Содержание
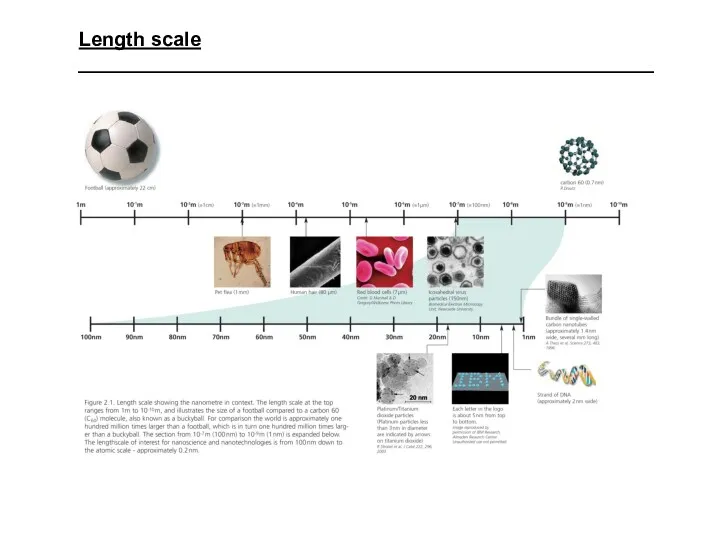
- 2. Length scale
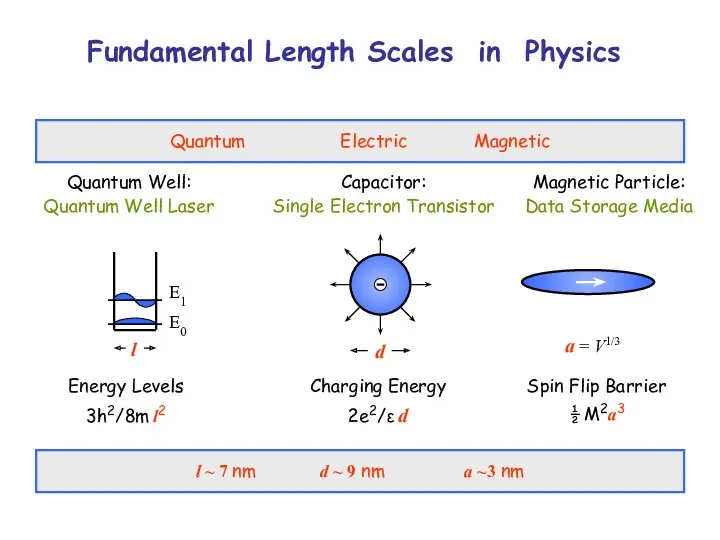
- 3. Fundamental Length Scales in Physics Quantum Electric Magnetic Quantum Well: Quantum Well Laser Capacitor: Single Electron
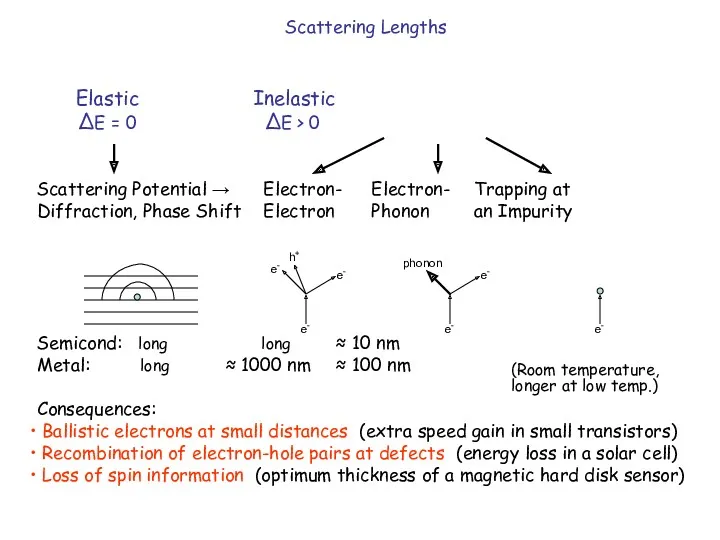
- 4. Elastic Inelastic ΔE = 0 ΔE > 0 Scattering Potential → Electron- Electron- Trapping at Diffraction,
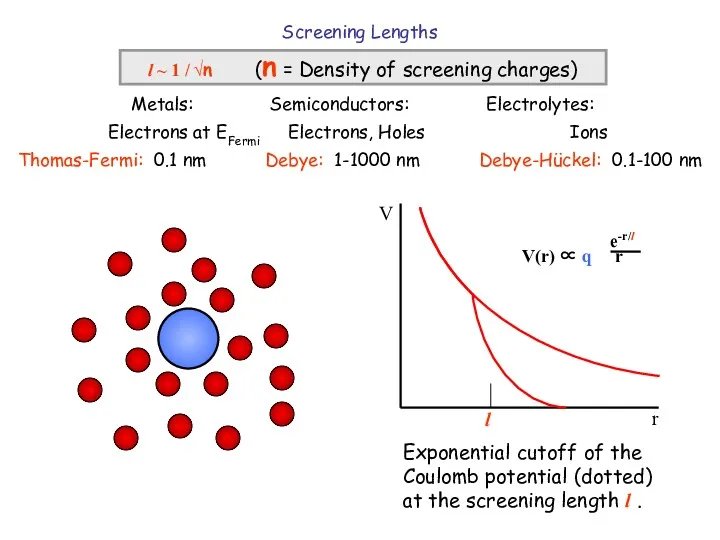
- 5. Screening Lengths l ~ 1 / √n (n = Density of screening charges) Metals: Semiconductors: Electrolytes:
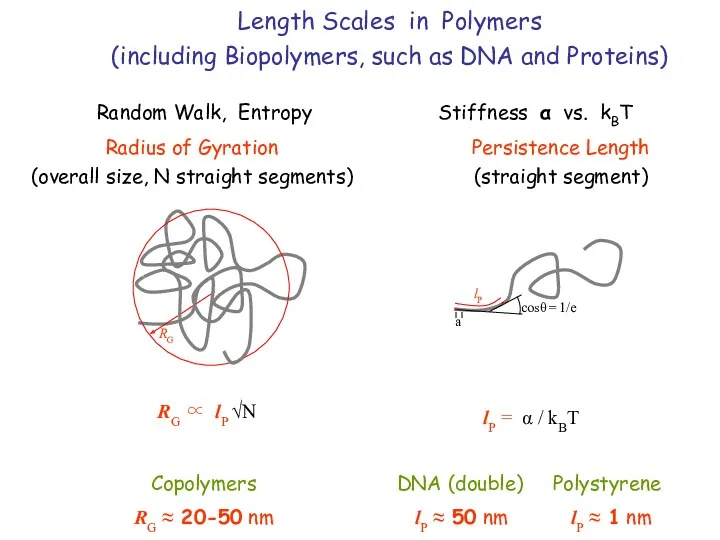
- 6. Length Scales in Polymers (including Biopolymers, such as DNA and Proteins) Random Walk, Entropy Stiffness α
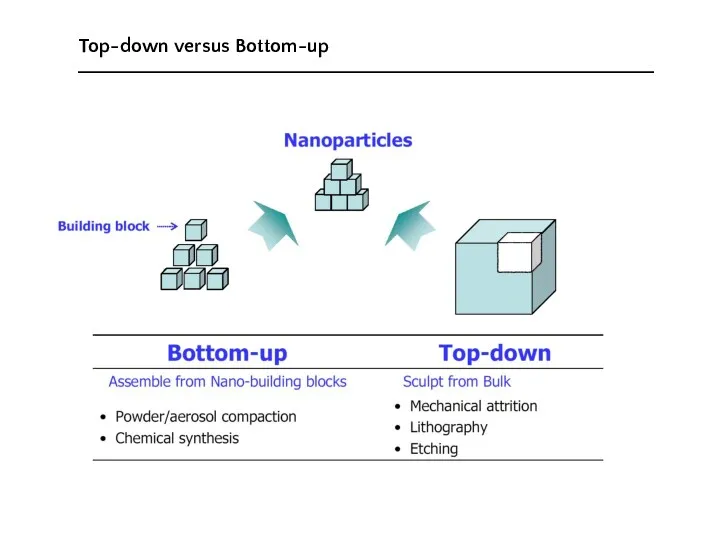
- 7. Top-down versus Bottom-up
