Содержание
- 2. Vocabulary Acidity, alkalinity, aqueous Donor, acceptor Dissociation Indicator
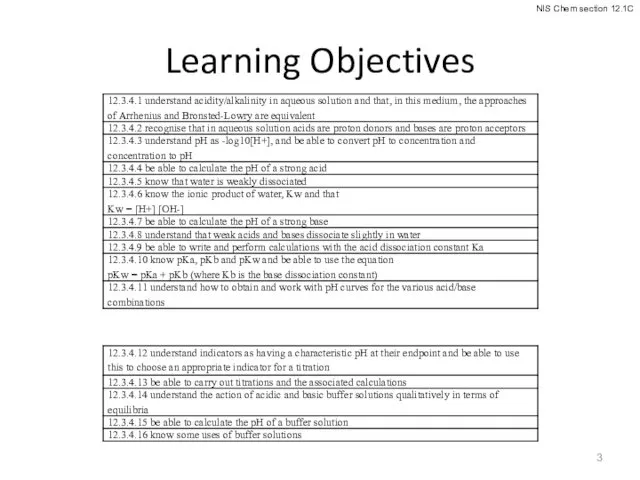
- 3. Learning Objectives
- 4. Acids and Bases Arrhenius definition: Classified in terms of formula and behaviour in water Acid: Base:
- 5. Acids and Bases Brønsted-Lowry definition: An acid-base reaction is a proton transfer process Acid: Base: Proton
- 6. Brønsted-Lowry Acid-Bases Conjugate acid-base pairs: Every acid-base reaction has two conjugate acid-base pairs. NH4+ is the
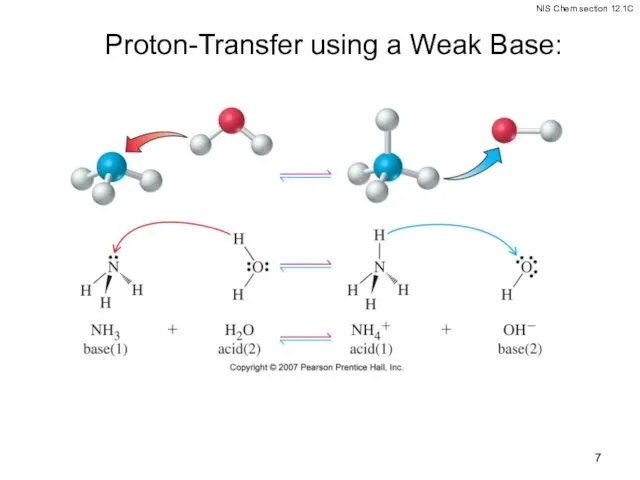
- 7. Proton-Transfer using a Weak Base:
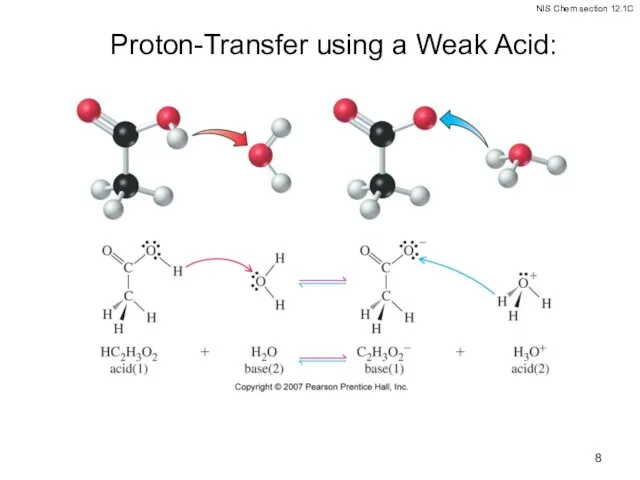
- 8. Proton-Transfer using a Weak Acid:
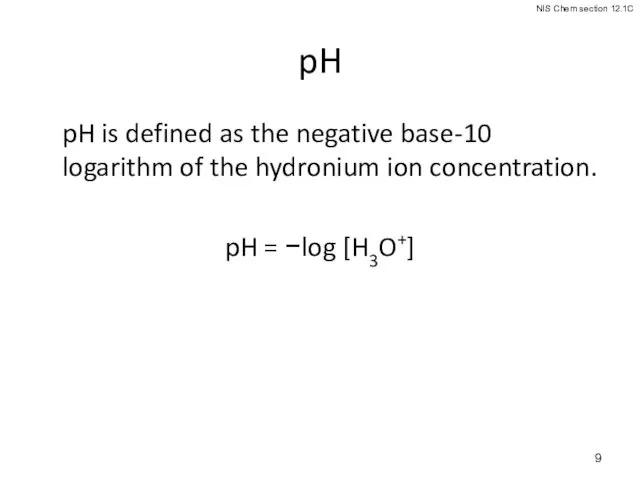
- 9. pH pH is defined as the negative base-10 logarithm of the hydronium ion concentration. pH =
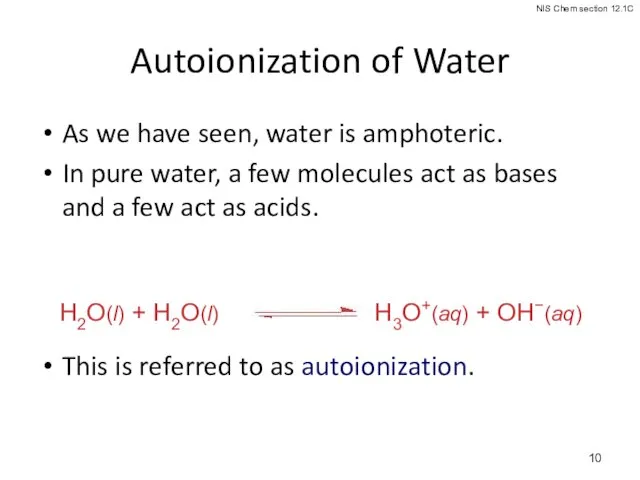
- 10. Autoionization of Water As we have seen, water is amphoteric. In pure water, a few molecules
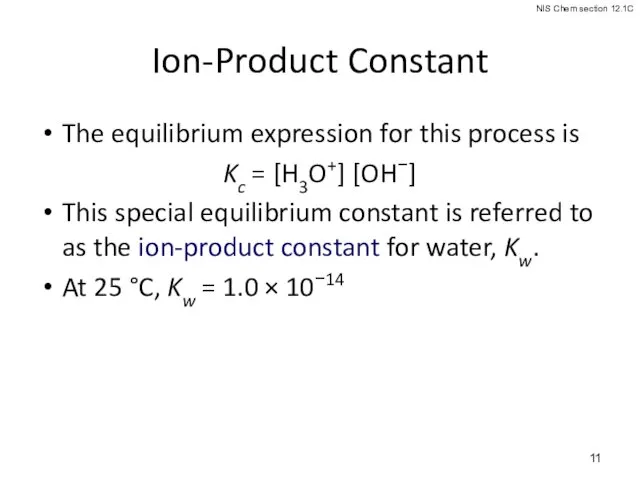
- 11. Ion-Product Constant The equilibrium expression for this process is Kc = [H3O+] [OH−] This special equilibrium
- 12. pH In pure water, Kw = [H3O+] [OH−] = 1.0 × 10−14 Because in pure water
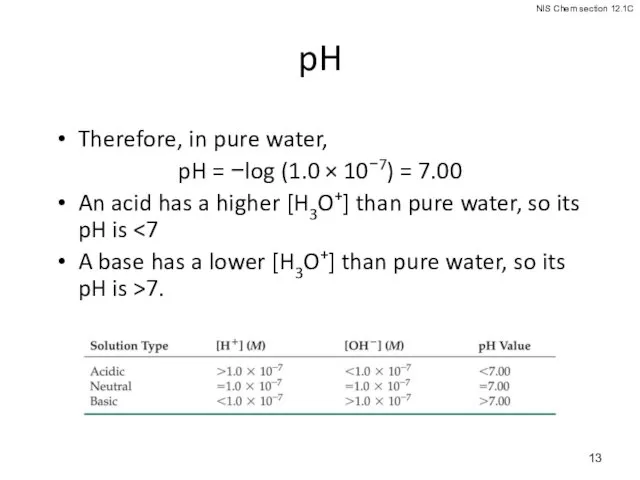
- 13. pH Therefore, in pure water, pH = −log (1.0 × 10−7) = 7.00 An acid has
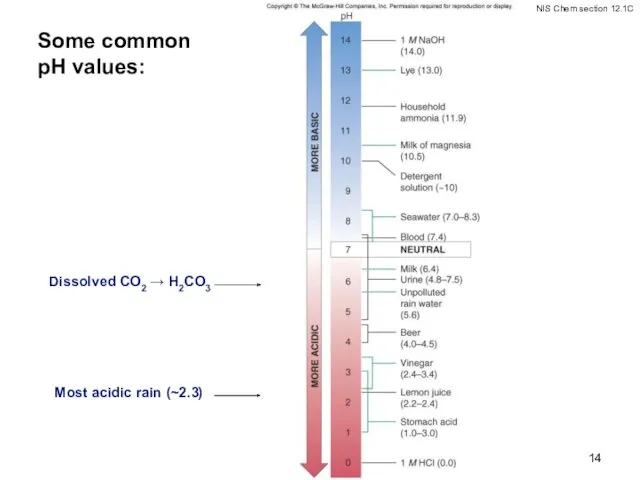
- 14. Some common pH values:
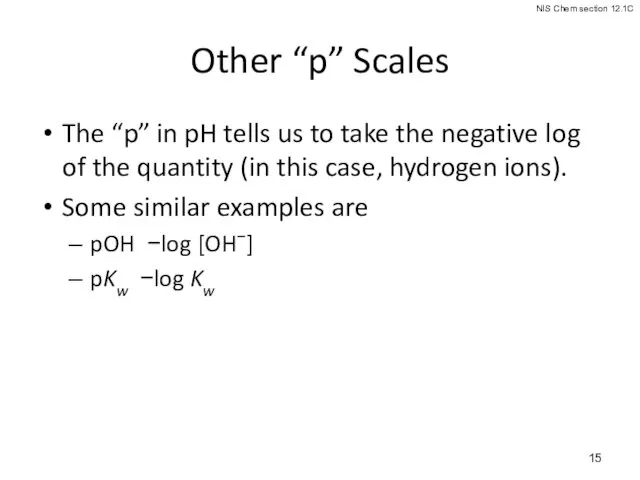
