Содержание
- 2. Protocol Code: MOR106-CL-102 Protocol Title: A parallel-design phase 1 study to assess safety, tolerability and pharmacokinetics
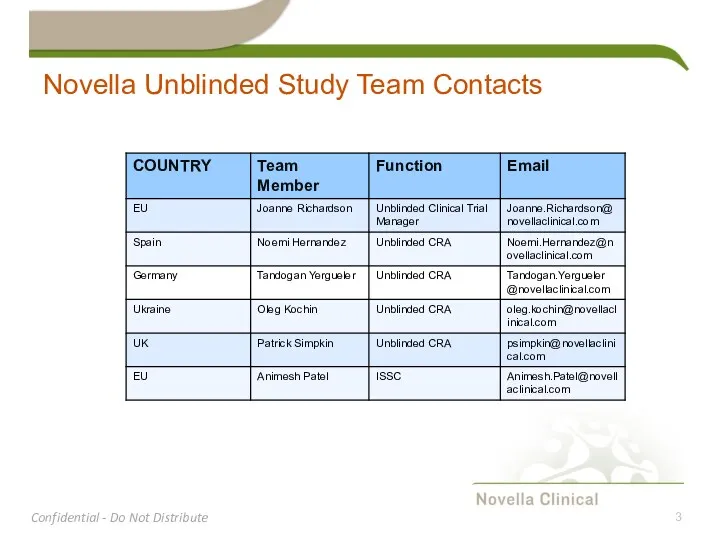
- 3. Novella Unblinded Study Team Contacts
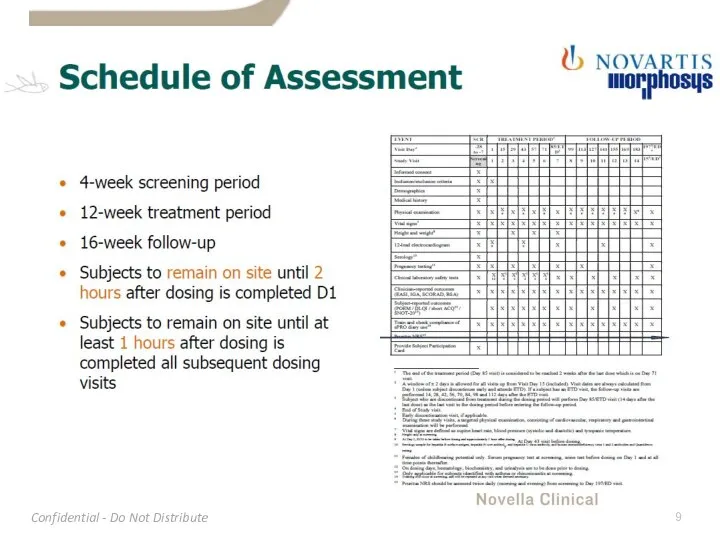
- 4. Protocol Overview
- 10. Investigational Product
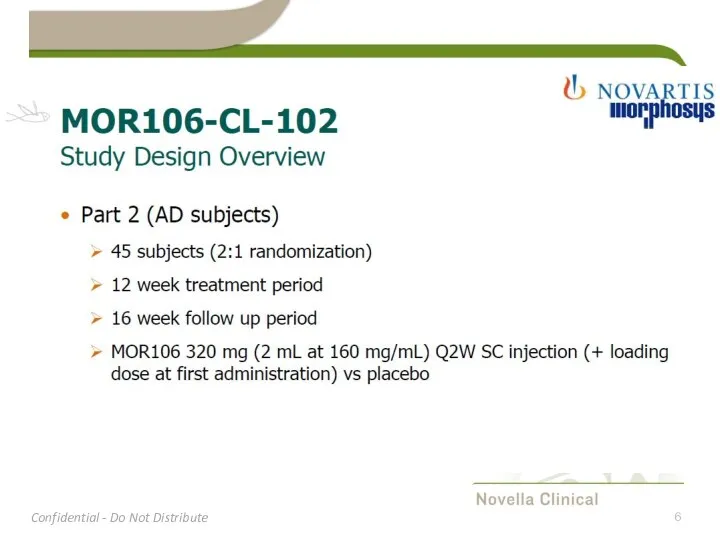
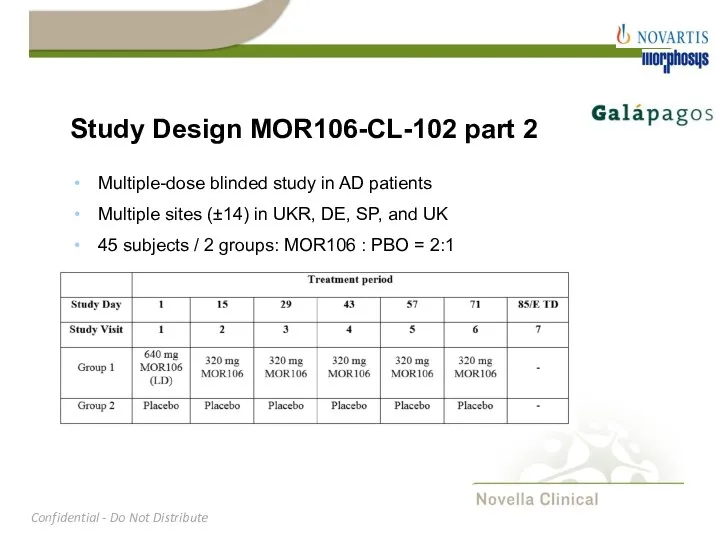
- 11. Study Design MOR106-CL-102 part 2 Multiple-dose blinded study in AD patients Multiple sites (±14) in UKR,
- 12. General Information Two IMPs used: active (MOR106) and Placebo Fixed dose Six doses given every 2
- 13. Site Blinding Plan A template Site Blinding Plan will be provided to each site for developing
- 14. Hazards of MOR106 Drug Product MOR106 is an experimental therapeutic humanized IgG1 antibody directed against IL-17C
- 15. Description of MOR106 and placebo MOR106 is a solution for SC injection Single-use vial of 160
