Содержание
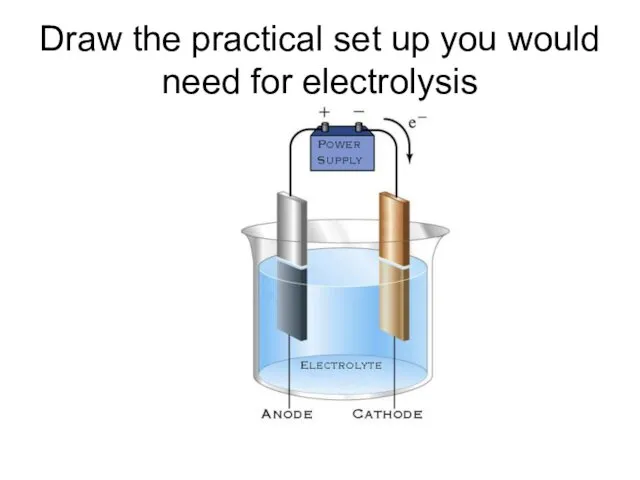
- 2. Draw the practical set up you would need for electrolysis
- 3. INDUSTRIAL USES OF ELECTROLYSIS To extract reactive metals such as ALUMINIUM, sodium, magnesium etc from their
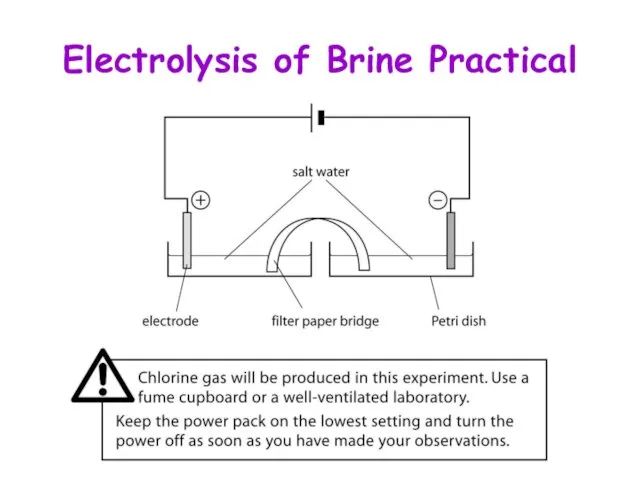
- 4. Electrolysis of Brine Practical
- 5. Questions 1 What did the universal indicator show you about the type of substance formed in:
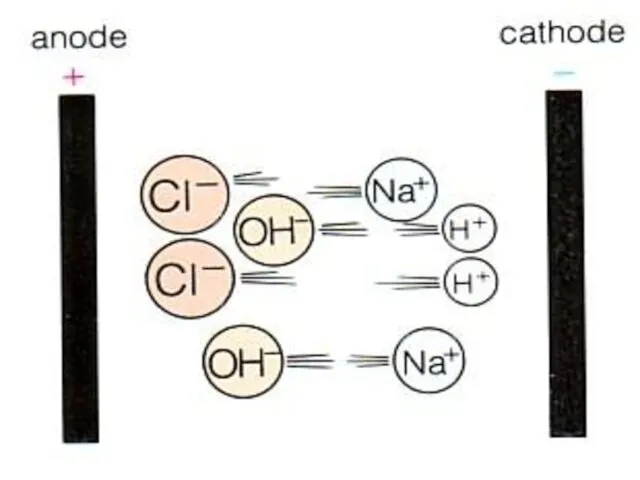
- 6. Electrolysis of brine The NaCl will split into Na+ ions and Cl- ions. Water splits into
- 8. Now let’s see what actually happens
- 9. Electrolysis of brine The H+ and Cl- ions are discharged at the electrodes.
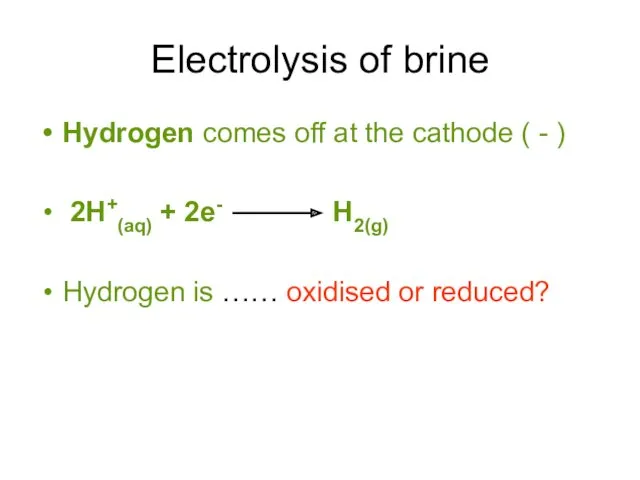
- 10. Electrolysis of brine Hydrogen comes off at the cathode ( - ) 2H+(aq) + 2e- H2(g)
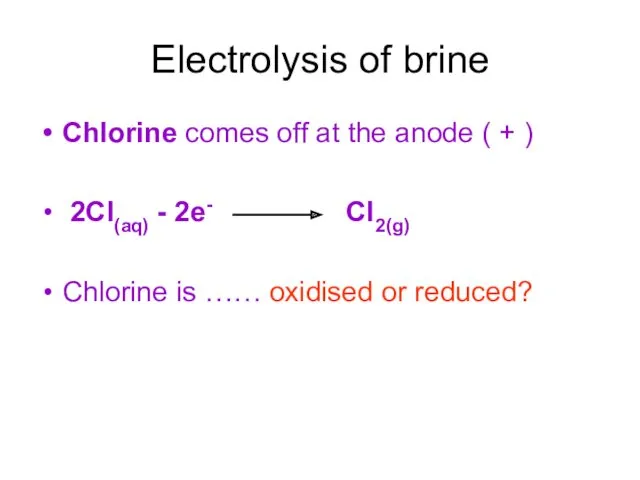
- 11. Electrolysis of brine Chlorine comes off at the anode ( + ) 2Cl(aq) - 2e- Cl2(g)
- 12. Electrolysis of brine The Na+ and OH- ions stay in solution. They join together to form
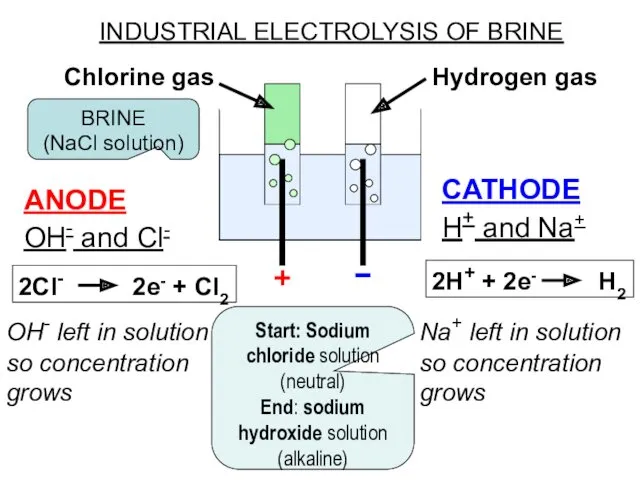
- 13. INDUSTRIAL ELECTROLYSIS OF BRINE ANODE OH- and Cl- 2Cl- 2e- + Cl2 OH- left in solution
- 14. Industrial chlorine production from electrolysis of brine
- 15. Hydrogen Used to make margarine (helps to make the oils in the margarine spread on your
- 16. Sodium hydroxide Detergents and soap Paper
- 17. Sodium hydroxide Purifying bauxite to extract aluminium Rayon and acetate fibres
- 18. Chlorine Bleach Killing bacteria in water
- 19. Chlorine Solvents (used in dry cleaning) Hydrochloric acid (HCl)
- 21. Скачать презентацию












 Анализ современных конструкторов школьных сайтов
Анализ современных конструкторов школьных сайтов Конспект открытого индивидуального занятия по развитию речевого слуха и формированию произносительной стороны речи у детей с нарушением слуха в 9 классе
Конспект открытого индивидуального занятия по развитию речевого слуха и формированию произносительной стороны речи у детей с нарушением слуха в 9 классе Однополосные и широкополосные сигналы в системах радиосвязи. К лекции 4