- 15. Other “p” Scales The “p” in pH tells us to take the negative log of the
- 16. Because [H3O+] [OH−] = Kw = 1.0 × 10−14, we know that −log [H3O+] + −log
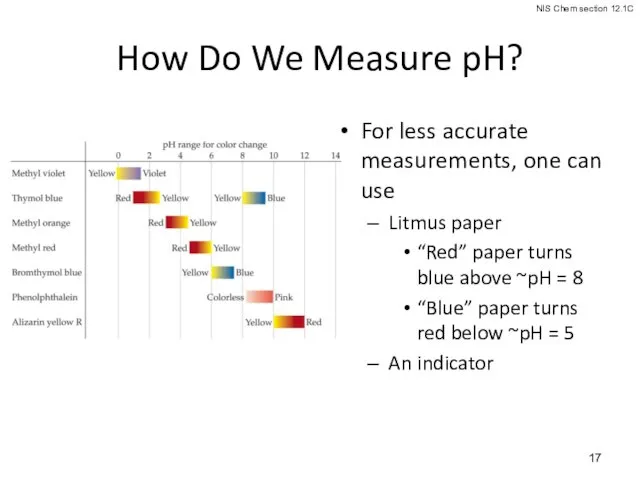
- 17. How Do We Measure pH? For less accurate measurements, one can use Litmus paper “Red” paper
- 18. How Do We Measure pH? For more accurate measurements, one uses a pH meter, which measures
- 19. How much is the equilibrium displaced towards the formation of the products (ionization) What is the
- 20. Strong Acids You will recall that the six strong acids are HCl, HBr, HI, HNO3, H2SO4
- 21. Strong Bases Strong bases are the soluble hydroxides, which are the alkali metal and heavier alkaline
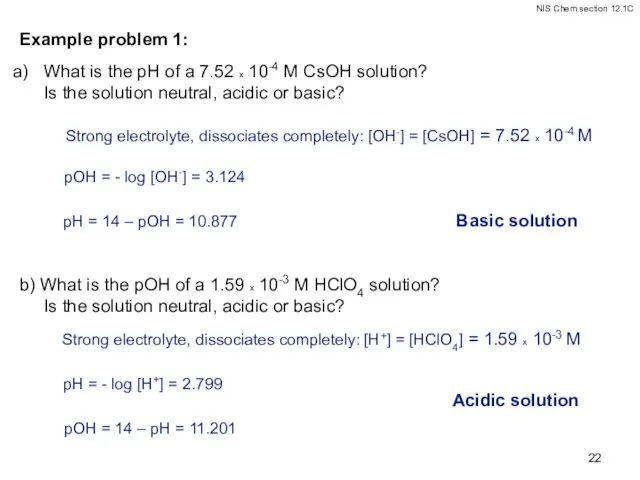
- 22. Example problem 1: What is the pH of a 7.52 x 10-4 M CsOH solution? Is
- 23. Example problem 2: What is the [H3O+] and [OH-] of a solution with a pH of
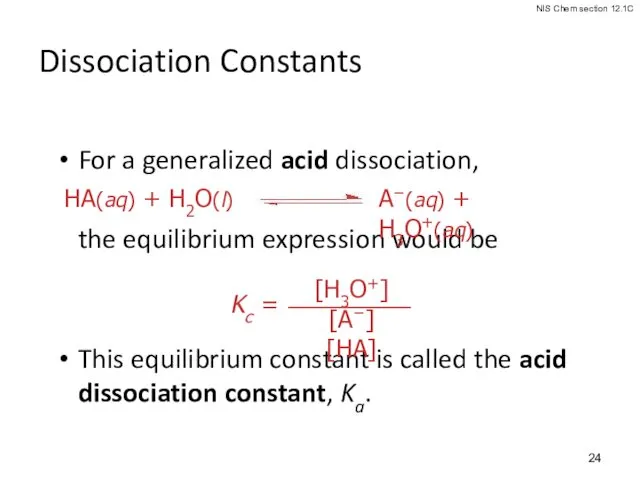
- 24. Dissociation Constants For a generalized acid dissociation, the equilibrium expression would be This equilibrium constant is
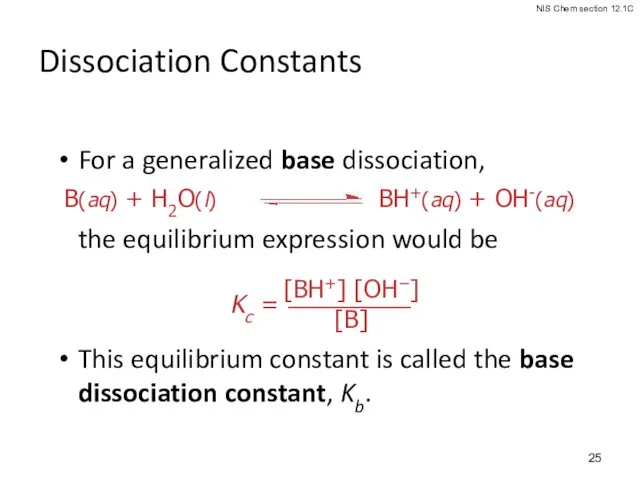
- 25. Dissociation Constants For a generalized base dissociation, the equilibrium expression would be This equilibrium constant is
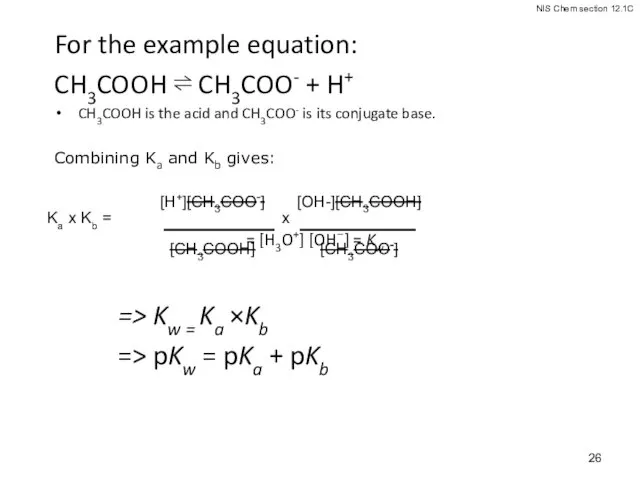
- 26. For the example equation: CH3COOH ⇌ CH3COO- + H+ CH3COOH is the acid and CH3COO- is
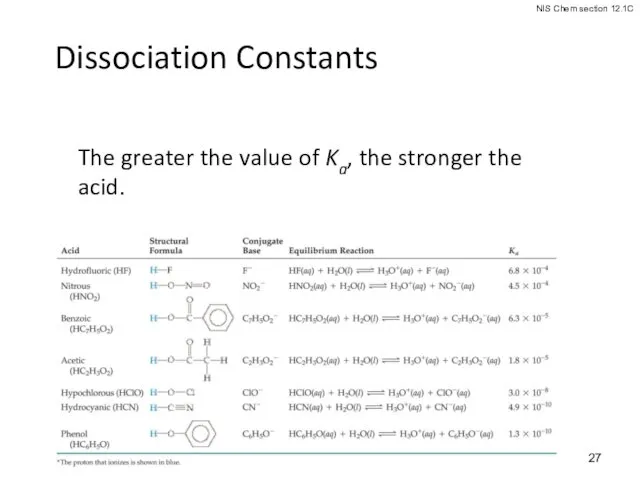
- 27. Dissociation Constants The greater the value of Ka, the stronger the acid.