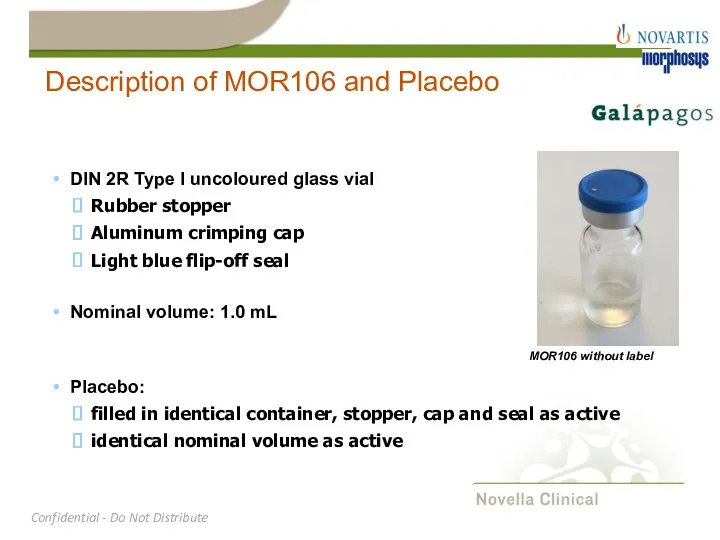
- 16. Description of MOR106 and Placebo DIN 2R Type I uncoloured glass vial Rubber stopper Aluminum crimping
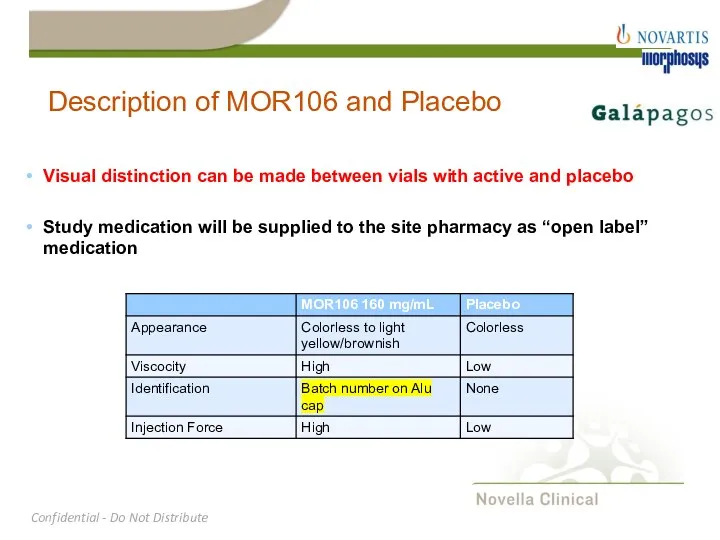
- 17. Description of MOR106 and Placebo Visual distinction can be made between vials with active and placebo
- 18. Description of MOR106 and Placebo MOR106 SC vials Manufactured by Rentschler (Germany) 2 vials / carton
- 19. IMP Shipment Triggered by regulatory green light and site activation in IWRS by GLPG/Novella Shipment of
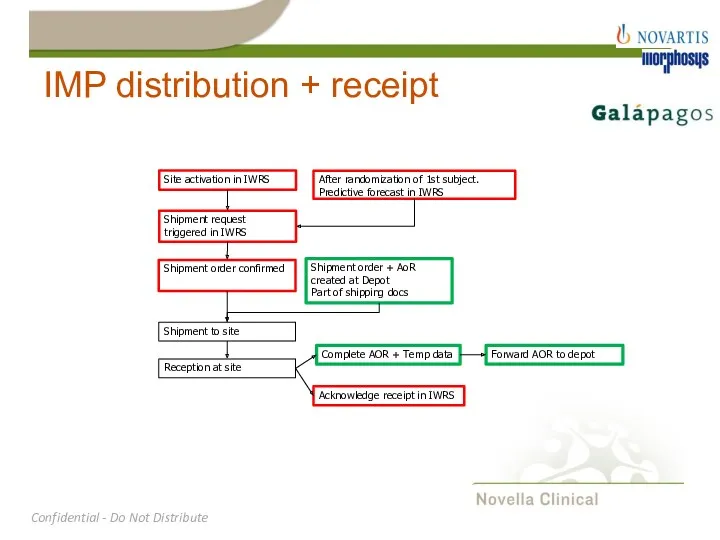
- 20. IMP distribution + receipt Site activation in IWRS Shipment request triggered in IWRS Shipment order confirmed
- 21. Ancillary supplies Site activation in IWRS Shipment request triggered in IWRS Shipment order confirmed Shipment to
