Содержание
- 2. Characteristics of air handling systems In the following slides, we will study alternatives in air handling
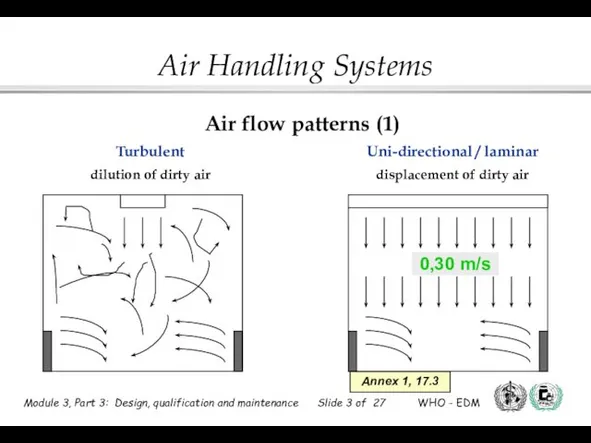
- 3. Uni-directional / laminar displacement of dirty air Turbulent dilution of dirty air 0,30 m/s Annex 1,
- 4. Air flow patterns (2) Filtered air entering a production room or covering a process can be
- 5. Annex 1, 17.3
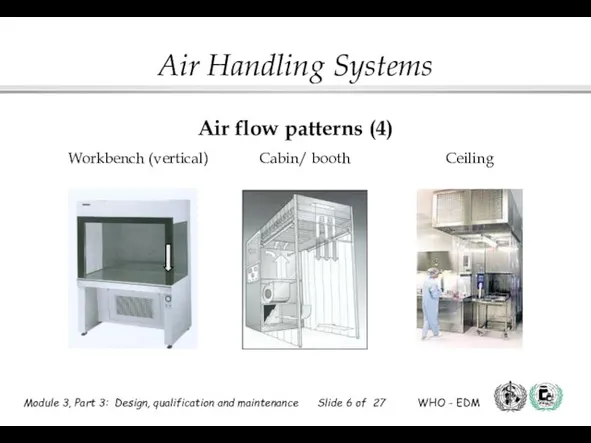
- 6. Workbench (vertical) Cabin/ booth Ceiling Air flow patterns (4)
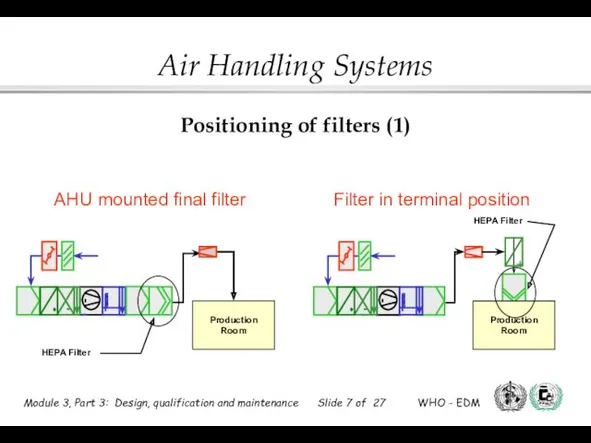
- 7. Positioning of filters (1) Filter in terminal position AHU mounted final filter HEPA Filter HEPA Filter
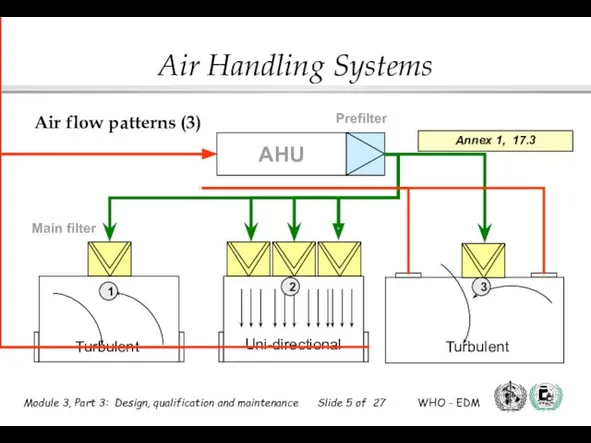
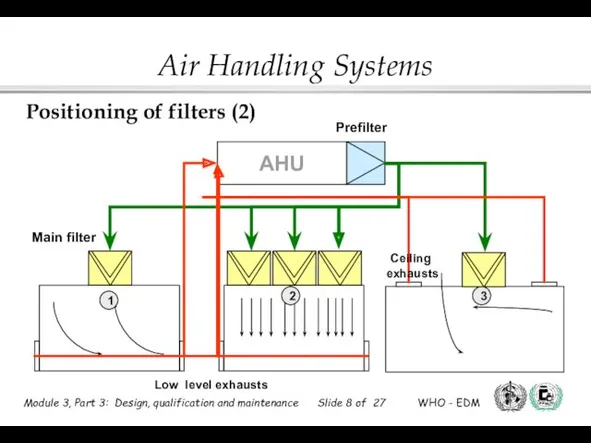
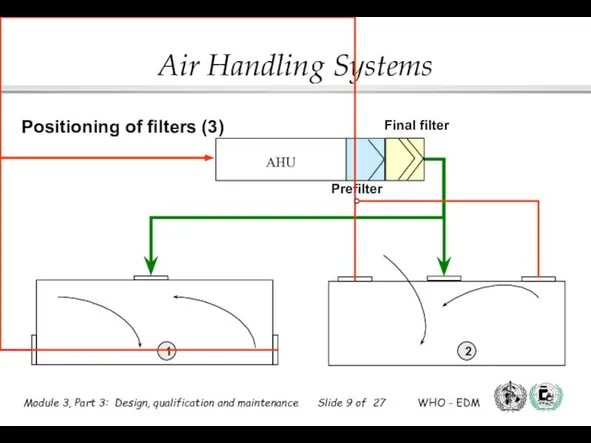
- 8. Prefilter AHU Main filter 1 2 3 Low level exhausts Ceiling exhausts Positioning of filters (2)
- 10. Air re-circulation The filtered air entering a production room can be 100% exhausted or a proportion
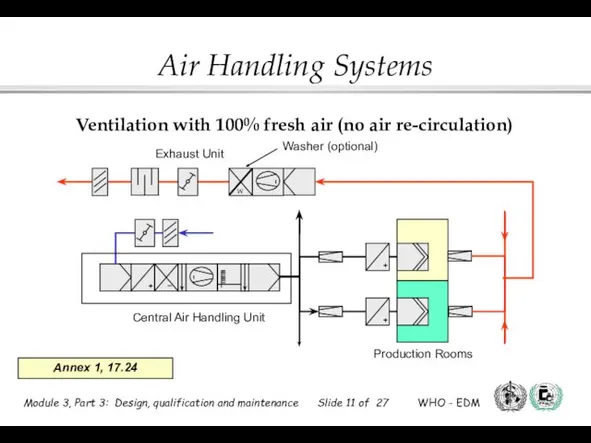
- 11. Ventilation with 100% fresh air (no air re-circulation) Annex 1, 17.24 W Washer (optional) Central Air