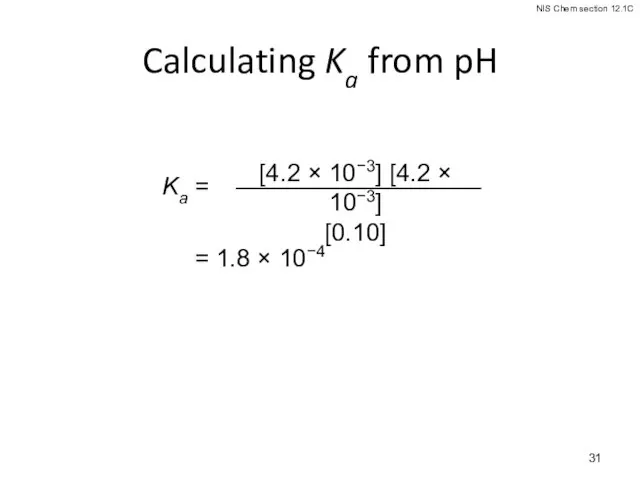
- 28. Calculating Ka from the pH The pH of a 0.10 M solution of formic acid, HCOOH,
- 29. Calculating Ka from the pH The pH of a 0.10 M solution of formic acid, HCOOH,
- 30. Calculating Ka from the pH pH = −log [H3O+] 2.38 = −log [H3O+] −2.38 = log
- 31. Calculating Ka from pH = 1.8 × 10−4
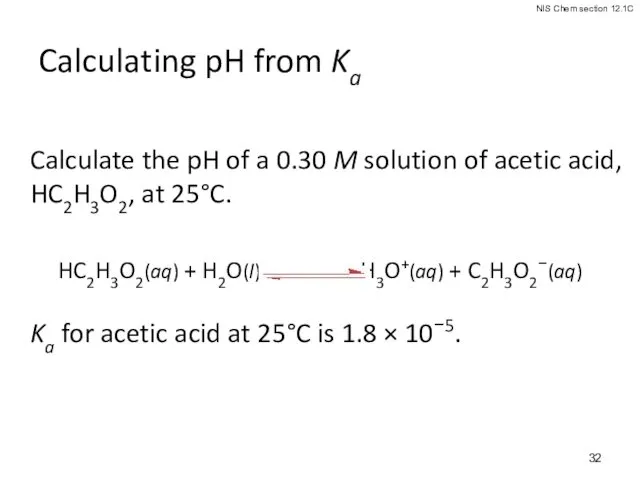
- 32. Calculating pH from Ka Calculate the pH of a 0.30 M solution of acetic acid, HC2H3O2,
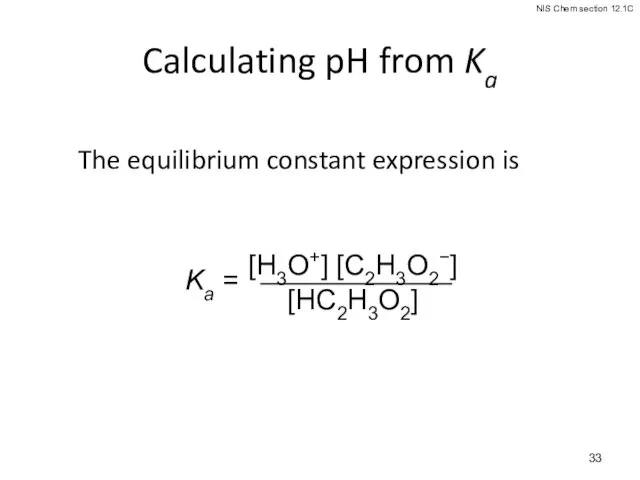
- 33. Calculating pH from Ka The equilibrium constant expression is
- 34. Calculating pH from Ka Now,x = [H3O+] = [C2H3O2−] (1.8 × 10−5) (0.30) = x2 5.4
- 35. Calculating pH from Ka pH = −log [H3O+] pH = −log (2.3 × 10−3) pH =
- 36. Titration A known concentration of base (or acid) is slowly added to a solution of acid
- 37. Titration A pH meter or indicators are used to determine when the solution has reached the
- 38. acid alkali end-point METHYL ORANGE