- 22. IMP supply management Clinical supply will be managed in IWRS (Endpoint Clinical) – initial supply and
- 23. IMP receipt, storage and re-order An accountability form will be maintained to document the receipt, dispensing
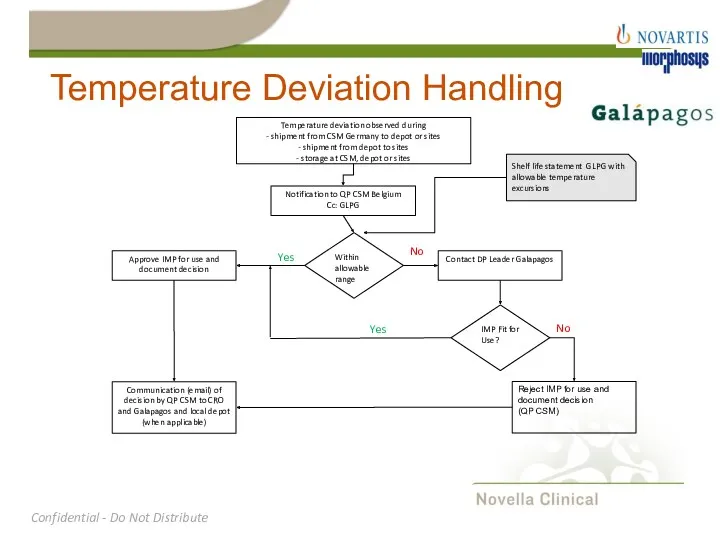
- 24. Temperature Deviation Handling Temperature deviation observed during - shipment from CSM Germany to depot or sites
- 25. Dosing Summary Subjects will be dosed with two times 2 mL of active (640 mg) or
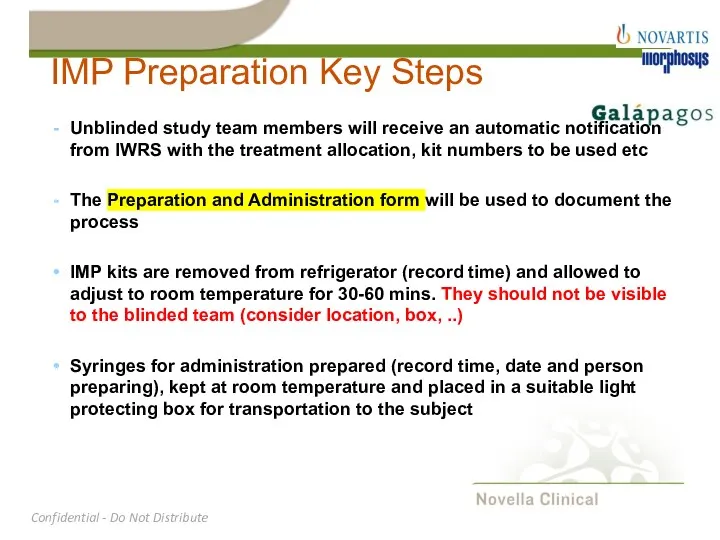
- 26. IMP Preparation Key Steps Unblinded study team members will receive an automatic notification from IWRS with
- 27. IMP Preparation Key Steps IMP should be administered within 2 hrs after start of preparation (time
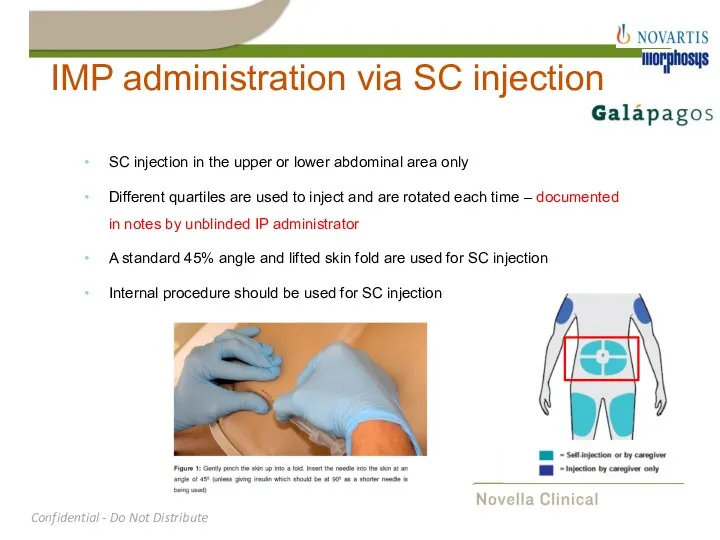
- 28. IMP administration via SC injection SC injection in the upper or lower abdominal area only Different
- 29. IWRS Endpoint