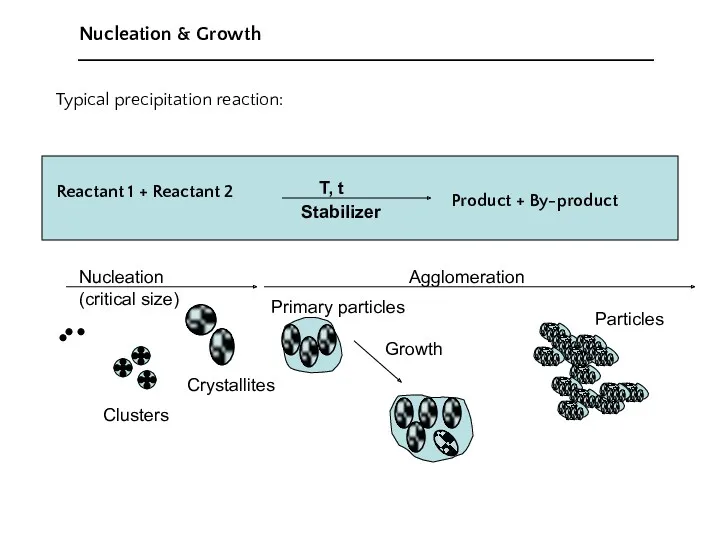
- 8. Nucleation and Growth of Crystals
- 9. Typical precipitation reaction: Reactant 1 + Reactant 2 Product + By-product Nucleation & Growth
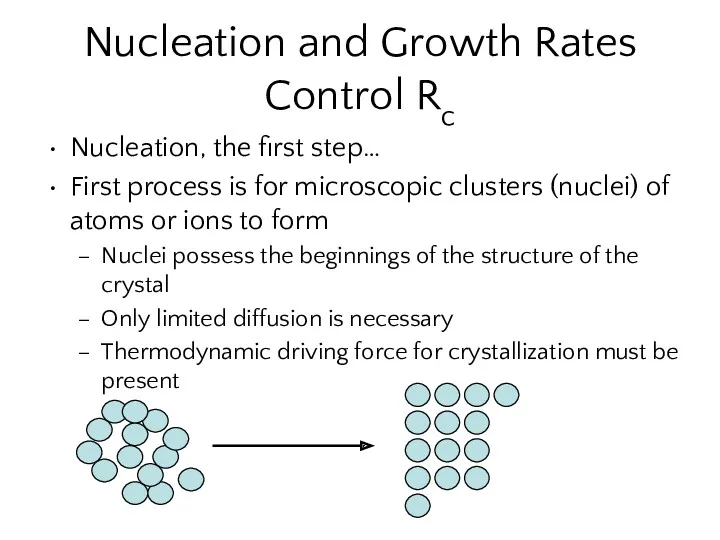
- 10. Nucleation and Growth Rates Control Rc Nucleation, the first step… First process is for microscopic clusters
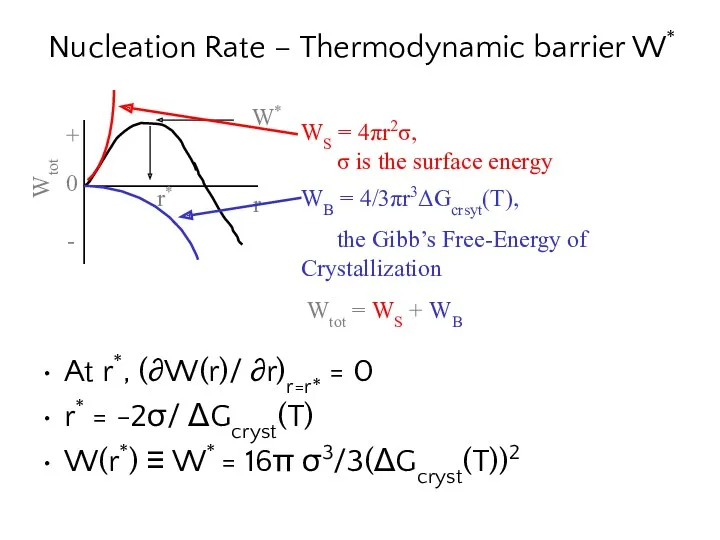
- 11. Nucleation Rate – Thermodynamic barrier W* At r*, (∂W(r)/ ∂r)r=r* = 0 r* = -2σ/ ΔGcryst(T)
- 12. Bottom-up Approaches Two approaches thermodynamic equilibrium approach generation of supersaturation nucleation subsequent growth kinetic approach limiting
- 13. Homogeneous nucleation Liquid, vapor or solid supersaturation temperature reduction metal quantum dots in glass matrix by