- 39. acid alkali end-point acid alkali PHENOLPHTHALEIN
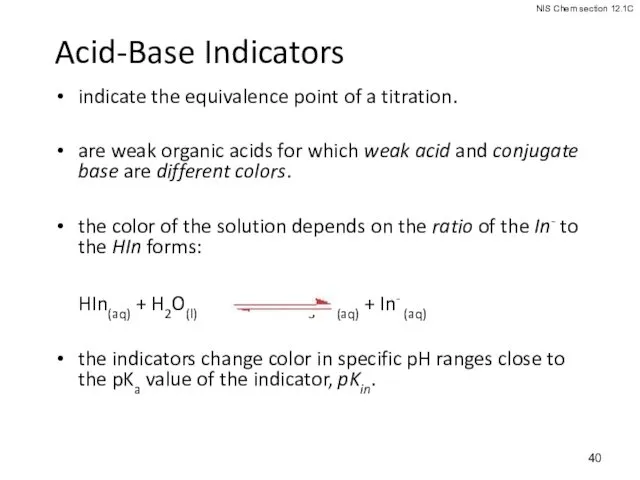
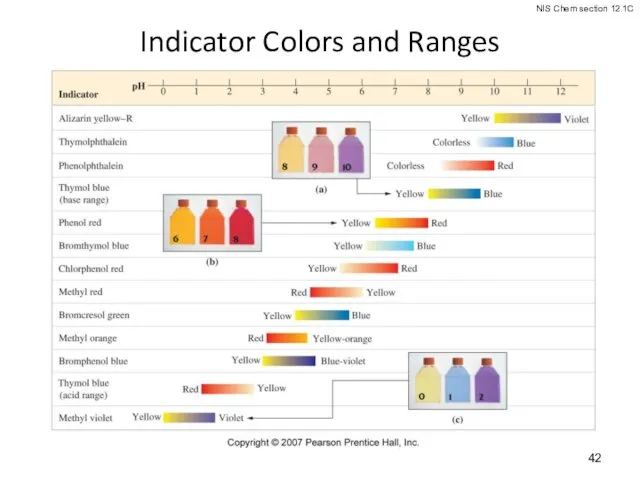
- 40. Acid-Base Indicators indicate the equivalence point of a titration. are weak organic acids for which weak
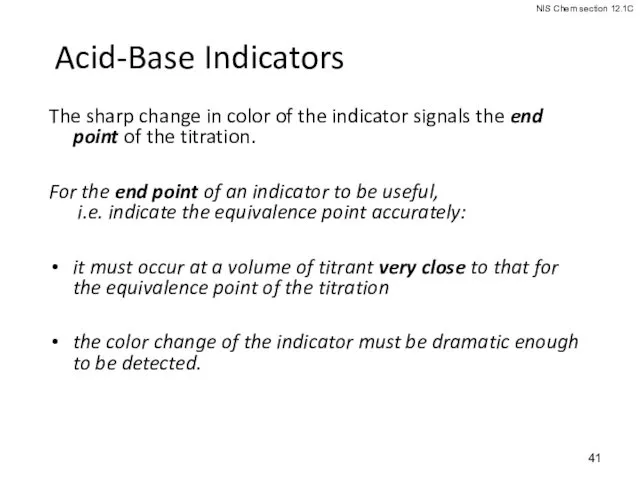
- 41. Acid-Base Indicators The sharp change in color of the indicator signals the end point of the
- 42. Indicator Colors and Ranges
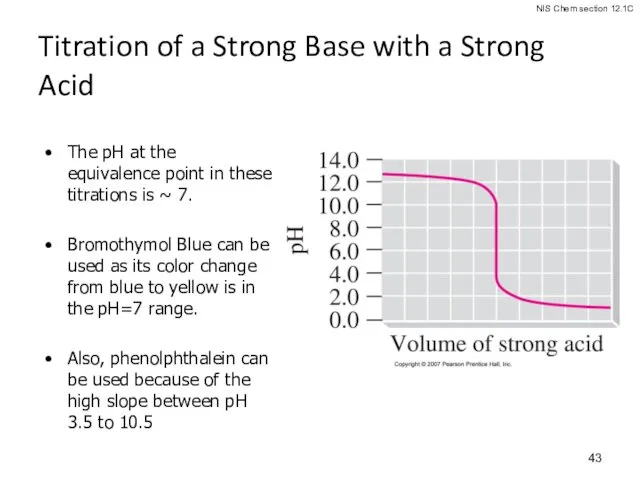
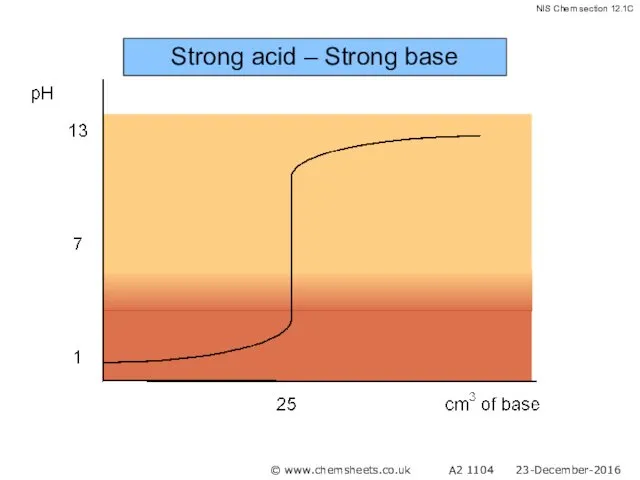
- 43. Titration of a Strong Base with a Strong Acid The pH at the equivalence point in
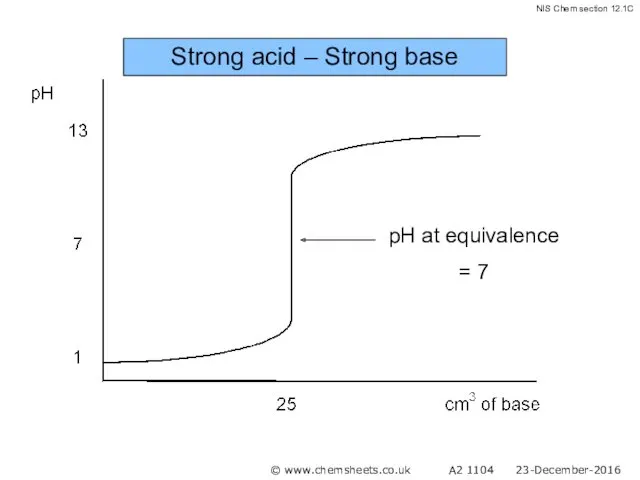
- 44. Strong acid – Strong base © www.chemsheets.co.uk A2 1104 23-December-2016
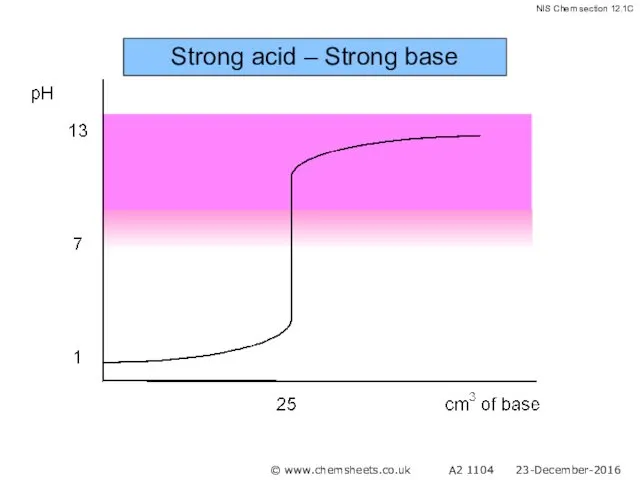
- 45. Strong acid – Strong base © www.chemsheets.co.uk A2 1104 23-December-2016
- 46. Strong acid – Strong base © www.chemsheets.co.uk A2 1104 23-December-2016
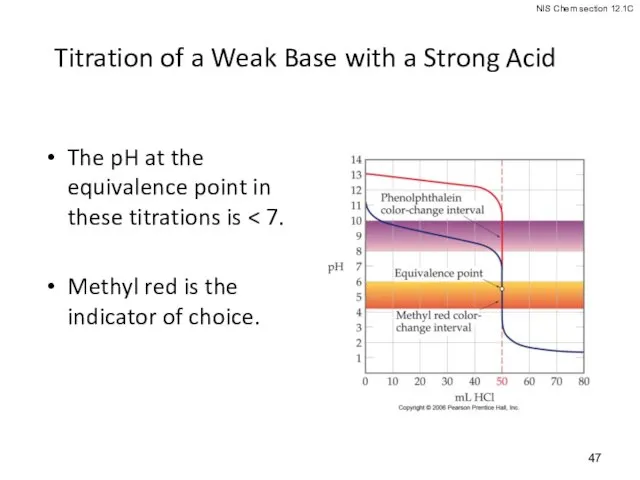