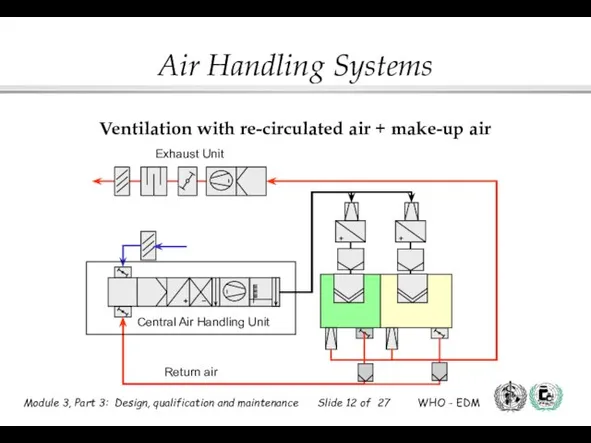
- 12. Ventilation with re-circulated air + make-up air Central Air Handling Unit Return air Exhaust Unit
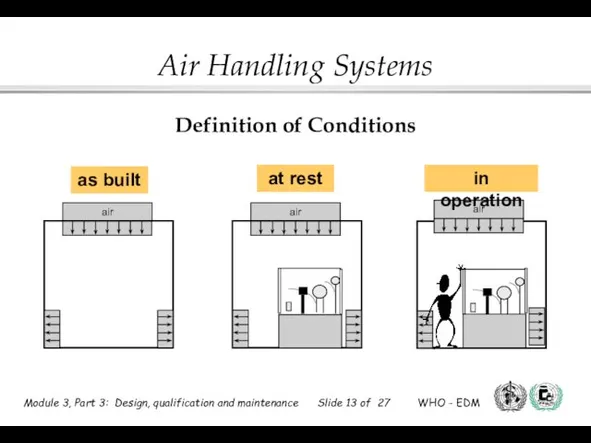
- 13. Definition of Conditions
- 14. Qualification / Validation issues A good design is essential, but it has to be complemented by:
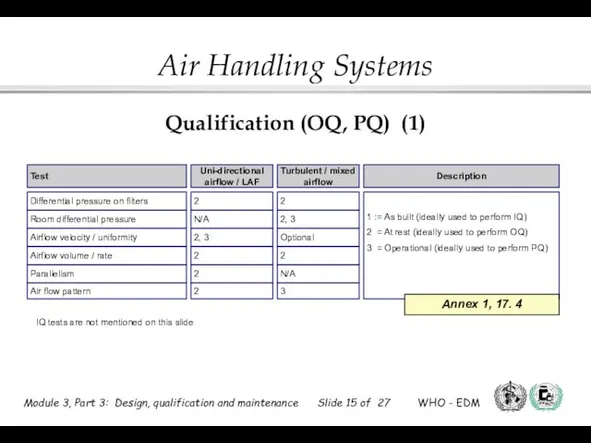
- 15. Qualification (OQ, PQ) (1)
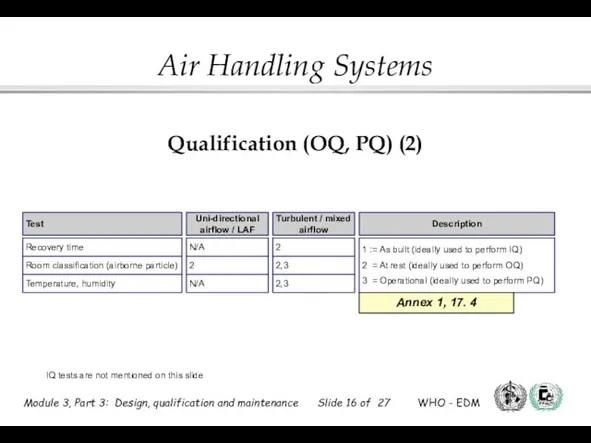
- 16. Qualification (OQ, PQ) (2) IQ tests are not mentioned on this slide
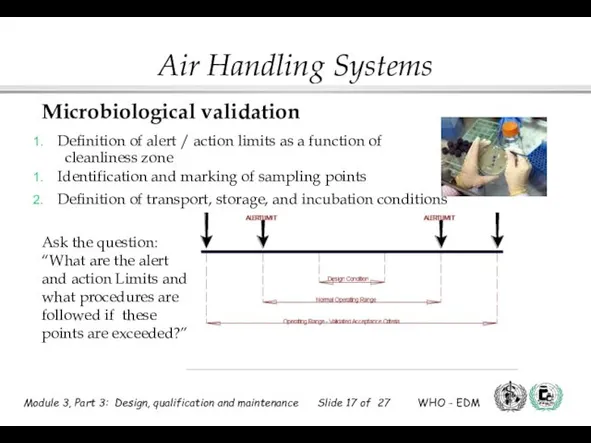
- 17. Microbiological validation Definition of alert / action limits as a function of cleanliness zone Identification and
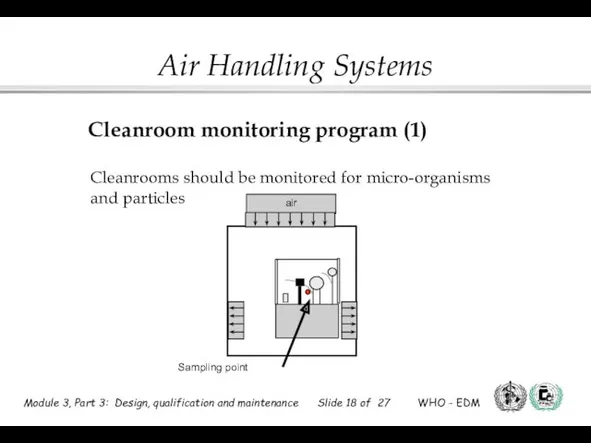
- 18. air Sampling point Cleanroom monitoring program (1) Cleanrooms should be monitored for micro-organisms and particles
- 19. Cleanroom monitoring program (2) Routine monitoring program as part of quality assurance Additional monitoring and triggers
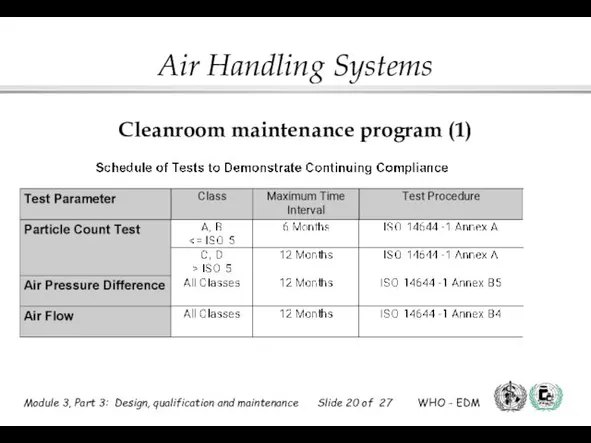
- 20. Cleanroom maintenance program (1)
- 21. Cleanroom maintenance program (2)