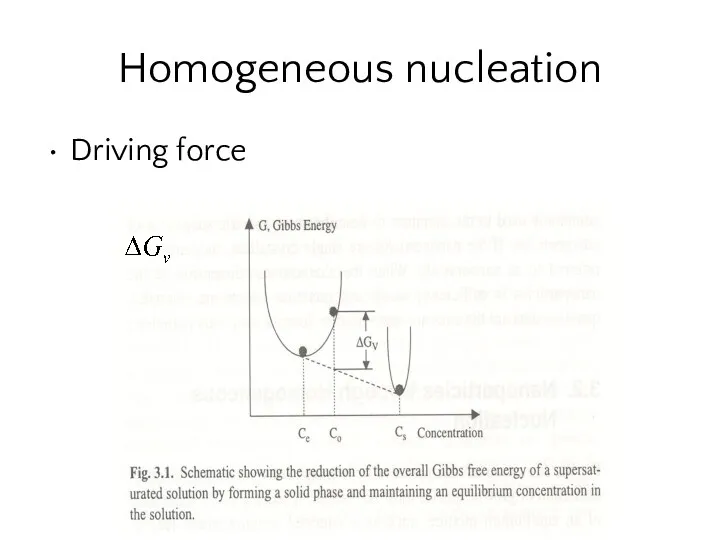
- 14. Homogeneous nucleation Driving force Fig 3.1
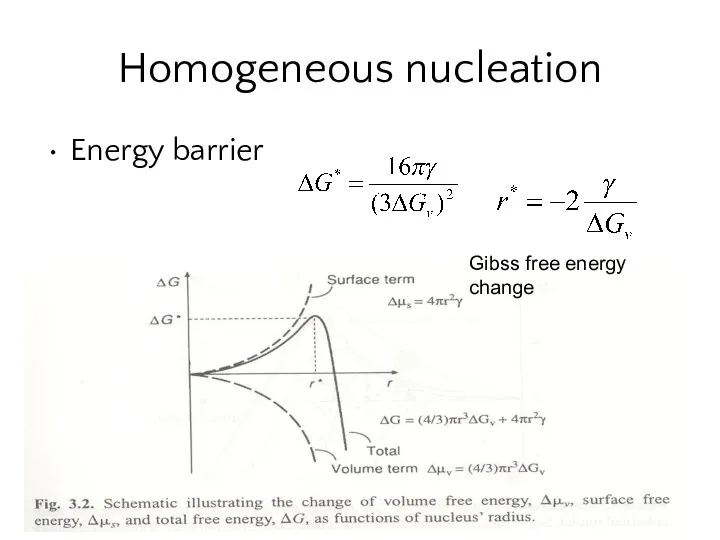
- 15. Homogeneous nucleation Energy barrier Gibss free energy change
- 16. Nuclei formation favor: high initial concentration or supersaturation low viscosity low critical energy barrier uniform nanoparticle
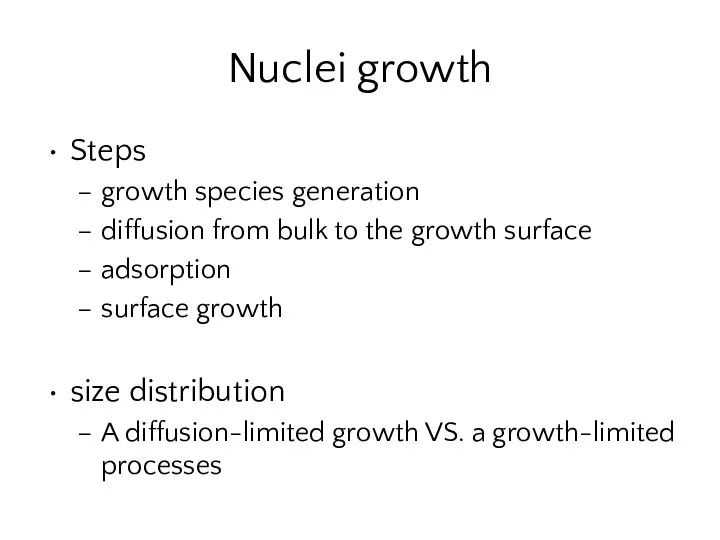
- 17. Nuclei growth Steps growth species generation diffusion from bulk to the growth surface adsorption surface growth
- 18. Ostwald ripening Many small crystals form in a system initially but slowly disappear except for a
- 19. “LEEM (Low-energy electron microscopy) images of ripening of single atomic layer height islands on Si(001) at