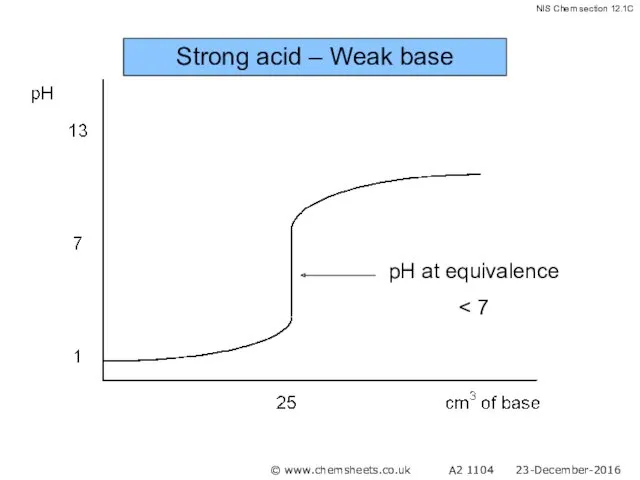
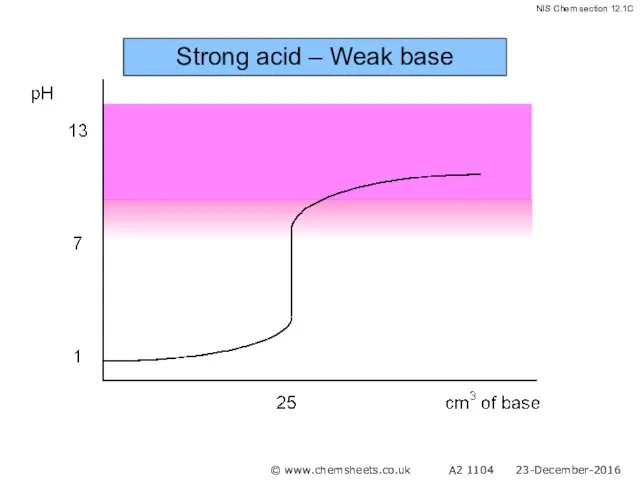
- 47. Titration of a Weak Base with a Strong Acid The pH at the equivalence point in
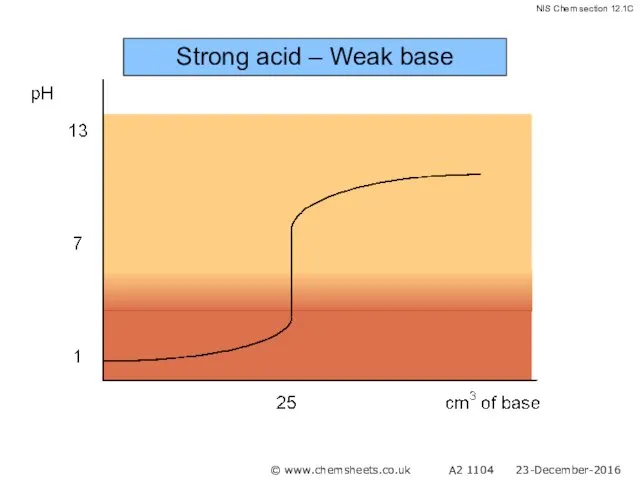
- 48. Strong acid – Weak base © www.chemsheets.co.uk A2 1104 23-December-2016
- 49. Strong acid – Weak base © www.chemsheets.co.uk A2 1104 23-December-2016
- 50. Strong acid – Weak base © www.chemsheets.co.uk A2 1104 23-December-2016
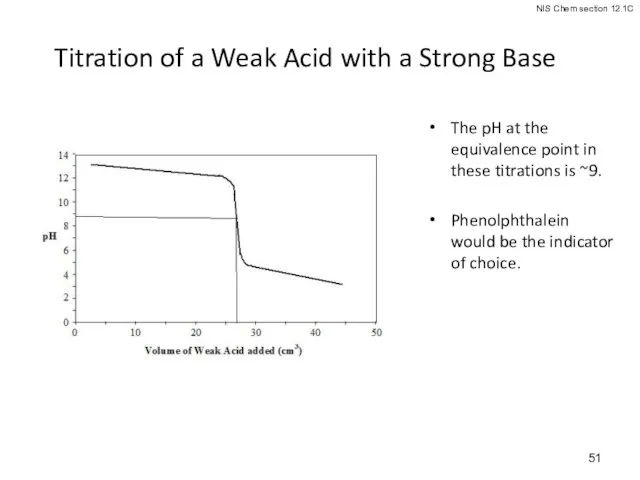
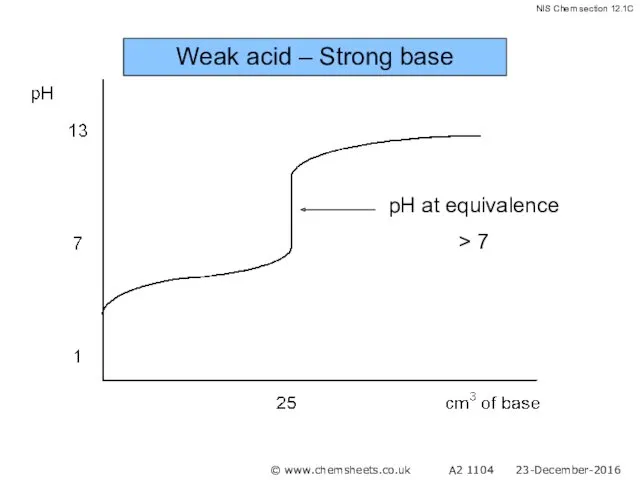
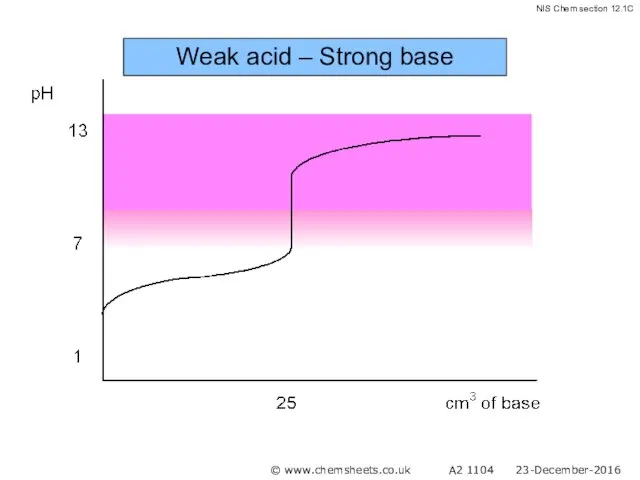
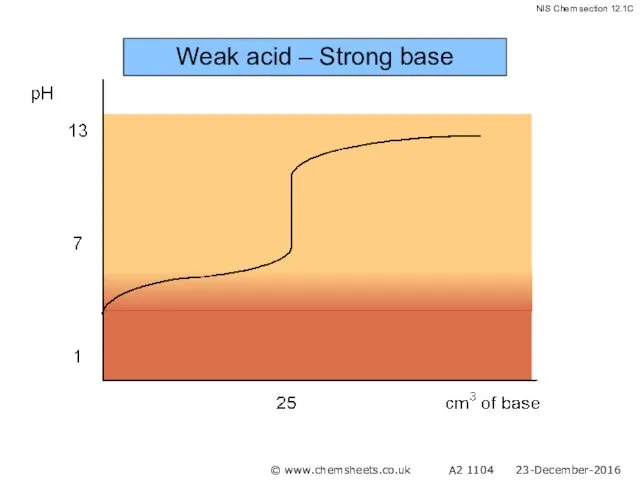
- 51. Titration of a Weak Acid with a Strong Base The pH at the equivalence point in
- 52. Weak acid – Strong base © www.chemsheets.co.uk A2 1104 23-December-2016
- 53. Weak acid – Strong base © www.chemsheets.co.uk A2 1104 23-December-2016
- 54. Weak acid – Strong base © www.chemsheets.co.uk A2 1104 23-December-2016
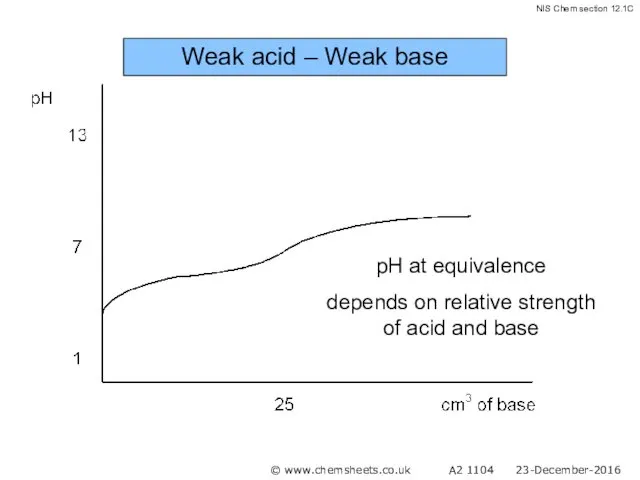