- 22. Description of installation and functions Specification of the requirements Operating procedures Instructions for performance control Maintenance
- 23. Verification of design documentation, including description of installation and functions specification of the requirements Operating procedures
- 24. Air handling systems: Play a major role in the quality of pharmaceuticals Must be designed properly,
- 25. This series of explanations will now be followed by: Group discussion, with a simple exercise Short
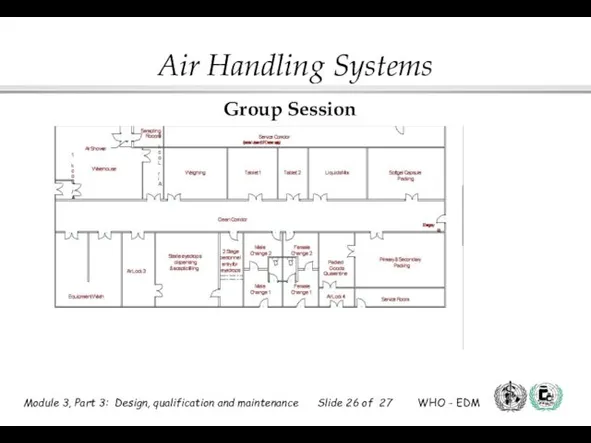
- 26. Group Session
- 28. Скачать презентацию









 Памятки по чтению
Памятки по чтению Белки. Свойства и функции белков в организме
Белки. Свойства и функции белков в организме Методы и приемы развития произностительной стороны у дошкольников
Методы и приемы развития произностительной стороны у дошкольников Инновационные методы по озеленению
Инновационные методы по озеленению Качество заканчивания скважин. Гидродинамически совершенная скважина
Качество заканчивания скважин. Гидродинамически совершенная скважина Правильные многоугольники