- 20. Metallic nanoparticles Reduction of metal complexes in dilute solution Diffusion-limited process maintaining Example: nano-gold particles chlorauric
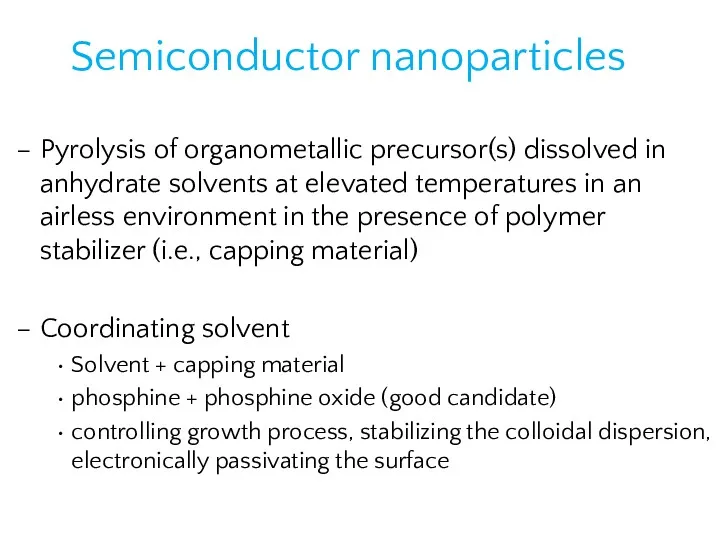
- 21. Semiconductor nanoparticles Pyrolysis of organometallic precursor(s) dissolved in anhydrate solvents at elevated temperatures in an airless
- 22. Oxide nanoparticles Several methods principles: burst of homogeneous nucleation + diffusion controlled growth most commonly: sol-gel
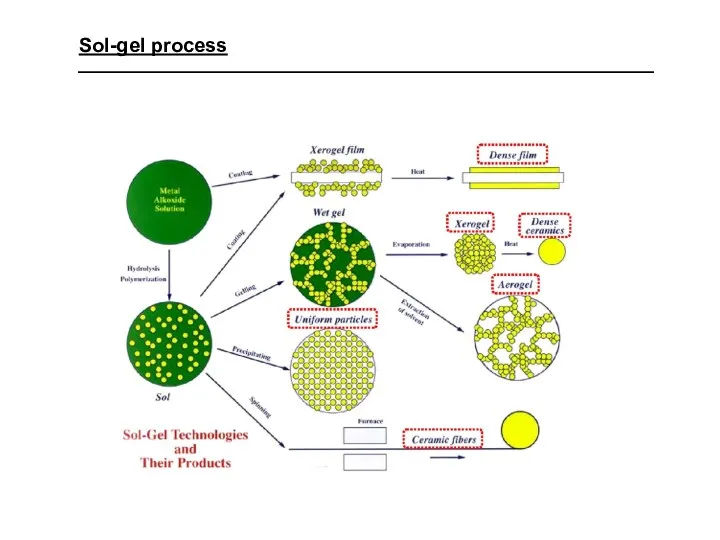
- 23. Sol-gel process
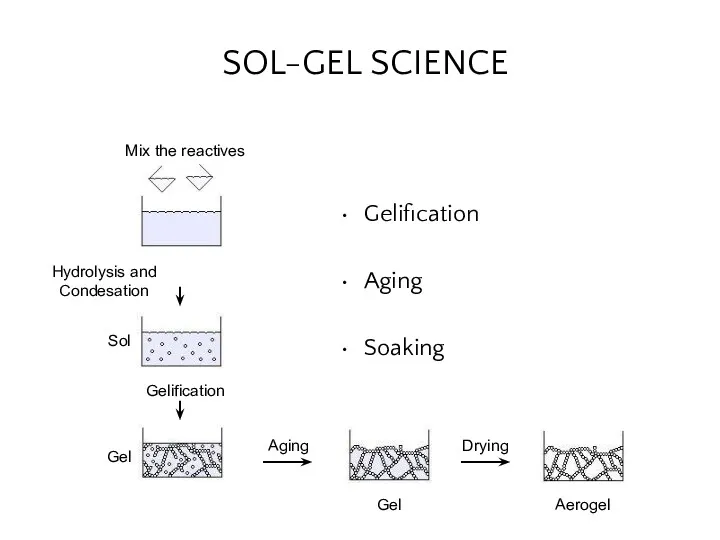
- 24. SOL-GEL SCIENCE Gelification Aging Soaking Mix the reactives Sol Gel Gel Aerogel Hydrolysis and Condesation Gelification
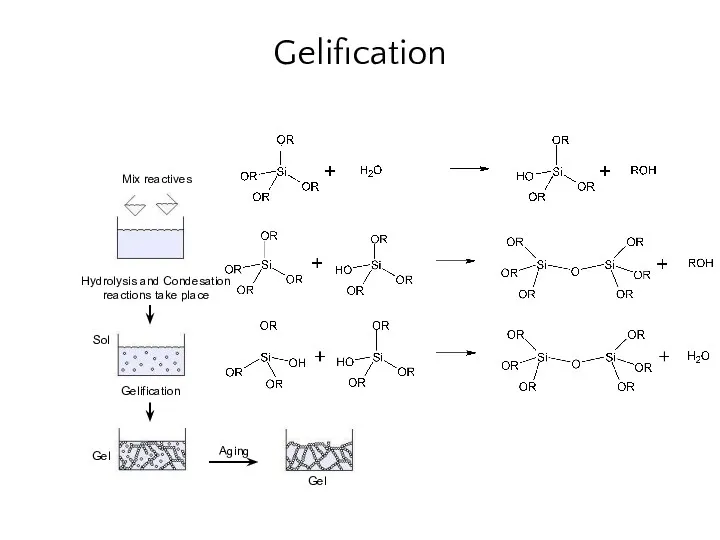
- 25. Gelification Mix reactives Sol Gel Gel Gelification Aging Hydrolysis and Condesation reactions take place