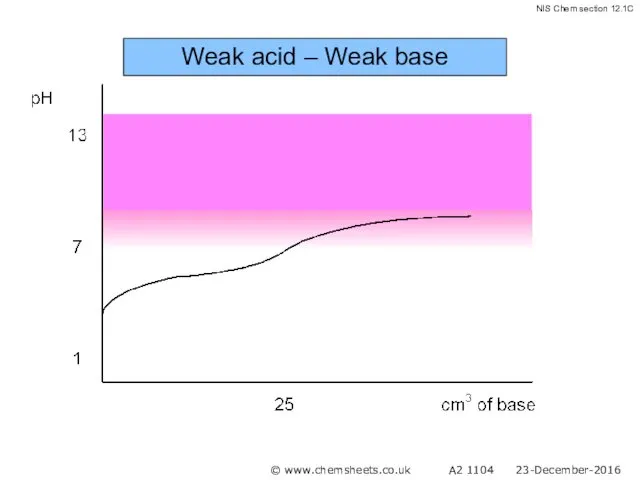
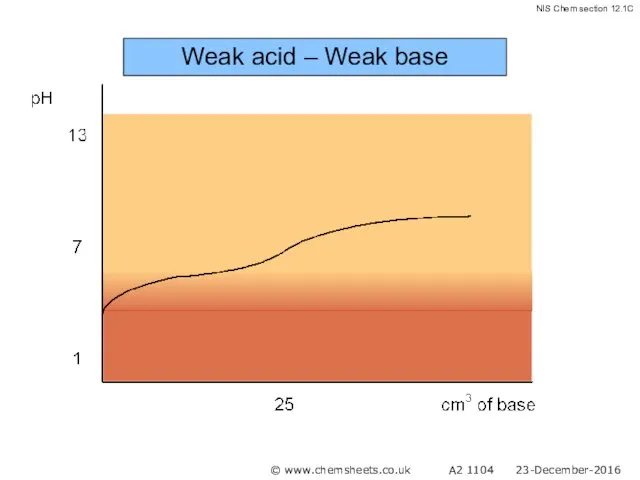
- 55. Weak acid – Weak base pH at equivalence depends on relative strength of acid and base
- 56. Weak acid – Weak base © www.chemsheets.co.uk A2 1104 23-December-2016
- 57. Weak acid – Weak base © www.chemsheets.co.uk A2 1104 23-December-2016
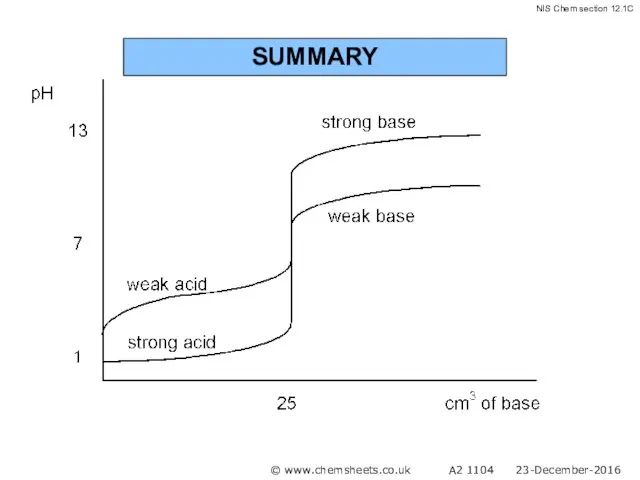
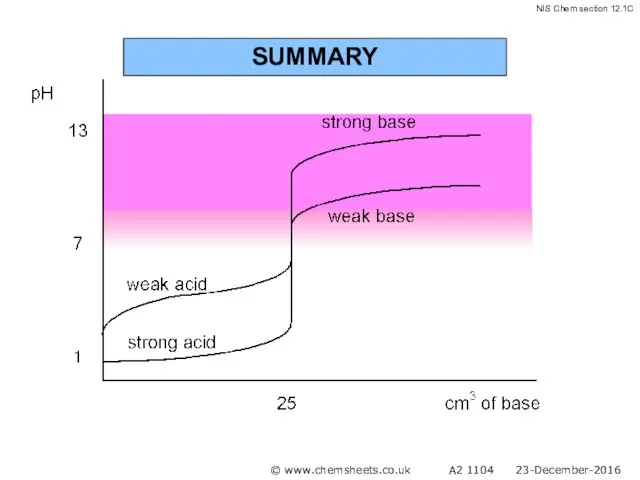
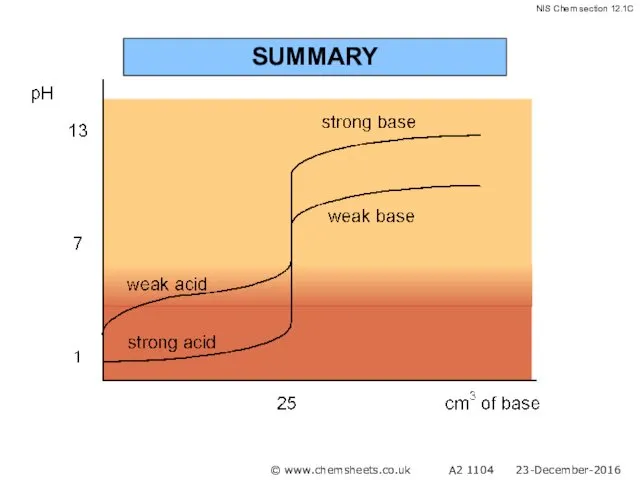
- 58. SUMMARY © www.chemsheets.co.uk A2 1104 23-December-2016
- 59. SUMMARY © www.chemsheets.co.uk A2 1104 23-December-2016
- 60. SUMMARY © www.chemsheets.co.uk A2 1104 23-December-2016
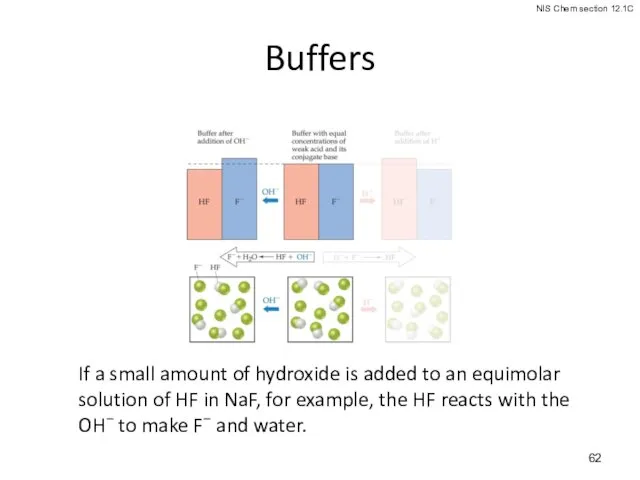
- 61. Buffers: Solutions of a weak conjugate acid-base pair. They are particularly resistant to pH changes, even
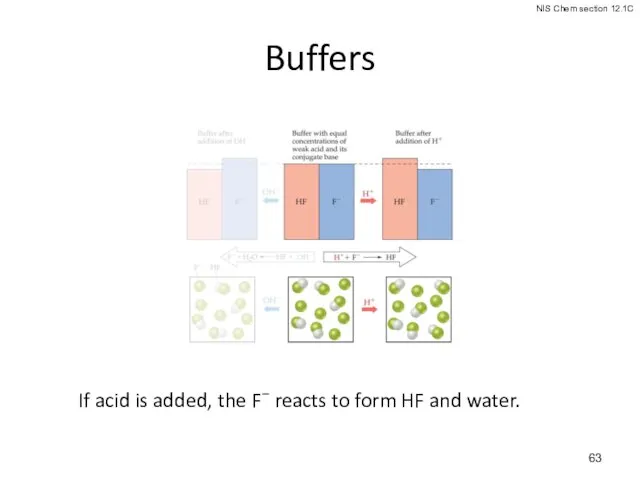
- 62. Buffers If a small amount of hydroxide is added to an equimolar solution of HF in
- 63. Buffers If acid is added, the F− reacts to form HF and water.