Правильные многоугольники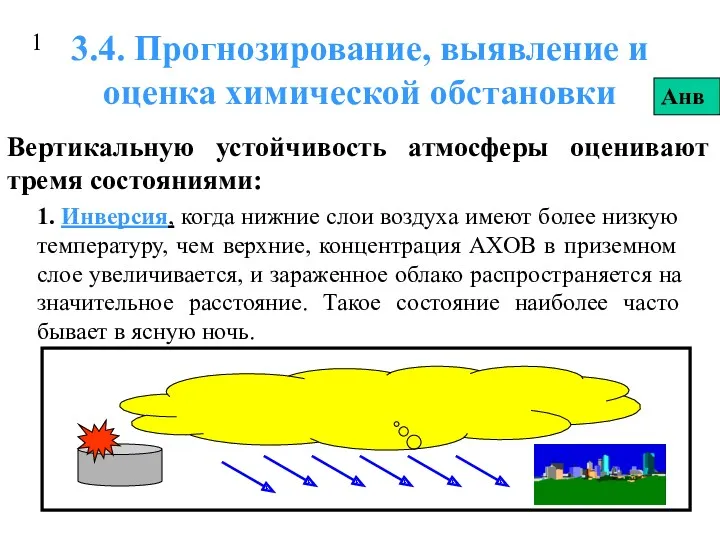 Прогнозирование, выявление и оценка химической обстановки. 4
Прогнозирование, выявление и оценка химической обстановки. 4 Игра Совет мудрейших (часть 1)
Игра Совет мудрейших (часть 1) Animals in our life
Animals in our life презентация к конспекту Гордость России- Михаил Илларионович Кутузов
презентация к конспекту Гордость России- Михаил Илларионович Кутузов Социальная дифференциация и социальная стратификация
Социальная дифференциация и социальная стратификация Алкоголь и здоровье. Интеллектуальная игра
Алкоголь и здоровье. Интеллектуальная игра Sobchak
Sobchak Обобщение опыта воспитательной работы
Обобщение опыта воспитательной работы Здоровье - не все, но все без здоровья - ничто!
Здоровье - не все, но все без здоровья - ничто! Презентация О домовых
Презентация О домовых Дизайн-проект благоустройства проспекта Ленина. Город Урюпинск Волгоградская область
Дизайн-проект благоустройства проспекта Ленина. Город Урюпинск Волгоградская область Эффективность управления компанией. Ключевые задачи оценки деятельности
Эффективность управления компанией. Ключевые задачи оценки деятельности Спиральды білім беру бағдарламасының ерекшеліктері
