Содержание
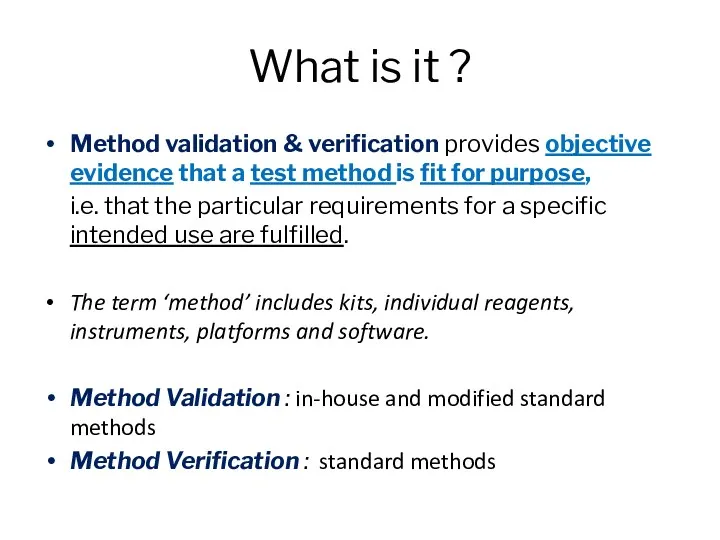
- 2. What is it ? Method validation & verification provides objective evidence that a test method is
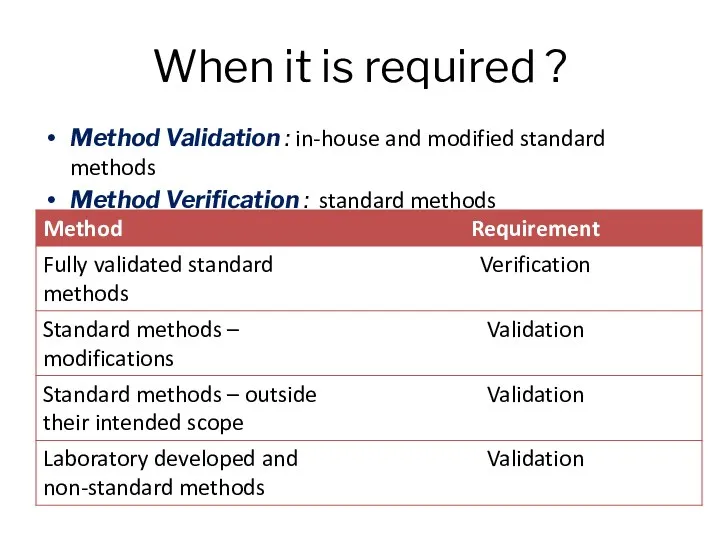
- 3. When it is required ? Method Validation : in-house and modified standard methods Method Verification :
- 4. Why it is necessary ? A test method must be shown to be fit for purpose
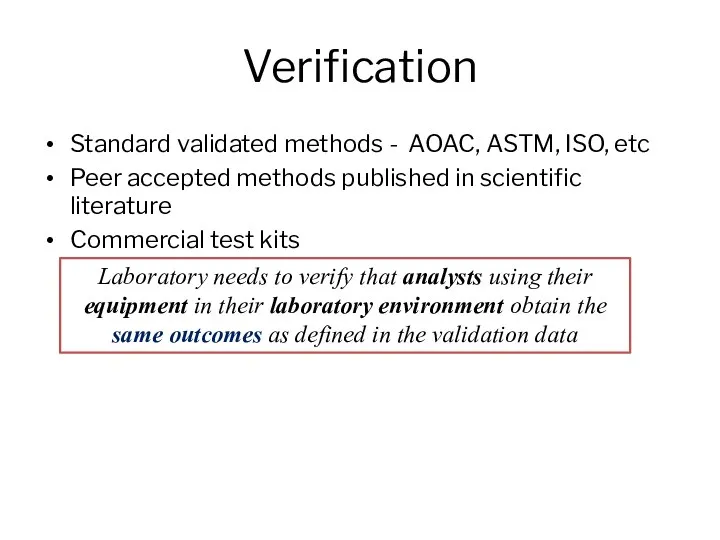
- 5. Verification Standard validated methods - AOAC, ASTM, ISO, etc Peer accepted methods published in scientific literature
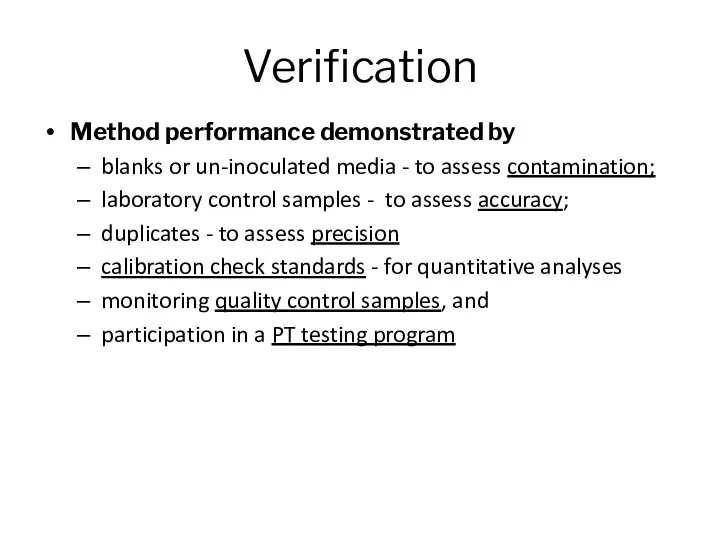
- 6. Verification Method performance demonstrated by blanks or un-inoculated media - to assess contamination; laboratory control samples
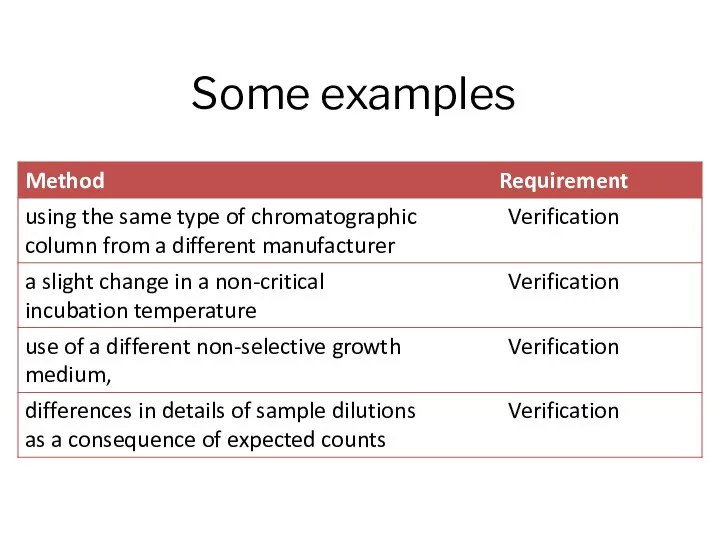
- 7. Some examples
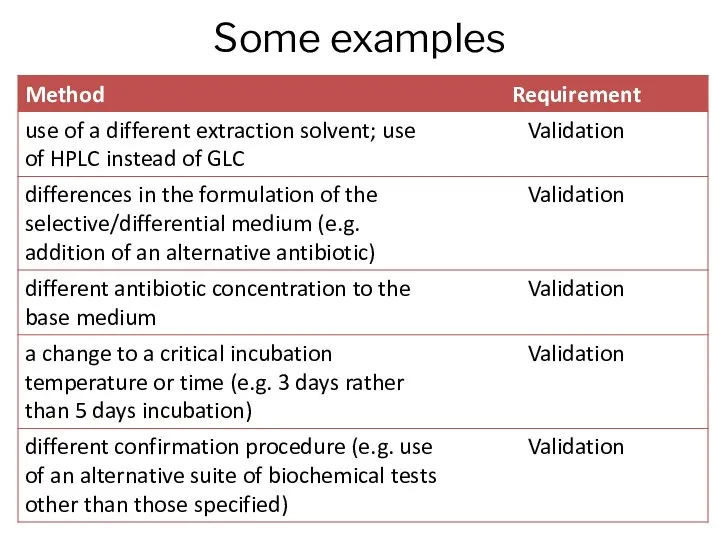
