Содержание
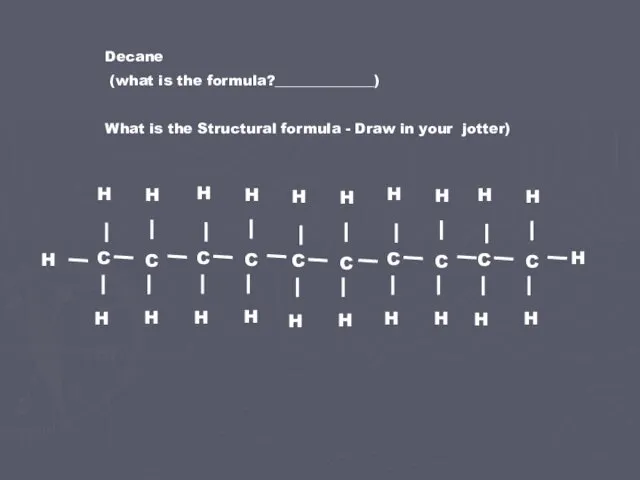
- 2. Decane (what is the formula?______________) What is the Structural formula - Draw in your jotter)
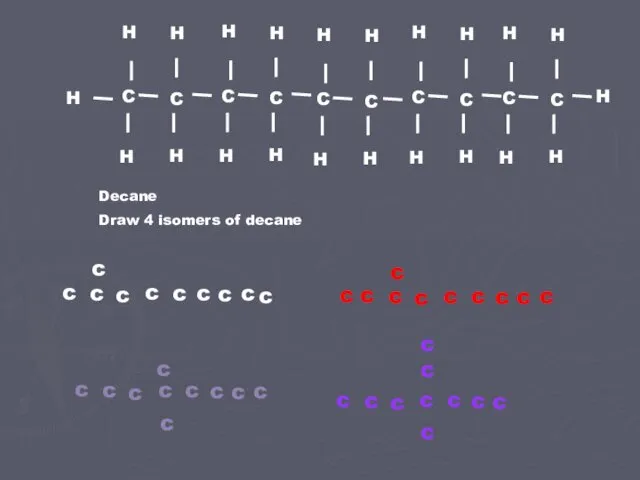
- 3. Decane Draw 4 isomers of decane
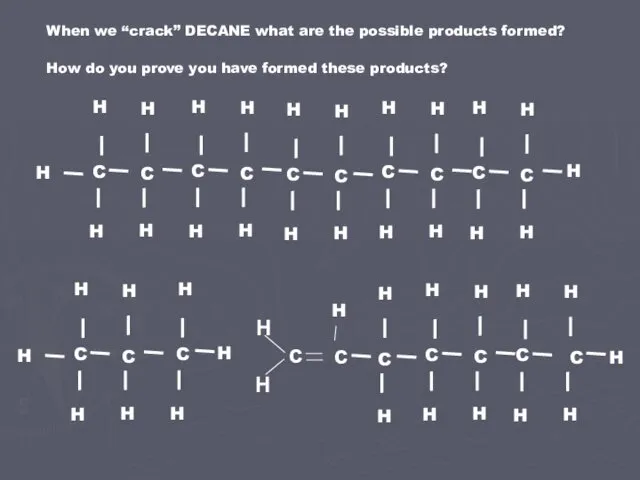
- 4. When we “crack” DECANE what are the possible products formed? How do you prove you have
- 5. Annapurna
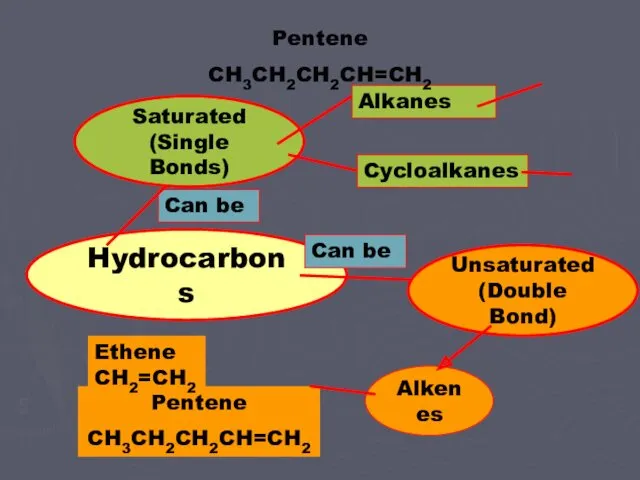
- 6. Pentene CH3CH2CH2CH=CH2 Alkenes Can be Hydrocarbons Saturated (Single Bonds) Can be Unsaturated (Double Bond) Ethene CH2=CH2
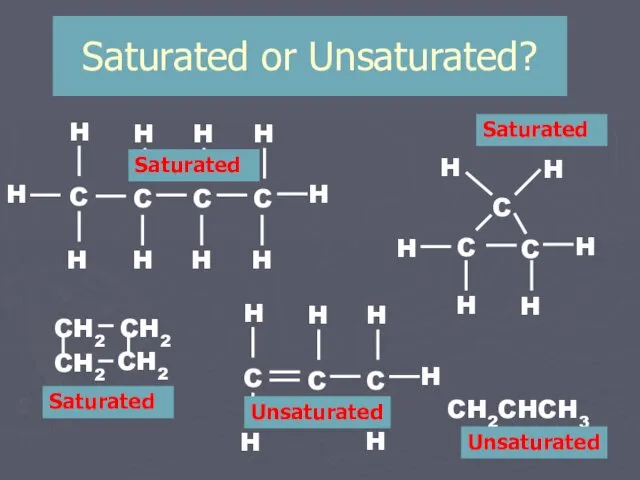