Спиральды білім беру бағдарламасының ерекшеліктері Творческие работы учеников 1 б класса .Конкурс на лучшую новогоднюю игрушку.2 четверть
Творческие работы учеников 1 б класса .Конкурс на лучшую новогоднюю игрушку.2 четверть Архитектура компьютеров. Основные характеристики компьютеров
Архитектура компьютеров. Основные характеристики компьютеров Гласные и согласные звуки. Тренажёр
Гласные и согласные звуки. Тренажёр Лесной пожар
Лесной пожар Мастер - класс Обереги - домовушки Худякова В.И.
Мастер - класс Обереги - домовушки Худякова В.И. 20191223_teoreticheskiy_test_po_teme_ploshchad
20191223_teoreticheskiy_test_po_teme_ploshchad КружокЯ и мой мир.Презентация для 2 класса.Человек и природа.Жизнь на планете Земля.Осторожно,жестокость!(о бережном отношении к животным).
КружокЯ и мой мир.Презентация для 2 класса.Человек и природа.Жизнь на планете Земля.Осторожно,жестокость!(о бережном отношении к животным). Фото отчет торгового зала. Шаблон
Фото отчет торгового зала. Шаблон Інтернет-сервіси для створення навчального контенту
Інтернет-сервіси для створення навчального контенту