- 8. Some examples
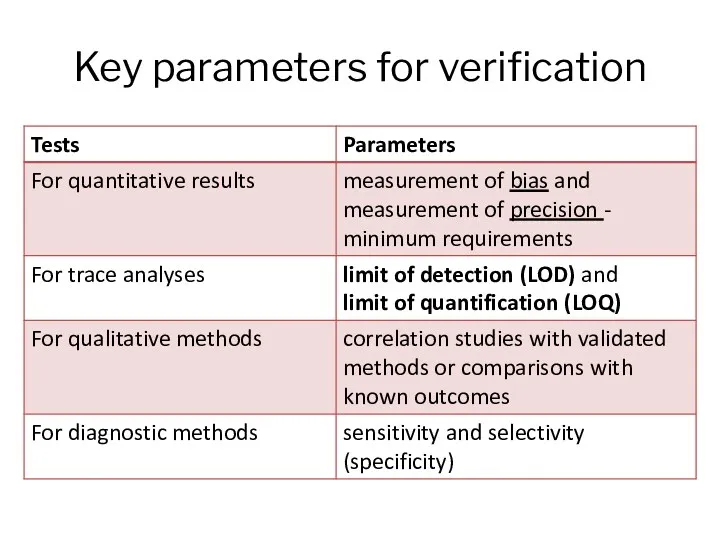
- 9. Key parameters for verification
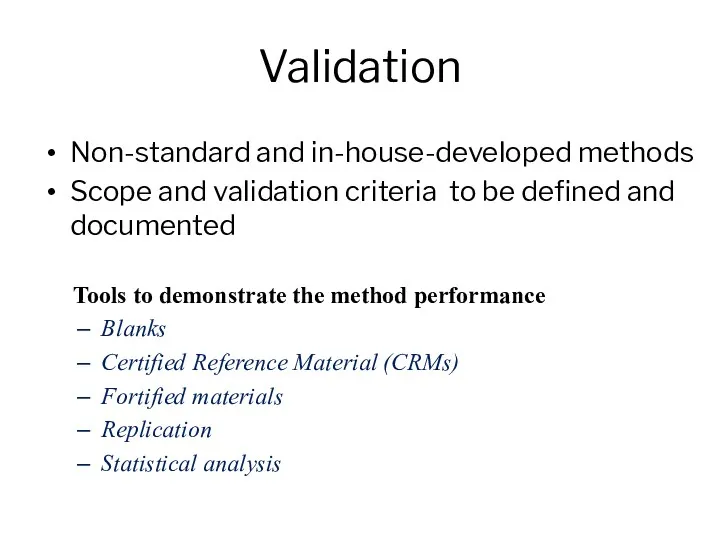
- 10. Validation Non-standard and in-house-developed methods Scope and validation criteria to be defined and documented Tools to
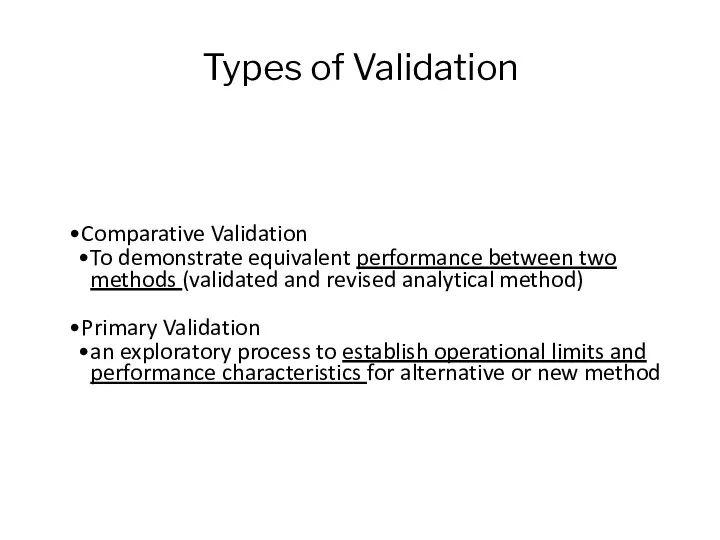
- 11. Types of Validation Comparative Validation To demonstrate equivalent performance between two methods (validated and revised analytical
- 12. Validation Two steps to specify what you intend to identify or measure to determine selected performance
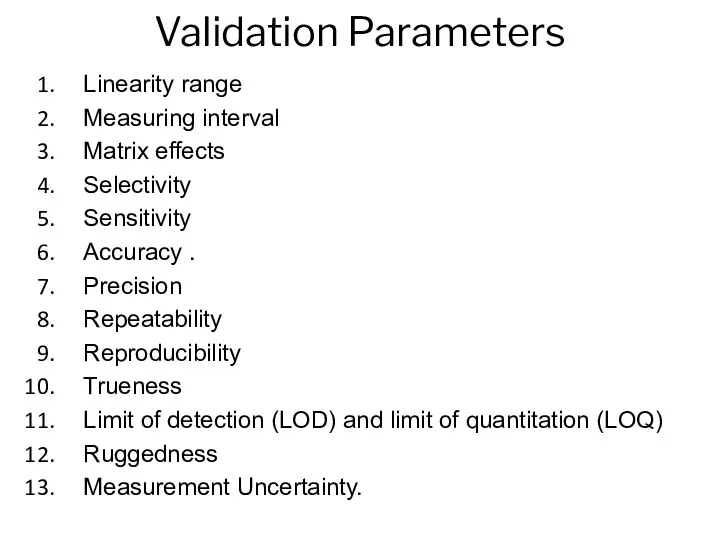
- 13. Validation Parameters Linearity range Measuring interval Matrix effects Selectivity Sensitivity Accuracy . Precision Repeatability Reproducibility Trueness
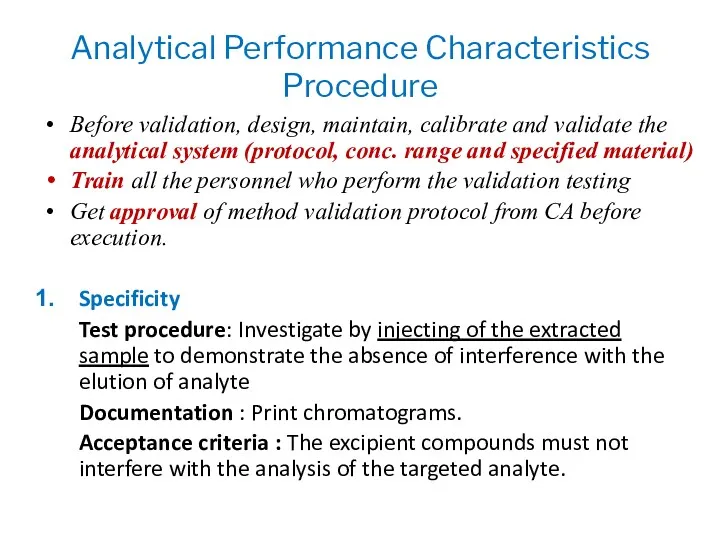