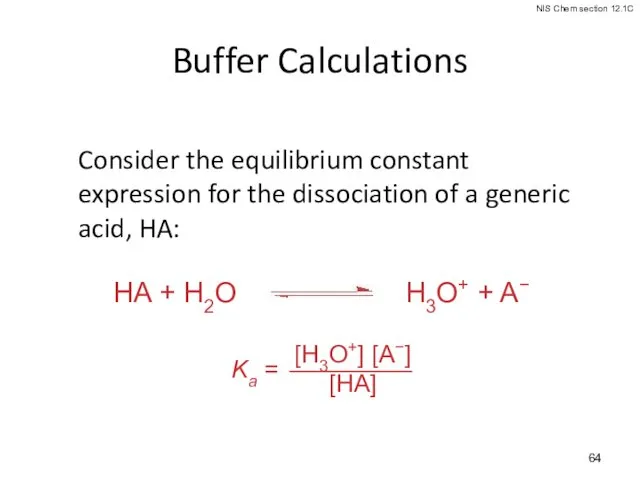
- 64. Buffer Calculations Consider the equilibrium constant expression for the dissociation of a generic acid, HA:
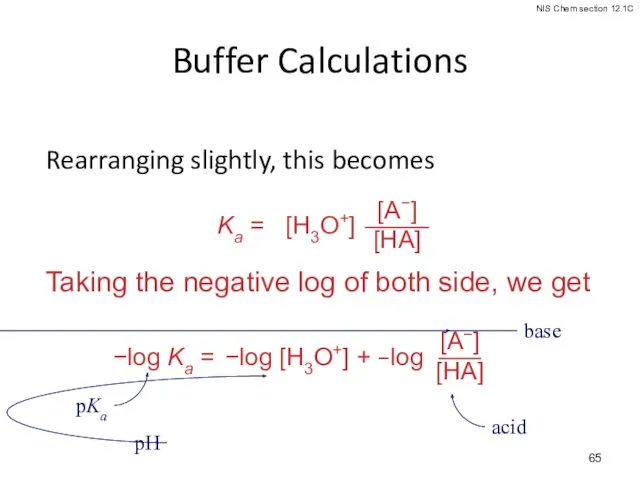
- 65. Buffer Calculations Rearranging slightly, this becomes Taking the negative log of both side, we get
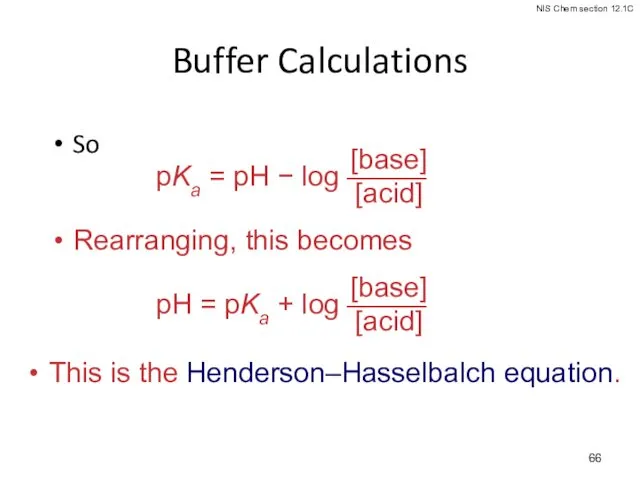
- 66. Buffer Calculations So Rearranging, this becomes This is the Henderson–Hasselbalch equation.
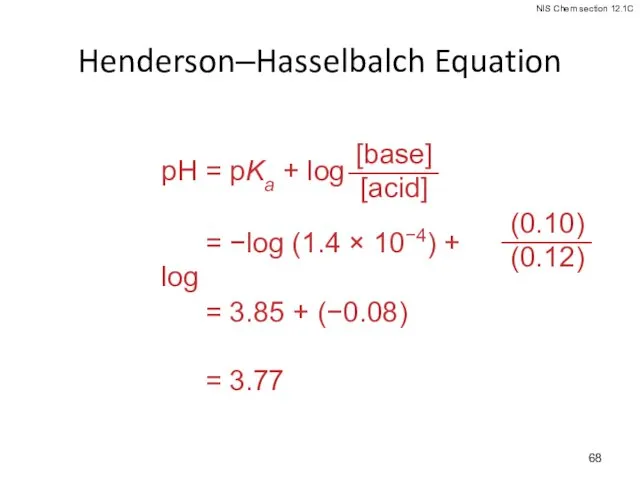
- 67. Henderson–Hasselbalch Equation What is the pH of a buffer that is 0.12 M in lactic acid,
- 68. Henderson–Hasselbalch Equation pH = 3.85 + (−0.08) pH = 3.77
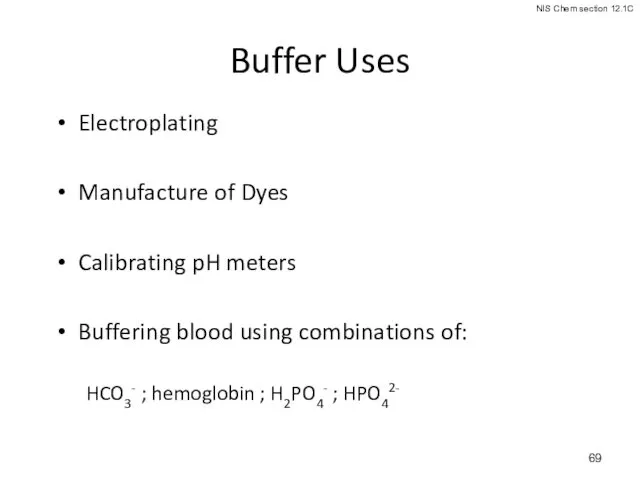
- 69. Buffer Uses Electroplating Manufacture of Dyes Calibrating pH meters Buffering blood using combinations of: HCO3- ;
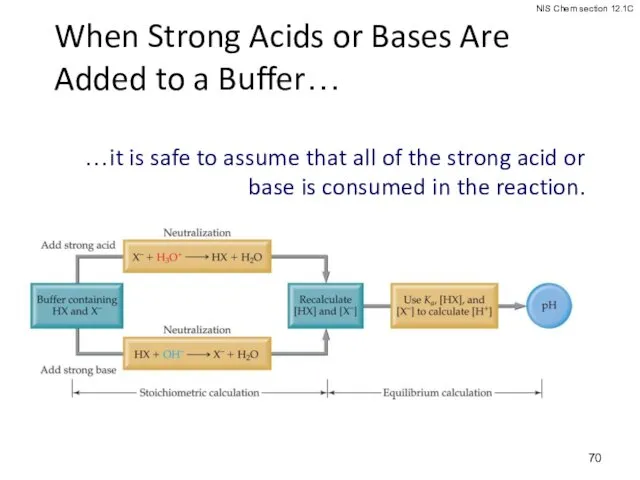
- 70. When Strong Acids or Bases Are Added to a Buffer… …it is safe to assume that
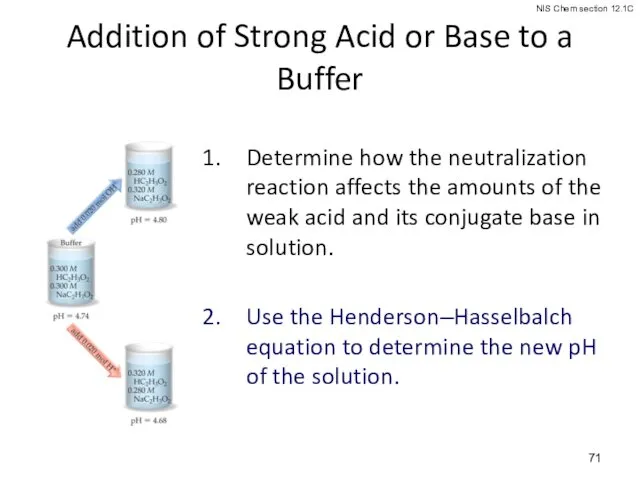
- 71. Addition of Strong Acid or Base to a Buffer Determine how the neutralization reaction affects the
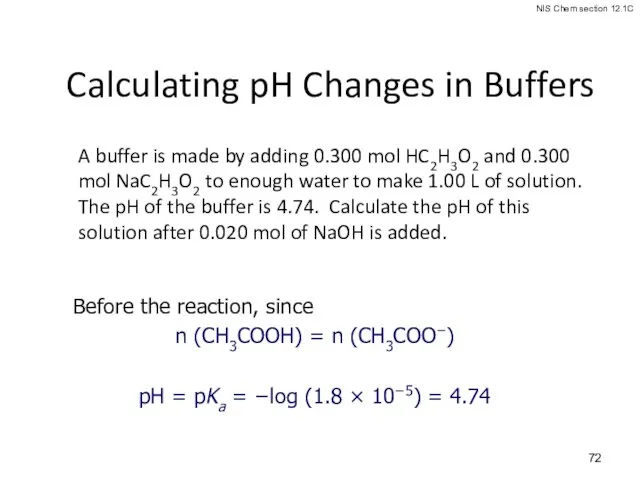
- 72. Calculating pH Changes in Buffers A buffer is made by adding 0.300 mol HC2H3O2 and 0.300
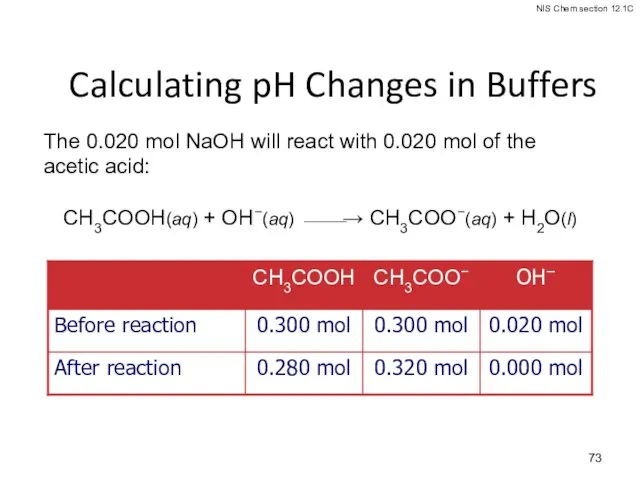
- 73. Calculating pH Changes in Buffers The 0.020 mol NaOH will react with 0.020 mol of the
- 75. Скачать презентацию




![pH In pure water, Kw = [H3O+] [OH−] = 1.0](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/70169/slide-11.jpg)
![Because [H3O+] [OH−] = Kw = 1.0 × 10−14, we](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/70169/slide-15.jpg)




![Example problem 2: What is the [H3O+] and [OH-] of](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/70169/slide-22.jpg)


![Calculating Ka from the pH pH = −log [H3O+] 2.38](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/70169/slide-29.jpg)
![Calculating pH from Ka Now,x = [H3O+] = [C2H3O2−] (1.8](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/70169/slide-33.jpg)
![Calculating pH from Ka pH = −log [H3O+] pH =](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/70169/slide-34.jpg)






 Консультация для воспитателей Использование приемов ТРИЗ-педагогики в развитие связной речи детей
Консультация для воспитателей Использование приемов ТРИЗ-педагогики в развитие связной речи детей Список вопросов по курсу Операционные системы
Список вопросов по курсу Операционные системы Презентация Синяя лента апреля в МБО УСОШ № 3
Презентация Синяя лента апреля в МБО УСОШ № 3 Дым вокруг от сигарет - мне в этом дыме места нет
Дым вокруг от сигарет - мне в этом дыме места нет Процессы, машины и аппараты пищевых производств