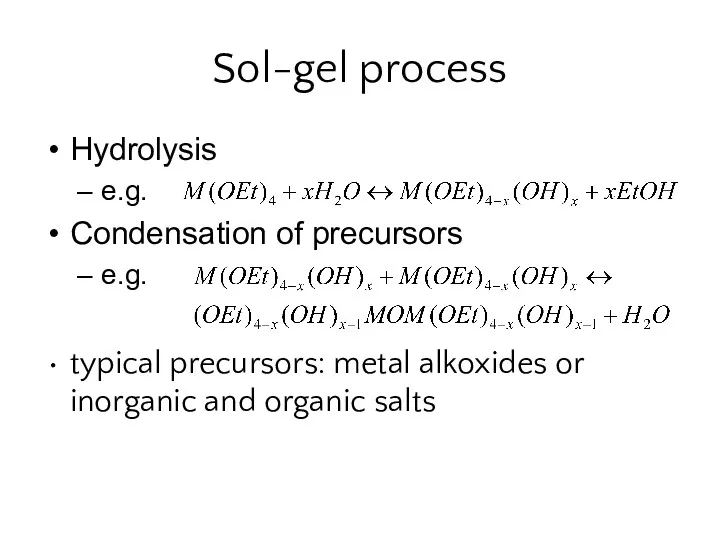
- 26. Sol-gel process Hydrolysis e.g. Condensation of precursors e.g. typical precursors: metal alkoxides or inorganic and organic
- 27. Sol-gel example: silica Precursors: silicone alkoxides with different alkyl ligand sizes catalyst: ammonia solvent: various alcohols
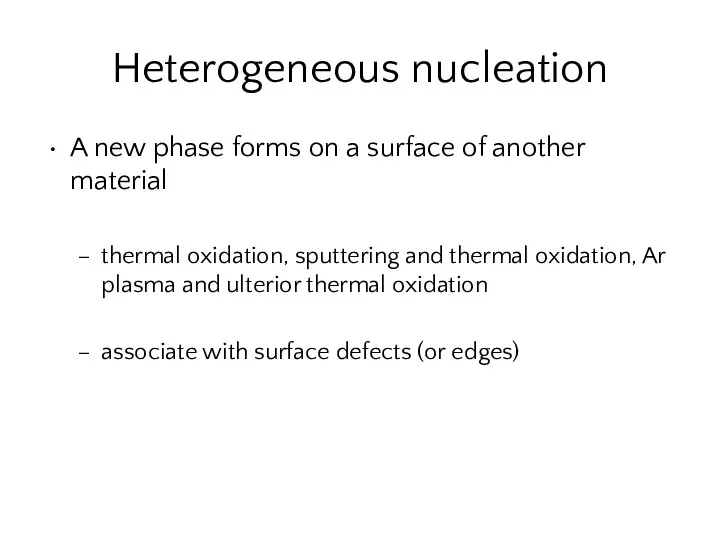
- 28. Heterogeneous nucleation A new phase forms on a surface of another material thermal oxidation, sputtering and
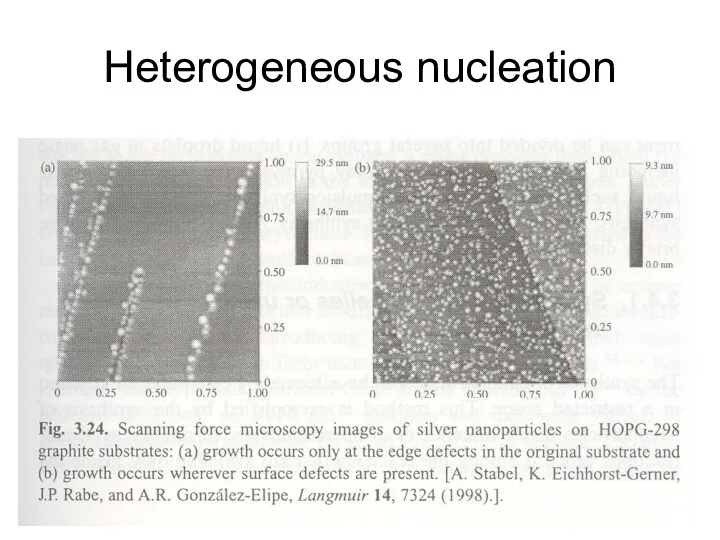
- 29. Heterogeneous nucleation
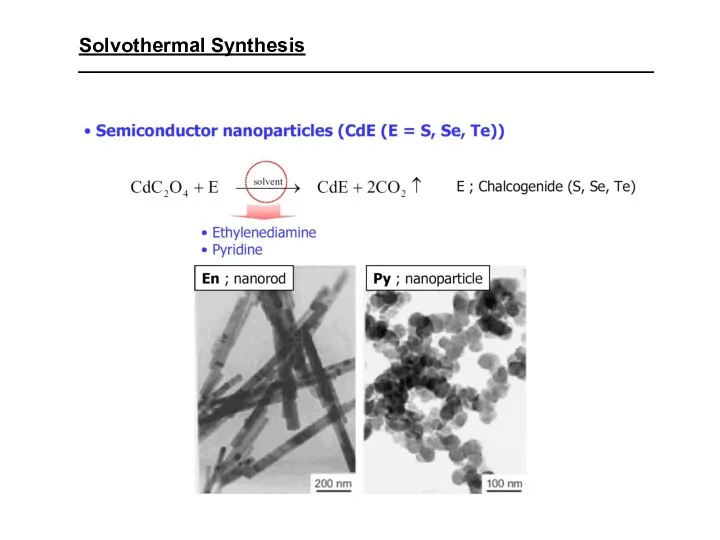
- 30. Solvothermal Synthesis
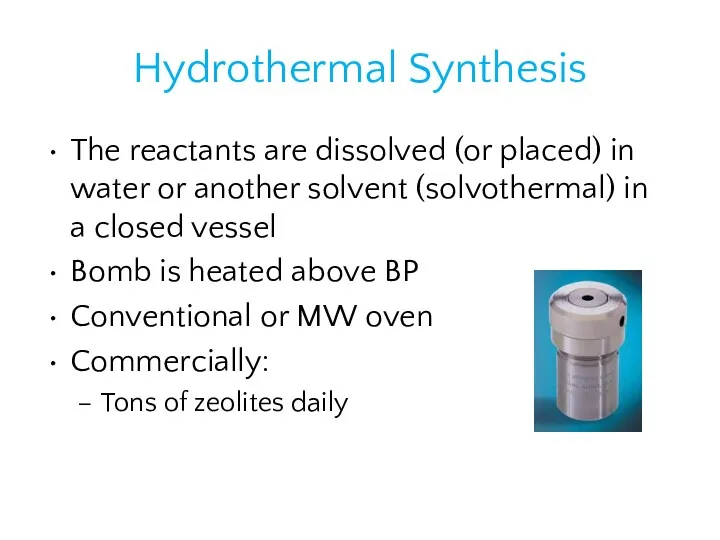
- 31. Hydrothermal Synthesis The reactants are dissolved (or placed) in water or another solvent (solvothermal) in a