- 8. Saturated or Unsaturated? CH2CHCH3 Saturated Saturated Saturated Unsaturated Unsaturated
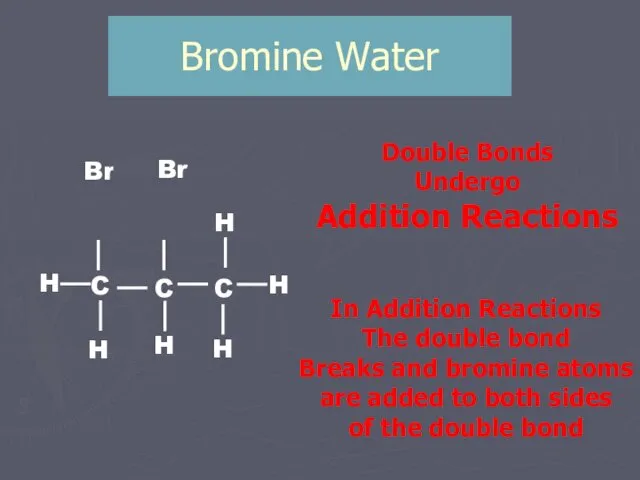
- 9. Bromine Water Br Br Double Bonds Undergo Addition Reactions In Addition Reactions The double bond Breaks
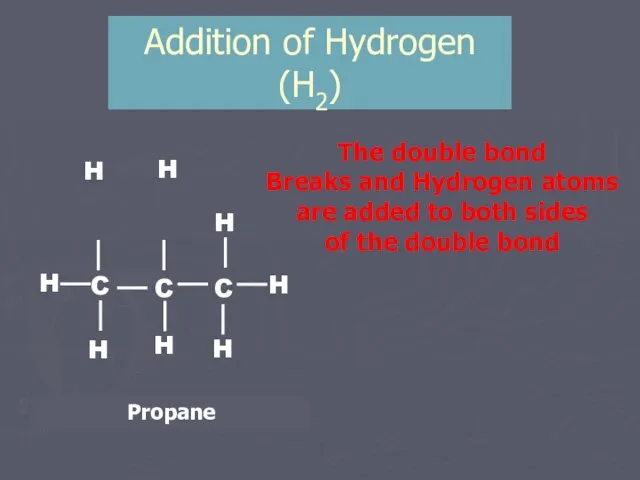
- 10. Addition of Hydrogen (H2) H H The double bond Breaks and Hydrogen atoms are added to
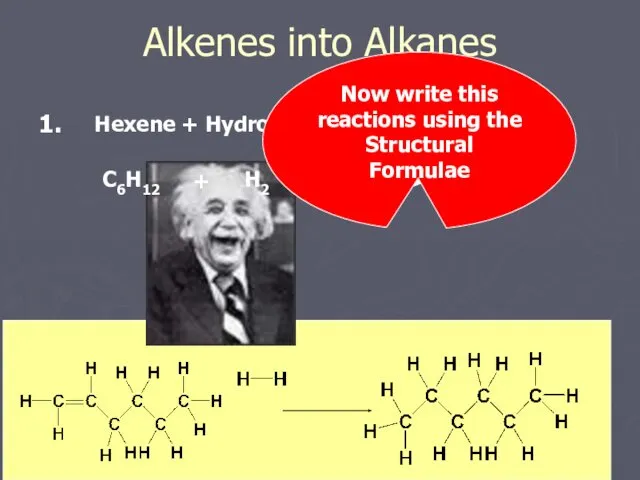
- 11. Alkenes into Alkanes Hexene + Hydrogen C6H12 H2 + Hexane C6H14 Now write this reactions using
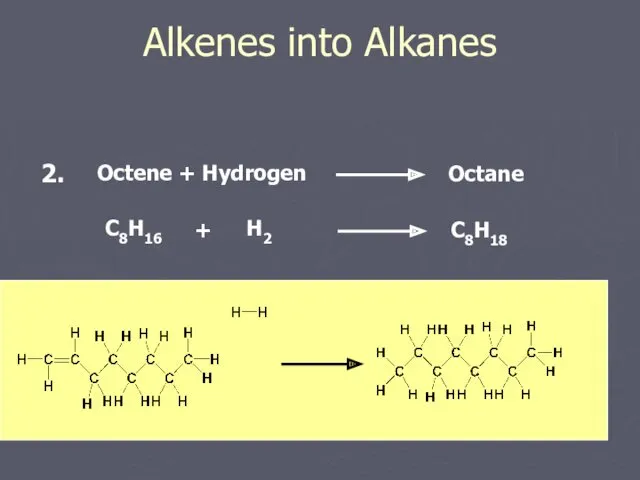
- 12. Alkenes into Alkanes Octene + Hydrogen Octane C8H16 H2 + C8H18 2.
