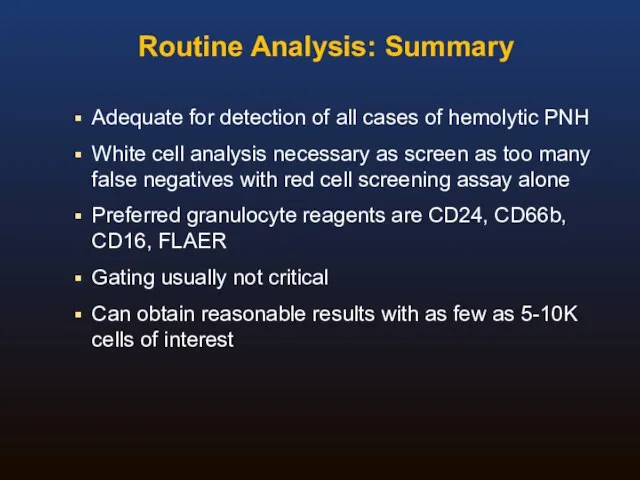
- 37. Routine Analysis: Summary Adequate for detection of all cases of hemolytic PNH White cell analysis necessary
- 38. High Sensitivity Assays: Special concerns Need to collect more events Requirement for an extensive study of
- 39. Guideline Summary I Broad agreement on the need for a consensus guideline Document reviews and clarifies
- 40. Guideline Summary II Granulocyte analysis provides better estimate of size of PNH clone than RBC analysis
- 41. Guideline Summary III For high sensitivity WBC analysis, essential to use an antibody for gating, and
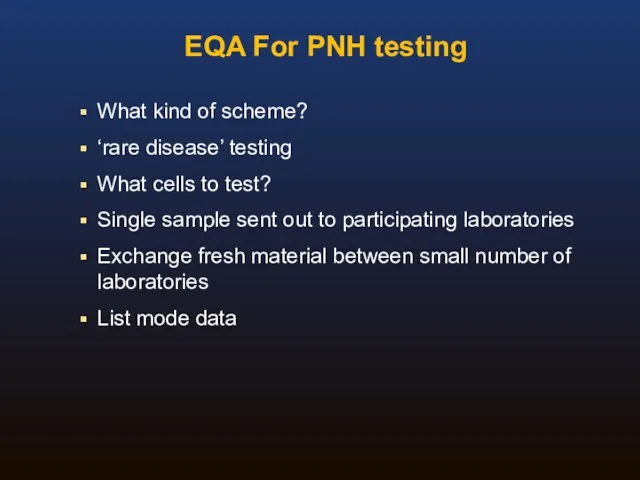
- 42. EQA For PNH testing What kind of scheme? Screening vs high sensitivity (MRD) testing What material?
- 43. EQA For PNH testing What kind of scheme? ‘rare disease’ testing What cells to test? Single
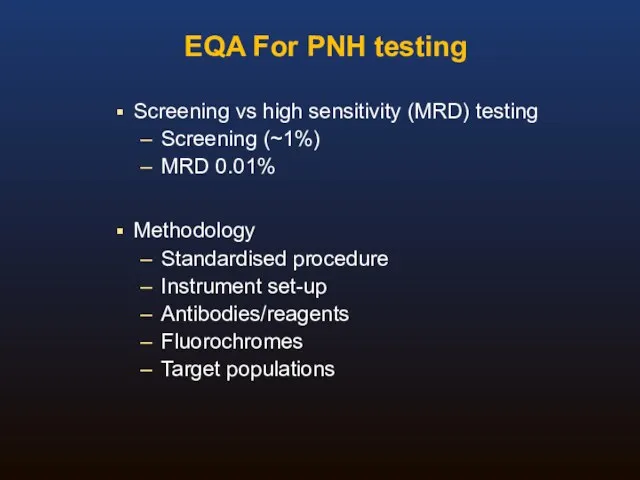
- 44. EQA For PNH testing Screening vs high sensitivity (MRD) testing Screening (~1%) MRD 0.01% Methodology Standardised
- 45. EQA For PNH testing What material? Small groups: exchange of known fresh patient samples Large International
- 46. EQA For PNH testing Educational aspects? Scoring/performance issues? How to assess performance? Poor performance – educational
- 48. Скачать презентацию
















 Травмы органов и тканей челюстнолицевой области у детей
Травмы органов и тканей челюстнолицевой области у детей Вирус гепатита А (ВГА, HAV)
Вирус гепатита А (ВГА, HAV) Загальна характеристика вірусних гепатитів. Вірусні гепатити з фекально-оральним механізмом зараження
Загальна характеристика вірусних гепатитів. Вірусні гепатити з фекально-оральним механізмом зараження Патофизиология системы внешнего дыхания
Патофизиология системы внешнего дыхания Жүйелі аурулар және зат алмасу ауруларында ауыз қуысы кілегей қабығының зақымдануы. Клиникасы, диагностикасы, емі
Жүйелі аурулар және зат алмасу ауруларында ауыз қуысы кілегей қабығының зақымдануы. Клиникасы, диагностикасы, емі Лучевая анатомия позвоночника
Лучевая анатомия позвоночника Лямблиоз
Лямблиоз Эргономические основы организации рабочего места врача-стоматолога. Работа врача с помощником в четыре руки