- 14. Analytical Performance Characteristics Procedure Before validation, design, maintain, calibrate and validate the analytical system (protocol, conc.
- 15. 2. Linearity Test procedure : Prepare standard solutions at six concentrations, typically 25, 50, 75, 100,
- 16. 2. Linearity Acceptance criteria : The correlation coefficient for six conc. levels will be ≥ 0.999
- 17. 3. Range Test procedure : Use the data obtained during linearity and accuracy studies to assess
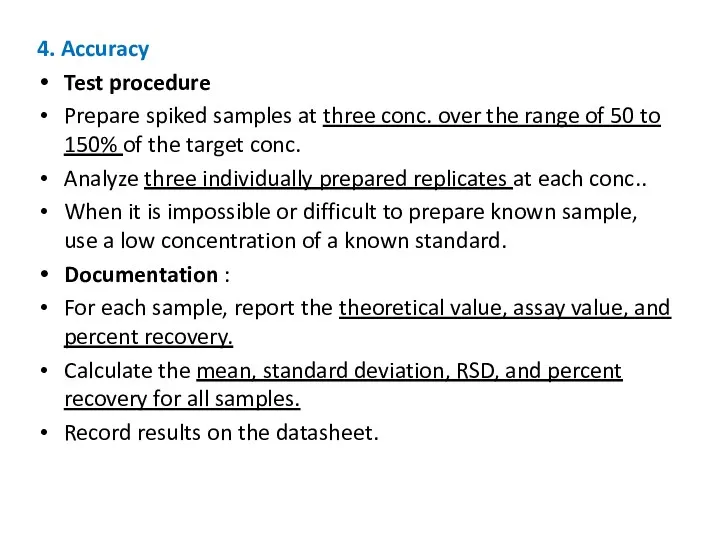
- 18. 4. Accuracy Test procedure Prepare spiked samples at three conc. over the range of 50 to
- 19. 4. Accuracy Acceptance criteria The mean recovery will be within 90 to 110% of the theoretical
- 20. 5. Precision - Repeatability Test procedure: Prepare one sample solution containing the target level of analyte