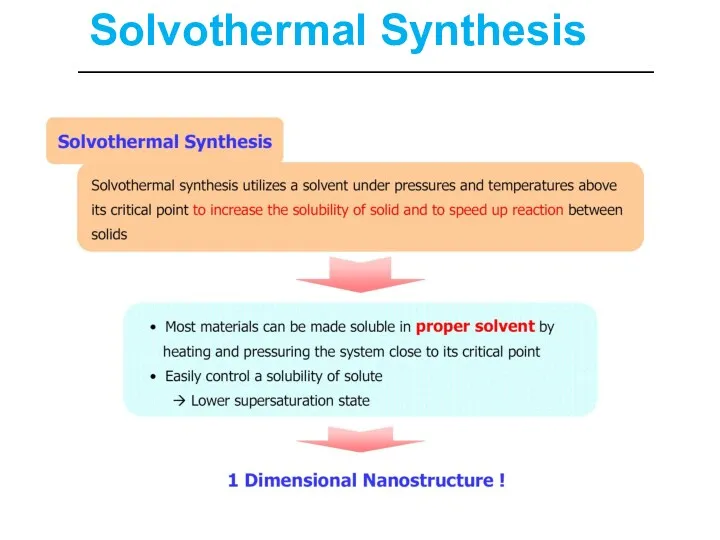
- 32. Solvothermal Synthesis
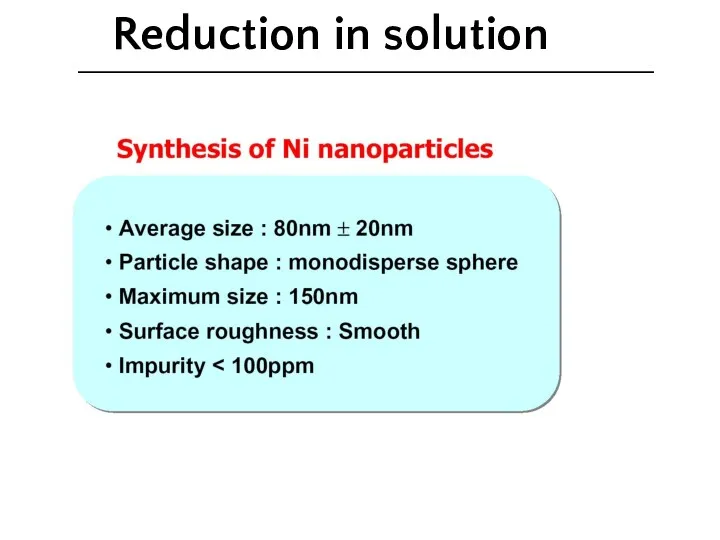
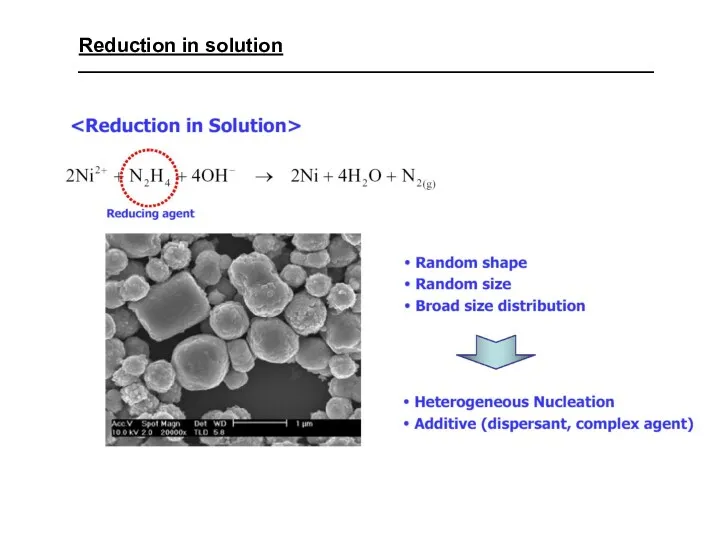
- 33. Reduction in solution
- 34. Reduction in solution
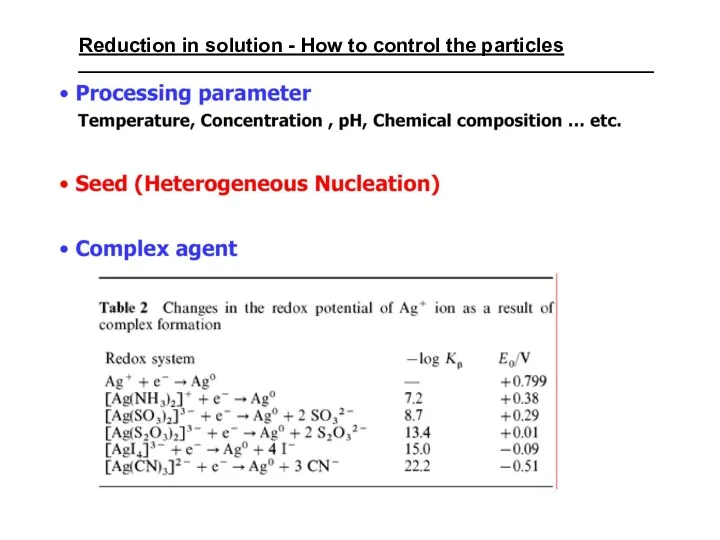
- 35. Reduction in solution - How to control the particles
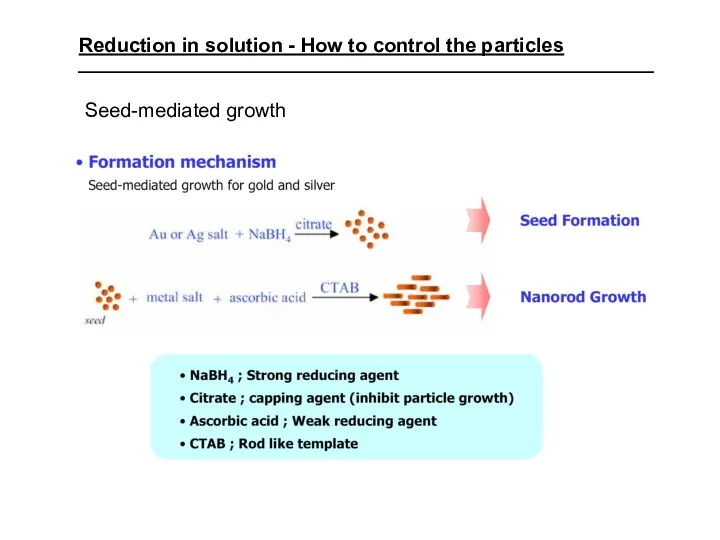
- 36. Reduction in solution - How to control the particles Seed-mediated growth
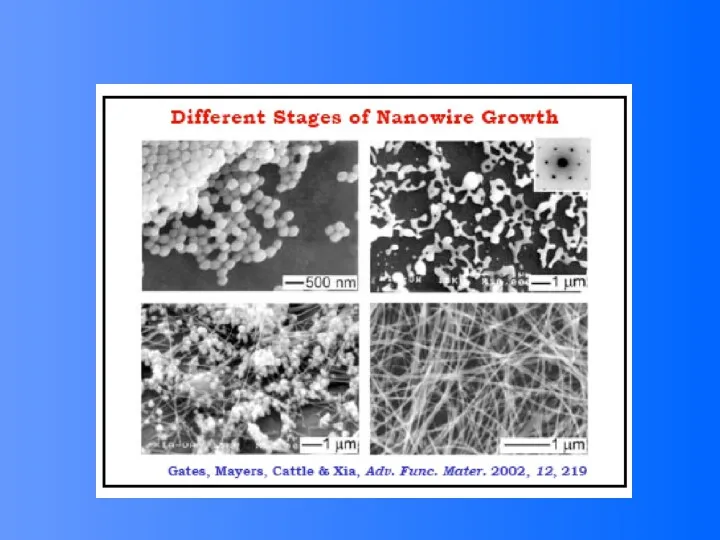
- 37. One dimensional nanostructures Nanowires Nanotubes “They represent the smallest dimension for efficient transport of electrons and
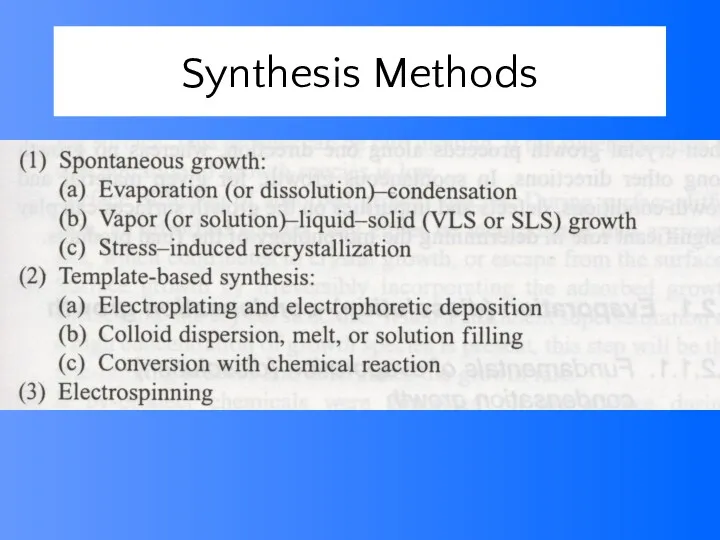
- 38. Synthesis Methods
- 39. Spontaneous Growth A growth driven by reduction of Gibbs free energy or chemical potential. This can