Эргономические основы организации рабочего места врача-стоматолога. Работа врача с помощником в четыре руки Антенатальды кезеңдегі инвазивті тексеру
Антенатальды кезеңдегі инвазивті тексеру Национальный институт рака им. Дж. Паскале
Национальный институт рака им. Дж. Паскале Хроническая венозная недостаточность
Хроническая венозная недостаточность Холиномиметические средства (Лекция № 4)
Холиномиметические средства (Лекция № 4) Сarec cистема, Cad/Cem система, свойства, применение
Сarec cистема, Cad/Cem система, свойства, применение Работа в очаге туберкулёзной инфекции
Работа в очаге туберкулёзной инфекции Пиодермии. Этиология. Классификация. Клиника
Пиодермии. Этиология. Классификация. Клиника Физиология эндокринной системы
Физиология эндокринной системы Жинақталған салыстыру. Бонферронидің түзетуімен. Стьюдент белгісі
Жинақталған салыстыру. Бонферронидің түзетуімен. Стьюдент белгісі Образ врача. Нравственные принципы профессии врача
Образ врача. Нравственные принципы профессии врача Дегидрогеназалардың нуклеотидті. Коферменттері. НАД, НАДФ, ФАД нуклеозидті коферменттер
Дегидрогеназалардың нуклеотидті. Коферменттері. НАД, НАДФ, ФАД нуклеозидті коферменттер Кожные проявления аллергии. Клинические аспекты и принципы лечения
Кожные проявления аллергии. Клинические аспекты и принципы лечения Роль медицинской сестры в преодолении деформации личности онкологических больных разных возрастов
Роль медицинской сестры в преодолении деформации личности онкологических больных разных возрастов Комплексна оцінка здоров’я населення. Методика вивчення та оцінка чинників, що впливають на здоров’я населення
Комплексна оцінка здоров’я населення. Методика вивчення та оцінка чинників, що впливають на здоров’я населення Принцип действия лазерного луча. Терапия сосудистых патологий
Принцип действия лазерного луча. Терапия сосудистых патологий Задачи принципы организации диетического питания
Задачи принципы организации диетического питания Развитие медицины в России в 19 веке. Хирургия
Развитие медицины в России в 19 веке. Хирургия Сердце. Основные диагностические алгоритмы
Сердце. Основные диагностические алгоритмы Зәр және жыныстық жүйесінің қатерсіз және қатерлі ісіктері
Зәр және жыныстық жүйесінің қатерсіз және қатерлі ісіктері Всемирный день здоровья
Всемирный день здоровья