Однополосные и широкополосные сигналы в системах радиосвязи. К лекции 4 Do you know these attractions?
Do you know these attractions? Особенности расчета местных сетей
Особенности расчета местных сетей Діннің болашағы: мәселелері мен перспективасы
Діннің болашағы: мәселелері мен перспективасы Старость в радость
Старость в радость Советский истребитель ЛА-5
Советский истребитель ЛА-5 Кадровое агентство Work&fun
Кадровое агентство Work&fun ВИКТОРИНА. БУГИ-ВУГИ
ВИКТОРИНА. БУГИ-ВУГИ Создание предметно –развивающей среды в ДОУ в первой младшей группе в соответствии ФГОС
Создание предметно –развивающей среды в ДОУ в первой младшей группе в соответствии ФГОС Підвищення енергоефективності транспортних засобів за рахунок впровадження імпульсних накопичувачів енергії
Підвищення енергоефективності транспортних засобів за рахунок впровадження імпульсних накопичувачів енергії Николай I
Николай I Формы выделения минералов
Формы выделения минералов Онорн Домье
Онорн Домье Высота. Медиана. Биссектриса
Высота. Медиана. Биссектриса Презентация Азбука ч.3 Диск
Презентация Азбука ч.3 Диск Презентация Аксай - город Ростовской области
Презентация Аксай - город Ростовской области Флора и фауна озера Байкал
Флора и фауна озера Байкал Статья Механизмы повышения качества образования в начальной школе
Статья Механизмы повышения качества образования в начальной школе Crude oil
Crude oil ОРГАНИЗАЦИЯ РАЗВИВАЮЩЕЙ СРЕДЫ В ВОСПИТАТЕЛЬНО-ОБРАЗОВАТЕЛЬНОМ ПРОЦЕССЕ В ДОО ПО РЕАЛИЗАЦИИ УМК.
ОРГАНИЗАЦИЯ РАЗВИВАЮЩЕЙ СРЕДЫ В ВОСПИТАТЕЛЬНО-ОБРАЗОВАТЕЛЬНОМ ПРОЦЕССЕ В ДОО ПО РЕАЛИЗАЦИИ УМК. Алюминиевые системы Schüco
Алюминиевые системы Schüco Малое и среднее предпринимательство Рязанской области
Малое и среднее предпринимательство Рязанской области Презентация Визитная карточка воспитателя Храмовой Елены Витальевны
Презентация Визитная карточка воспитателя Храмовой Елены Витальевны Индийский океан
Индийский океан История Земли. Геохронология
История Земли. Геохронология Правдолюбцы и лжецы. Решение олимпиадных математических задач
Правдолюбцы и лжецы. Решение олимпиадных математических задач