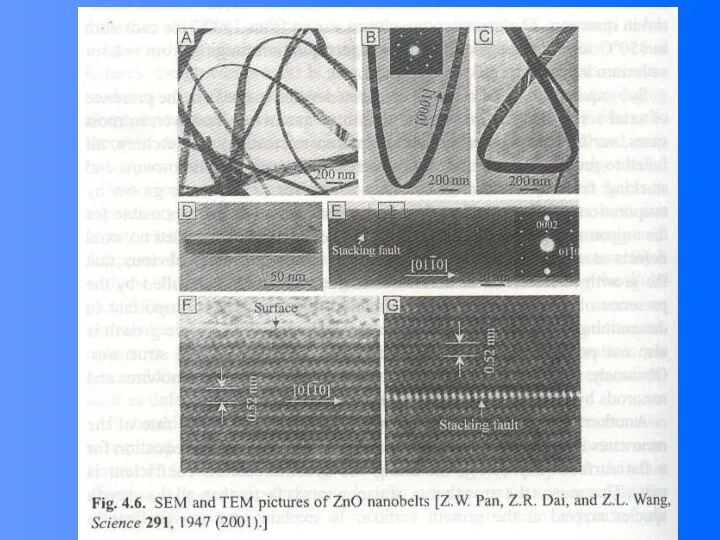
- 40. Growth of Single Crystal Nanobelts of Semiconducting or metal oxides Evaporating the metal oxides (ZnO, SnO2,
- 43. By controlling growth kinetics, a consequence of minimizing the total energy attributed by spontaneous polarization and
- 44. Dissolution and Condensation Growth The growth species first dissolve into a solvent or a solution, and
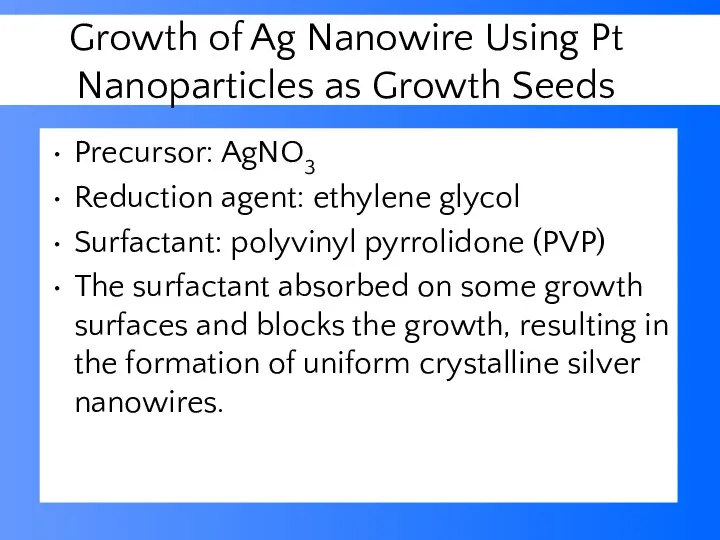
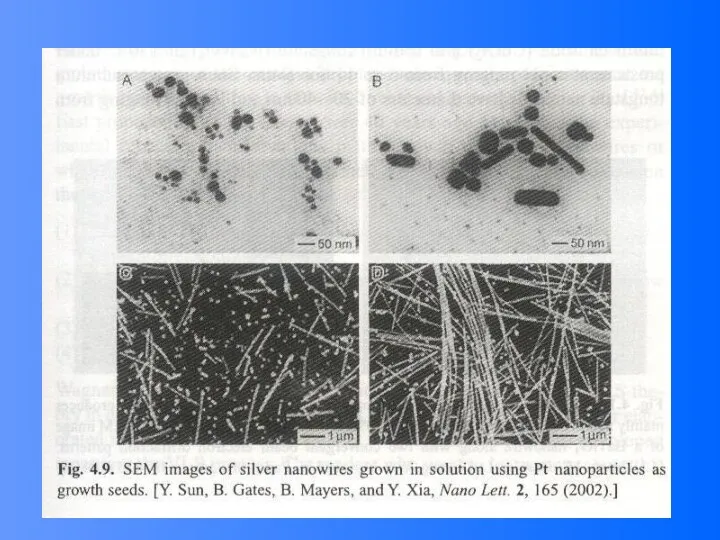
- 45. Growth of Ag Nanowire Using Pt Nanoparticles as Growth Seeds Precursor: AgNO3 Reduction agent: ethylene glycol
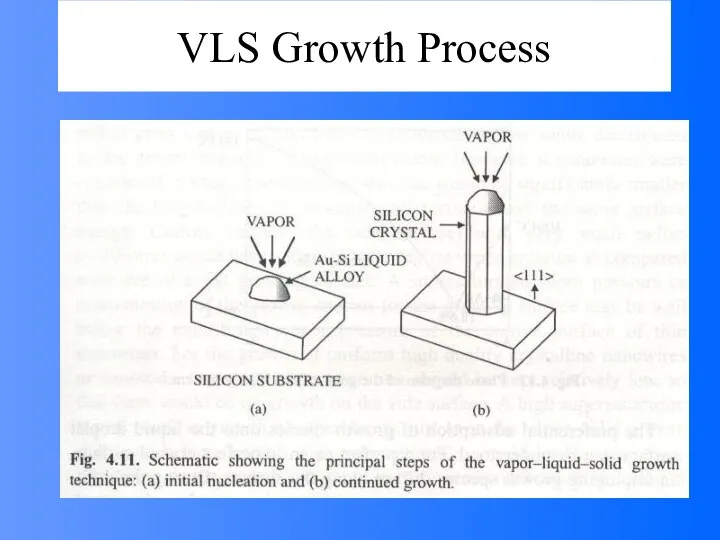
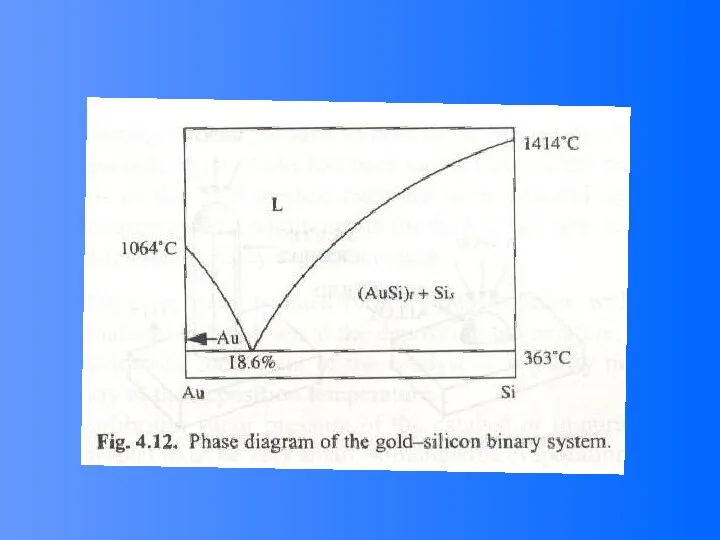
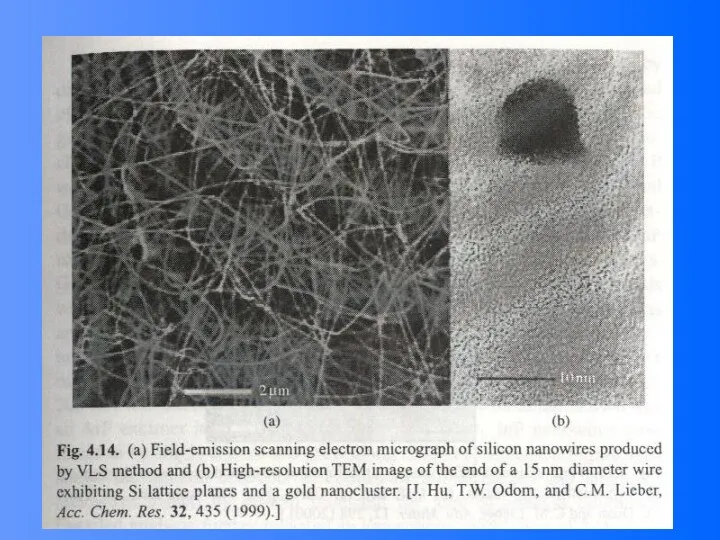
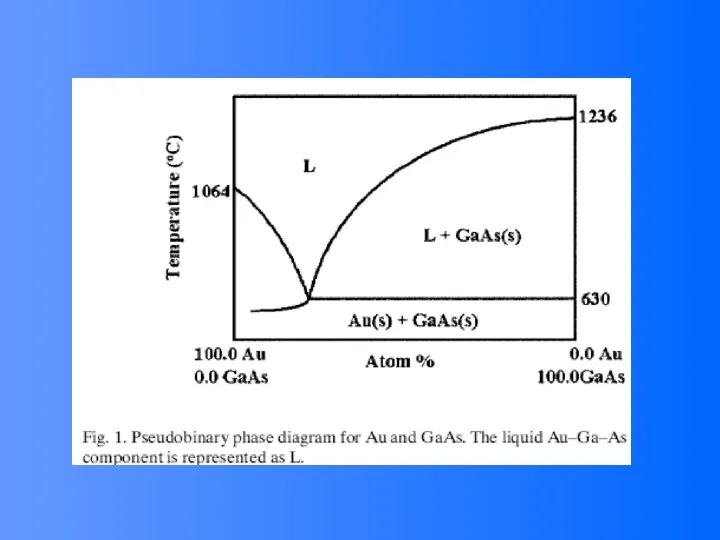