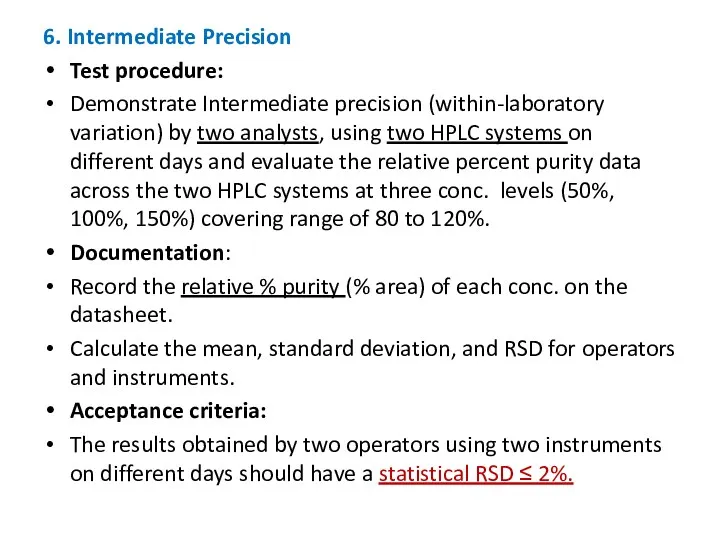
- 21. 6. Intermediate Precision Test procedure: Demonstrate Intermediate precision (within-laboratory variation) by two analysts, using two HPLC
- 22. 7. Limit of Detection Test procedure Determine the lowest concentration of the standard solution by sequentially
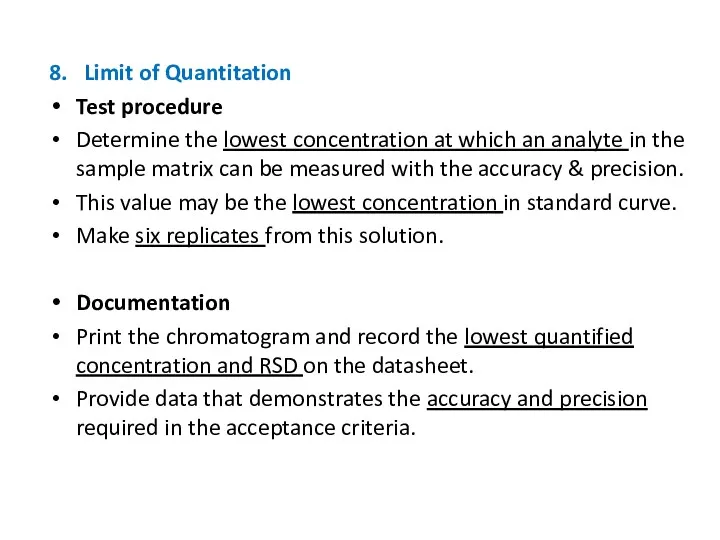
- 23. 8. Limit of Quantitation Test procedure Determine the lowest concentration at which an analyte in the
- 24. 8. Limit of Quantitation Acceptance criteria: The limit of quantitation for chromatographic methods is described as
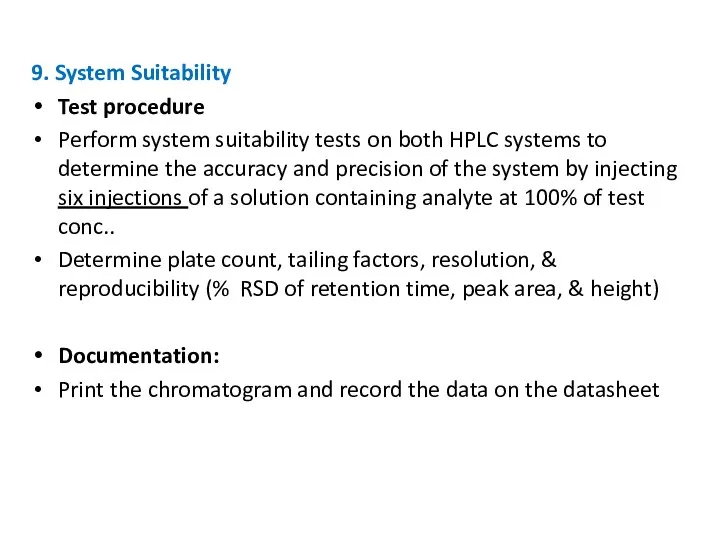
- 25. 9. System Suitability Test procedure Perform system suitability tests on both HPLC systems to determine the
- 26. 9. System Suitability Acceptance criteria: Retention factor (k): the peak of interest be well resolved from