- 30. IRT Basics IRT Access: https://secure.endpointclinical.com After documented training IRT (Interactive Response Technology) is an integrated web
- 31. System Overview
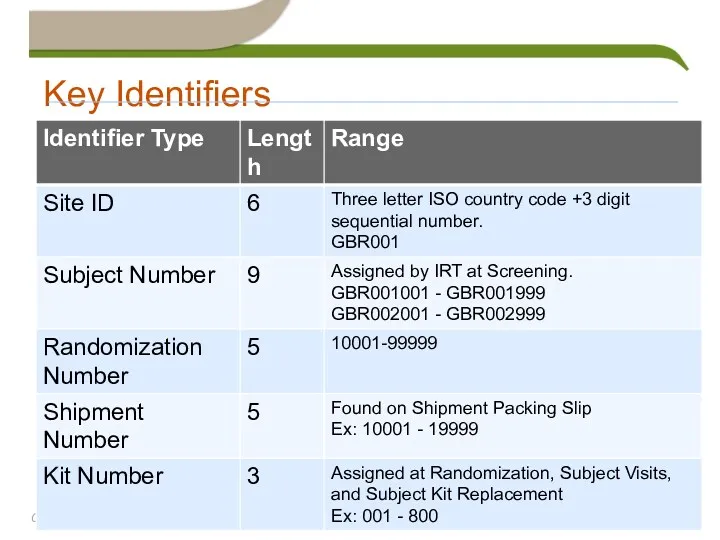
- 32. Key Identifiers
- 33. IRT Access
- 34. Randomization Notifications Dispensation information is NOT displayed on IWRS screen Notifications: Part 2 Blinded Notification Part
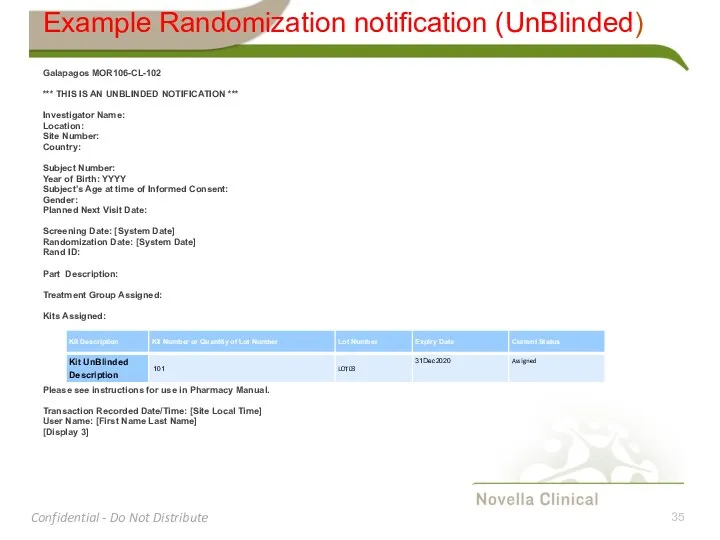
- 35. Example Randomization notification (UnBlinded) Galapagos MOR106-CL-102 *** THIS IS AN UNBLINDED NOTIFICATION *** Investigator Name: Location:
- 36. Example Subject Visit Notification (UnBlinded) Galapagos MOR106-CL-201 *** THIS IS AN UNBLINDED NOTIFICATION *** Investigator Name:
- 37. Drug Receipt Note: shipments must be acknowledged in the IWR system prior to any randomizations
- 38. Manage Inventory Select Site Number, Kit Status, and Kit Type Select Lot Number and Batch Number