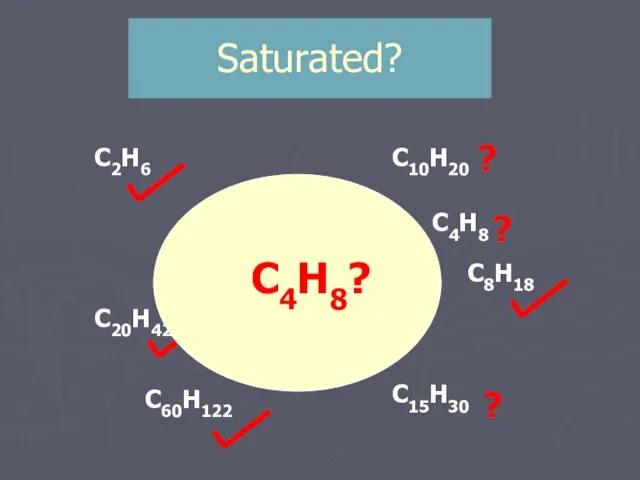
- 13. Saturated? C2H6 C4H10 C20H42 C10H20 C15H30 C8H18 C7H14 C60H122 C11H22 ? ? ? ? C4H8 ?
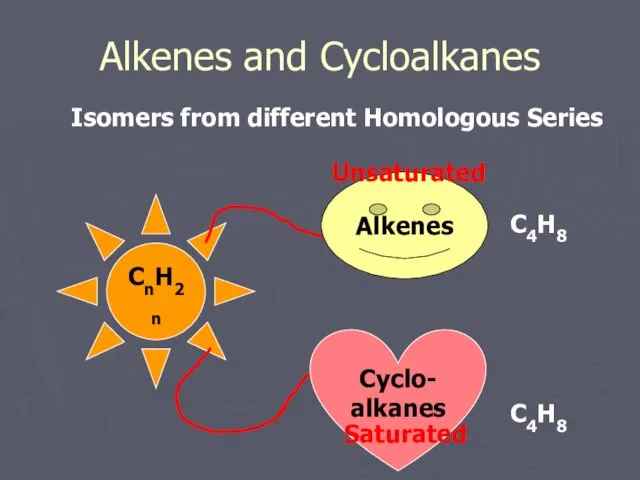
- 14. Alkenes and Cycloalkanes Isomers from different Homologous Series CnH2n Alkenes Cyclo- alkanes Unsaturated Saturated C4H8 C4H8
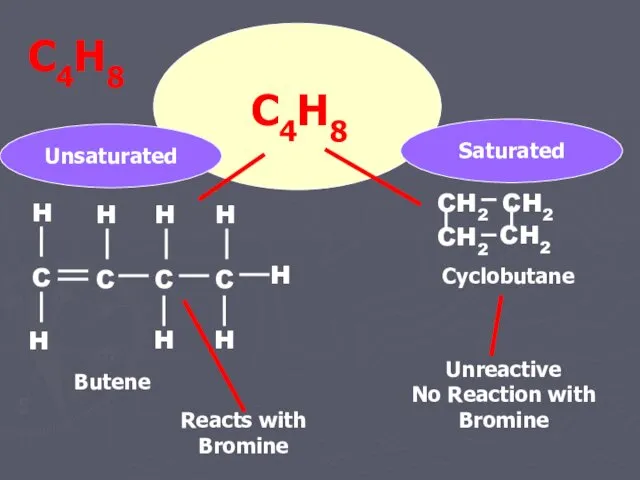
- 15. C4H8 Butene Cyclobutane Unsaturated Saturated Reacts with Bromine Unreactive No Reaction with Bromine
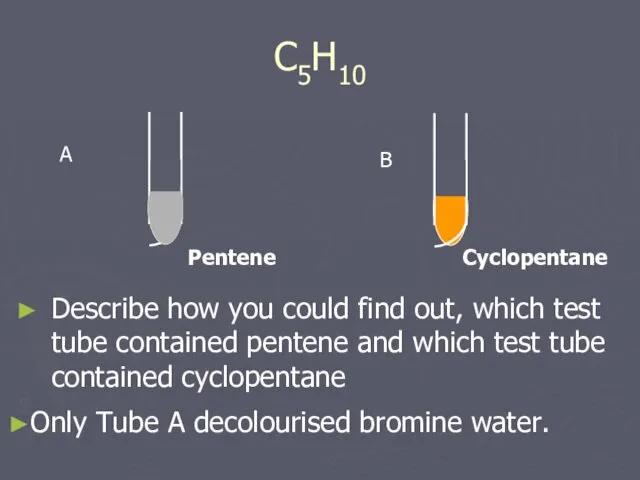
- 16. C5H10 Describe how you could find out, which test tube contained pentene and which test tube
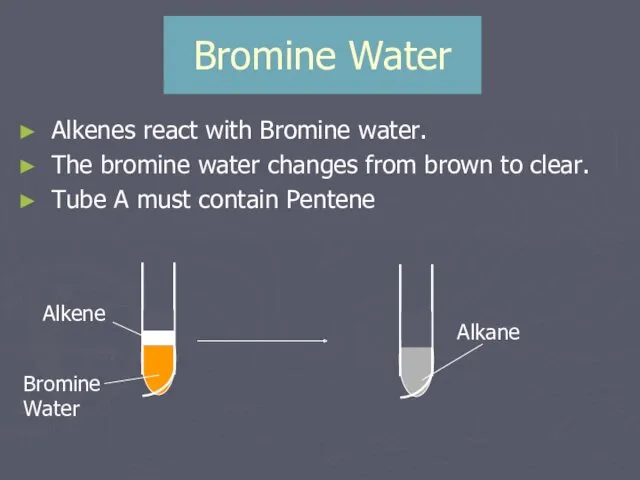
- 17. Bromine Water Alkenes react with Bromine water. The bromine water changes from brown to clear. Tube
- 19. Скачать презентацию


 Культура и духовная жизнь общества
Культура и духовная жизнь общества Ассамлеи и гулянья в эпоху Петра
Ассамлеи и гулянья в эпоху Петра Работа в microsoft powerpoint 2007
Работа в microsoft powerpoint 2007 Personality disorders and behavior-related diseases, damage and dysfunction of the brain
Personality disorders and behavior-related diseases, damage and dysfunction of the brain Экономическая политика Петра I
Экономическая политика Петра I Экологическая политика Японии в годы кризиса
Экологическая политика Японии в годы кризиса Архитектура компьютера. Виды памяти
Архитектура компьютера. Виды памяти Строительство общеобразовательной школы на территории округа Город Калининград в Восточном жилом районе
Строительство общеобразовательной школы на территории округа Город Калининград в Восточном жилом районе Adaptive type of population. Features of biological and social adaptation of arctic indigenous people