Процессы, машины и аппараты пищевых производств Понятие информации и ее свойства. Неопределенность и количество информации
Понятие информации и ее свойства. Неопределенность и количество информации Родная литература 2-4 класс Ф. Иҫәнғолов Ҡыҙҙар менән уйнарға яраймы.Г.Юнысова
Родная литература 2-4 класс Ф. Иҫәнғолов Ҡыҙҙар менән уйнарға яраймы.Г.Юнысова Сенсорный анализ продовольственных товаров. Впечатлительность дегустатора
Сенсорный анализ продовольственных товаров. Впечатлительность дегустатора Последние годы жизни Пушкина
Последние годы жизни Пушкина Презентация к уроку химии в 8 классе по теме Соли
Презентация к уроку химии в 8 классе по теме Соли Многофункциональное пособие
Многофункциональное пособие Работа в САДД Дело
Работа в САДД Дело Эффективность работы установки предварительного сброса воды на месторождении “Дружный” ЦДНГ-1
Эффективность работы установки предварительного сброса воды на месторождении “Дружный” ЦДНГ-1 Мастер-класс Коррекция агрессивности методом директивной игровой терапии
Мастер-класс Коррекция агрессивности методом директивной игровой терапии Бадминтон как средство развития ловкости у детей старшего дошкольного возраста
Бадминтон как средство развития ловкости у детей старшего дошкольного возраста Методы развития памяти.
Методы развития памяти. Волейбол. Стойки, перемещения, подводящие упражнения для приема и передач сверху двумя руками стоя на месте и в движении
Волейбол. Стойки, перемещения, подводящие упражнения для приема и передач сверху двумя руками стоя на месте и в движении Қала тұрғындарына емдеу-сақтандырудағы көмекті ұйымдастыру. Емдеу сақтандыру мекемелерінің ІС-эрекетін талдау
Қала тұрғындарына емдеу-сақтандырудағы көмекті ұйымдастыру. Емдеу сақтандыру мекемелерінің ІС-эрекетін талдау Урок обобщения и систематизации знаний по теме Дифференциальные уравнения
Урок обобщения и систематизации знаний по теме Дифференциальные уравнения Основы расчета ж/б конструкций и ж/б балочных ПС
Основы расчета ж/б конструкций и ж/б балочных ПС Means of communication. Non-Finite of the verb. The Gerund
Means of communication. Non-Finite of the verb. The Gerund Презентация к уроку технологии по теме Открытка-ландшафт.
Презентация к уроку технологии по теме Открытка-ландшафт. Презентация(игра-викторина) Умники и умницы
Презентация(игра-викторина) Умники и умницы Шаблон 8 Марта (цветы 4)
Шаблон 8 Марта (цветы 4) Викторина по сказке Г.Х.Андерсена Бузинная матушка
Викторина по сказке Г.Х.Андерсена Бузинная матушка Классификация чугунов
Классификация чугунов Мифическое существо Дракон, или крылатый змей
Мифическое существо Дракон, или крылатый змей Сосуды под давлением ЗАО Силд Эйр Каустик
Сосуды под давлением ЗАО Силд Эйр Каустик