- 39. Manage Inventory by Kit Number Select new kit status Select ‘Next’ Review all data then select
- 40. Subject Kit Replacement
- 41. Subject Replacement Visit Notification Dispensation information is NOT displayed on IWR screen Notifications: Blinded Notification Unblinded
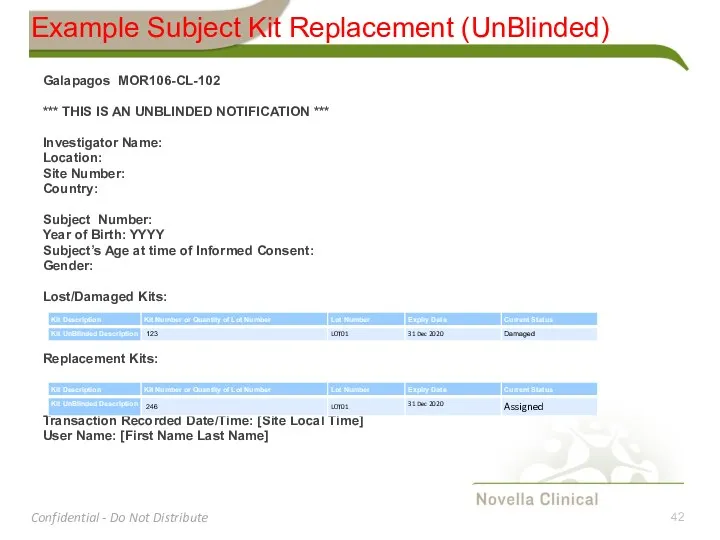
- 42. Example Subject Kit Replacement (UnBlinded) Galapagos MOR106-CL-102 *** THIS IS AN UNBLINDED NOTIFICATION *** Investigator Name:
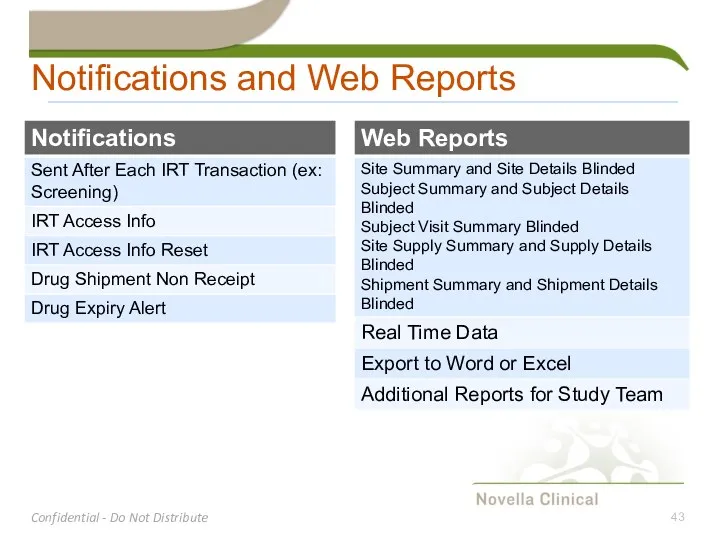
- 43. Notifications and Web Reports
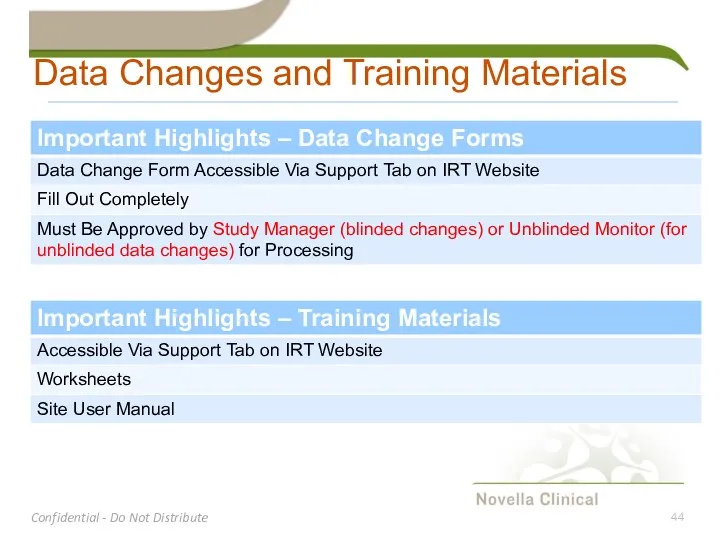
- 44. Data Changes and Training Materials
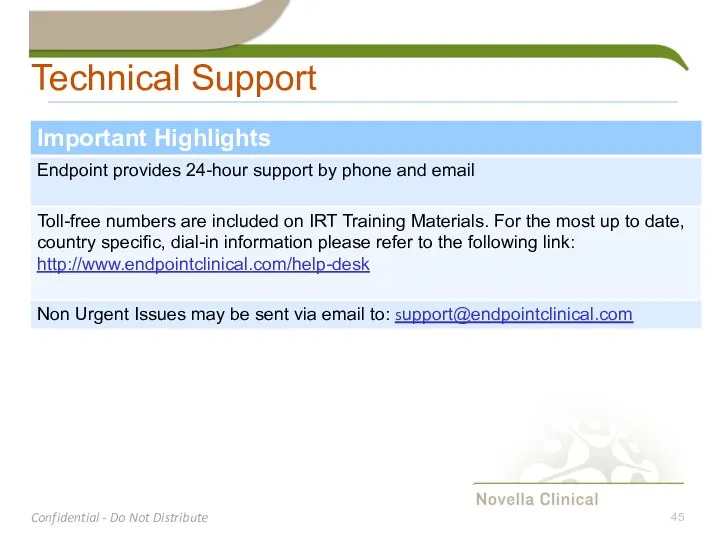
- 45. Technical Support
- 46. Monitoring Expectations
- 47. Blinded vs Unblinded Both at Galapagos and Novella there will exist two separate teams for this
- 48. Source Documentation and GCP Document it! All original documentation must be retained – the first place