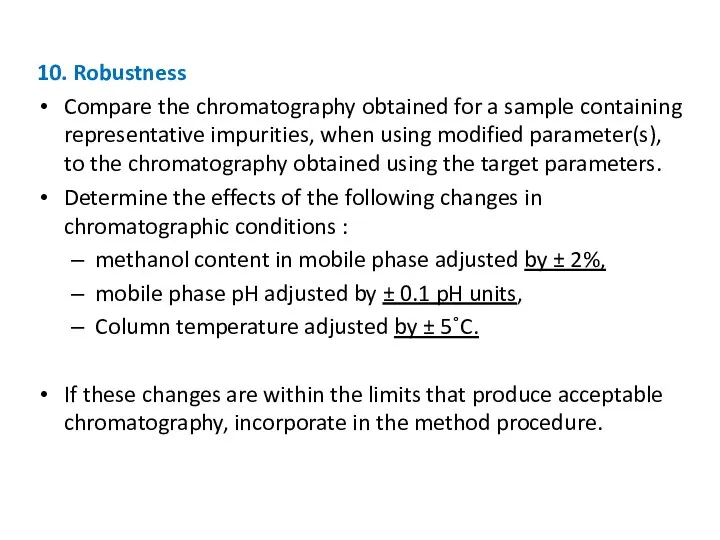
- 27. 10. Robustness Measures the capacity of an analytical method to remain unaffected by small but deliberate
- 28. 10. Robustness Compare the chromatography obtained for a sample containing representative impurities, when using modified parameter(s),
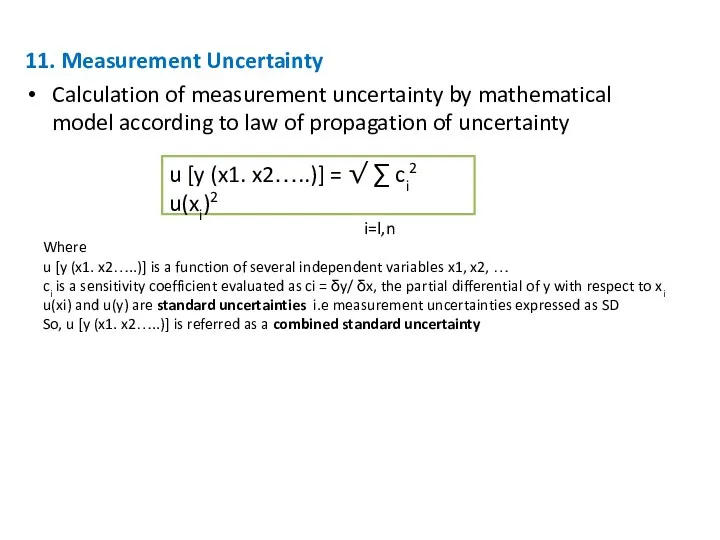
- 29. 11. Measurement Uncertainty Calculation of measurement uncertainty by mathematical model according to law of propagation of
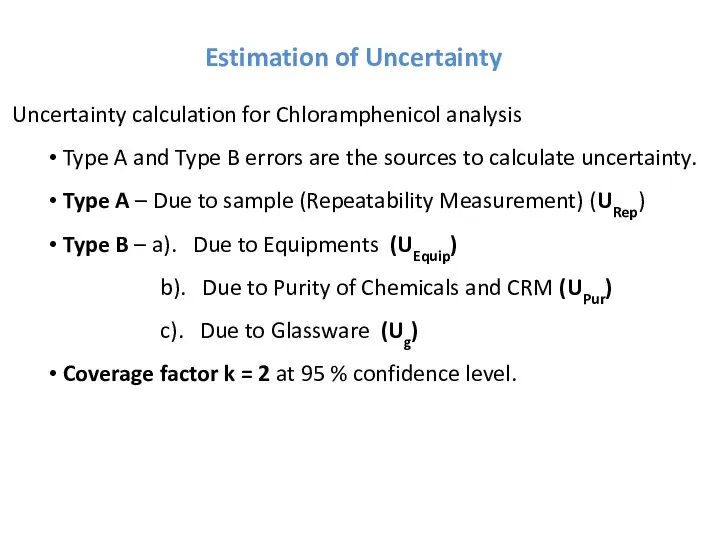
- 30. Estimation of Uncertainty Uncertainty calculation for Chloramphenicol analysis Type A and Type B errors are the
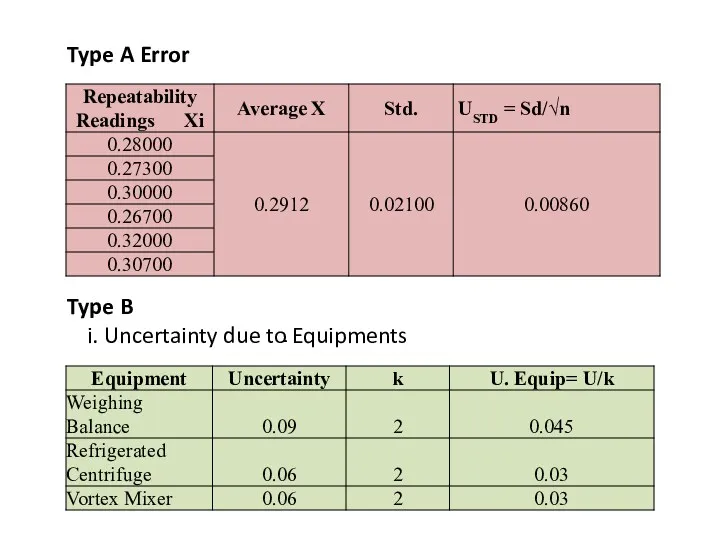
- 31. Type A Error Type B Uncertainty due to Equipments
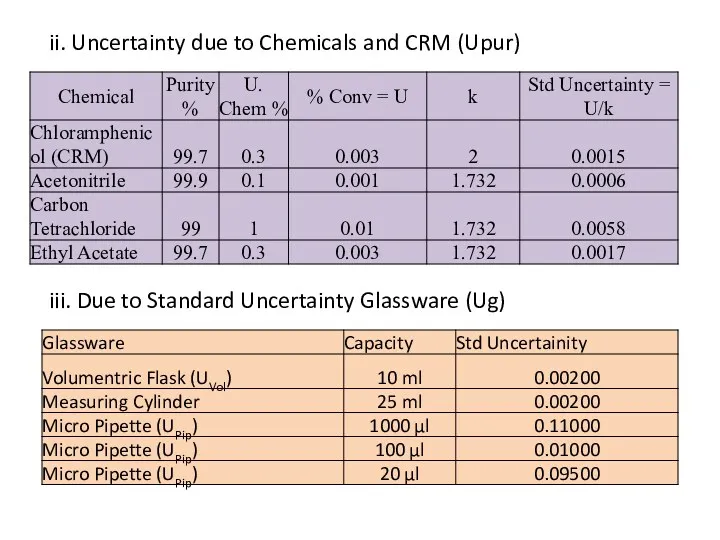