Adaptive type of population. Features of biological and social adaptation of arctic indigenous people Брейн-ринг воспитанников православных школ Красноярской Епархии по учебному предмету Основы православной культуры
Брейн-ринг воспитанников православных школ Красноярской Епархии по учебному предмету Основы православной культуры Сестринский процесс при бронхоэктатической болезни (бронхоэктазах)
Сестринский процесс при бронхоэктатической болезни (бронхоэктазах) Функции Постоянного комитета СФС по соблюдению международных стандартов
Функции Постоянного комитета СФС по соблюдению международных стандартов Условные линии на карте
Условные линии на карте Современные представления о происхождении и эволюции Солнца
Современные представления о происхождении и эволюции Солнца Структура Web-страницы. Форматирование текста на Web-странице
Структура Web-страницы. Форматирование текста на Web-странице Сочинский государственный университет. Факультет туризма и сервиса
Сочинский государственный университет. Факультет туризма и сервиса Толерантность
Толерантность Сибирская язва
Сибирская язва Сопротивление в цепи переменного тока
Сопротивление в цепи переменного тока Умножение дробей. 6 класс
Умножение дробей. 6 класс Цветные сплавы
Цветные сплавы зеленая экономика 17
зеленая экономика 17 Показательные уравнения и неравенства
Показательные уравнения и неравенства Склады и складские операции
Склады и складские операции Федеративна Республіка Німеччина
Федеративна Республіка Німеччина Д.И. Менделеев
Д.И. Менделеев Перечень тем рефератов. Корпоративные финансы
Перечень тем рефератов. Корпоративные финансы Затраты и их классификация. Учет и анализ: управленческий учет
Затраты и их классификация. Учет и анализ: управленческий учет