- 50. Скачать презентацию





























 Teaching listening
Teaching listening Language and speech. Types of speech
Language and speech. Types of speech Rules of procedure
Rules of procedure Smartphones in space
Smartphones in space Grooming talk
Grooming talk The Modal Verbs
The Modal Verbs This, that, these, those
This, that, these, those Studying in Denmark
Studying in Denmark Klass viktorina. Play-game
Klass viktorina. Play-game Armenian language
Armenian language Grammar of the text. Lecture 12
Grammar of the text. Lecture 12 russian Cuisine
russian Cuisine Summer fun
Summer fun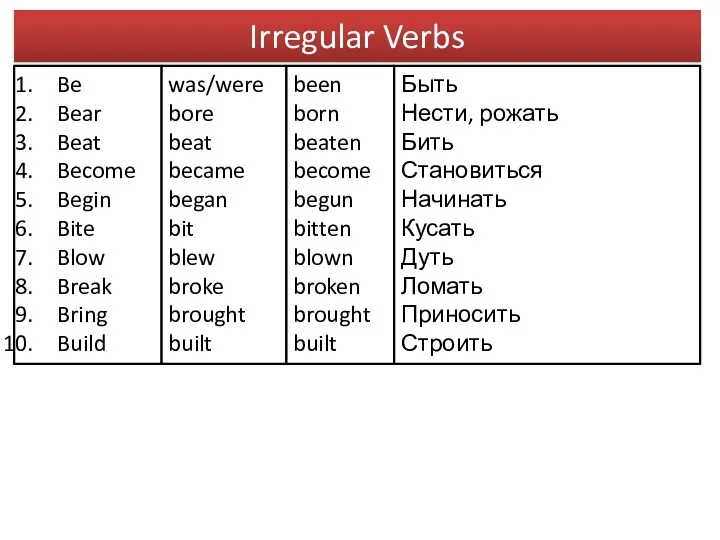 Irregular Verbs
Irregular Verbs Использование мультимедийных средств обучения и игровых приемов на уроках английского языка
Использование мультимедийных средств обучения и игровых приемов на уроках английского языка Интерактивные методы обучения как способ формирования коммуникативной компетенции учащихся при изучении английского языка
Интерактивные методы обучения как способ формирования коммуникативной компетенции учащихся при изучении английского языка Direct and reported speech (Прямая и косвенная речь)
Direct and reported speech (Прямая и косвенная речь) Consumer protection abroad returns and refunds regulation
Consumer protection abroad returns and refunds regulation Past Tenses
Past Tenses Еда. В. Эванс Спотлайт. 2 класс
Еда. В. Эванс Спотлайт. 2 класс The english alphabet s-z. Game 2
The english alphabet s-z. Game 2 Passive Voice
Passive Voice English for academic purposes (lesson 1)
English for academic purposes (lesson 1) The Prado Museum
The Prado Museum 12 months make a year
12 months make a year St. Valentine’s Day
St. Valentine’s Day Мероприятия по английскому языку
Мероприятия по английскому языку Mass Media
Mass Media