- 32. ii. Uncertainty due to Chemicals and CRM (Upur) iii. Due to Standard Uncertainty Glassware (Ug)
- 34. Скачать презентацию











 Gerund and Infinitive
Gerund and Infinitive The present indefinite tense
The present indefinite tense English. Oral texts
English. Oral texts Уровни перевода
Уровни перевода Comparatives and Superlatives
Comparatives and Superlatives Beautiful places
Beautiful places Teenagers and their problem
Teenagers and their problem Good and bad habits
Good and bad habits READ and LEARN
READ and LEARN Speakout Pre - Intermediate. Question forms
Speakout Pre - Intermediate. Question forms Instructions for writing the solution paragraph
Instructions for writing the solution paragraph Impressionizm
Impressionizm Young Heroes of the War 1941-1945
Young Heroes of the War 1941-1945 Snow
Snow Habits. Used to
Habits. Used to Verb to have got
Verb to have got Education abroad I had like
Education abroad I had like TV programmes
TV programmes Sport facilities of my university
Sport facilities of my university School What is school for you?
School What is school for you? Police of the Russian Federation
Police of the Russian Federation Present perfect tense
Present perfect tense Choice of profession
Choice of profession Peter’s family
Peter’s family Animals and parts of the body
Animals and parts of the body Customs and traditions in Great Britain. Обычаи и традиции
Customs and traditions in Great Britain. Обычаи и традиции About the person that is writing in this place. Something about
About the person that is writing in this place. Something about Yale university
Yale university