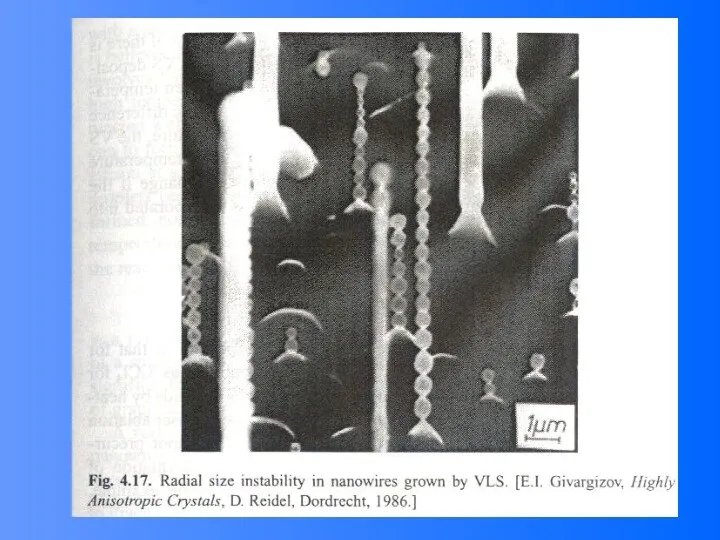
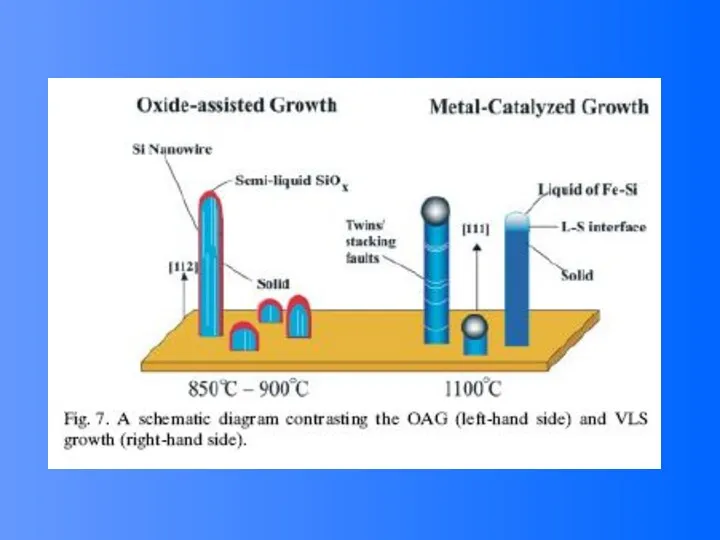
- 48. Vapor (or solution)-Liquid-solid (VLS) Growth It is noted that the surface of liquid has a large
- 49. VLS Growth Process
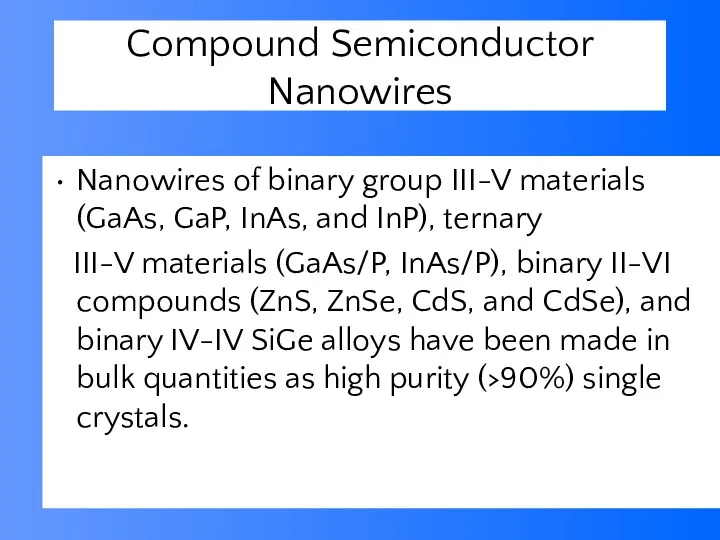
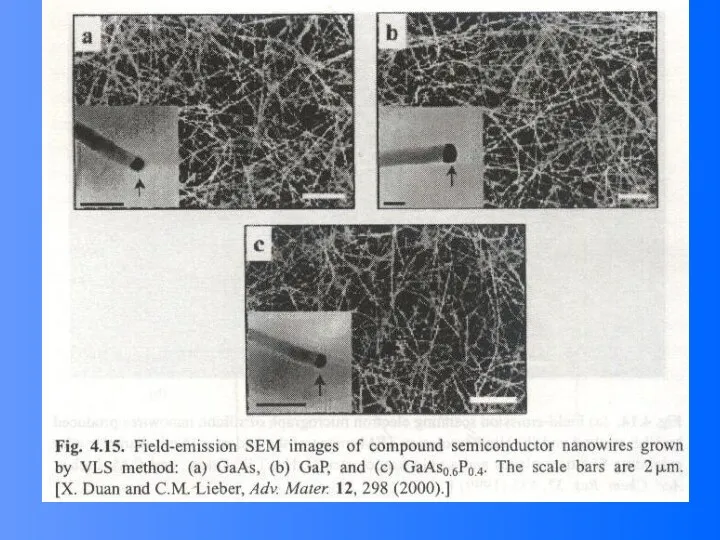
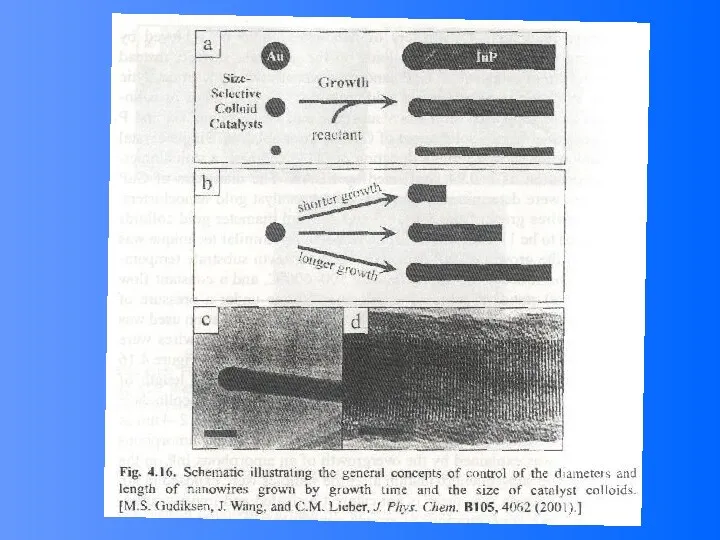
- 52. Compound Semiconductor Nanowires Nanowires of binary group III-V materials (GaAs, GaP, InAs, and InP), ternary III-V
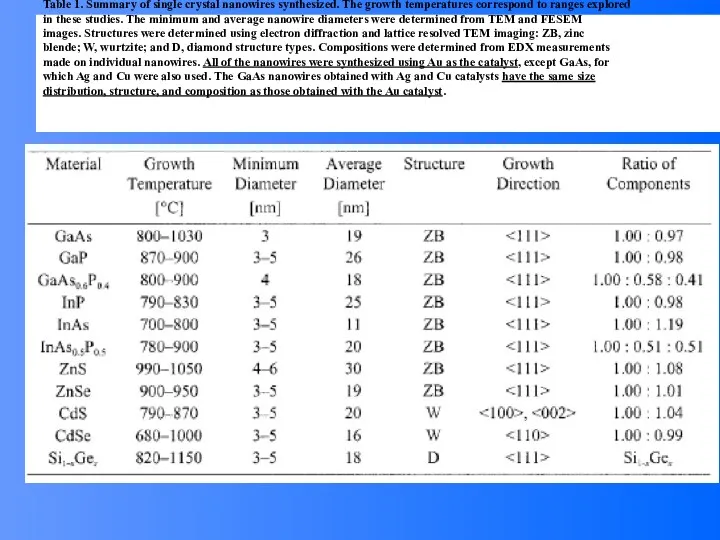
- 54. Table 1. Summary of single crystal nanowires synthesized. The growth temperatures correspond to ranges explored in
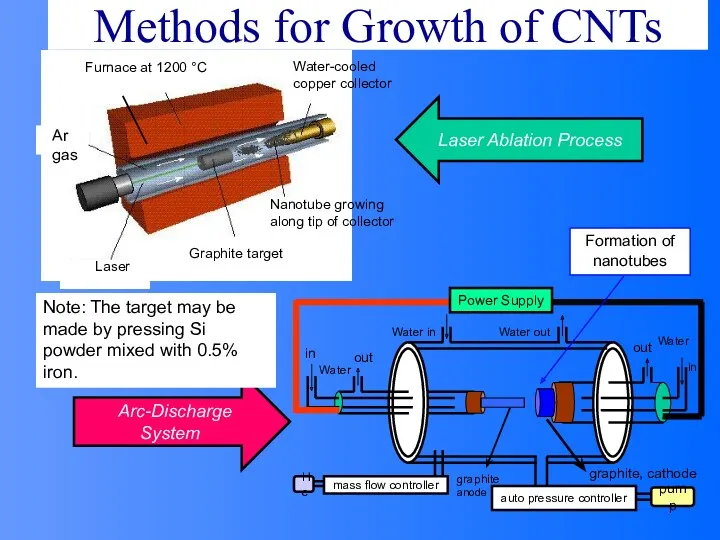
- 59. Methods for Growth of CNTs Formation of nanotubes Note: The target may be made by pressing
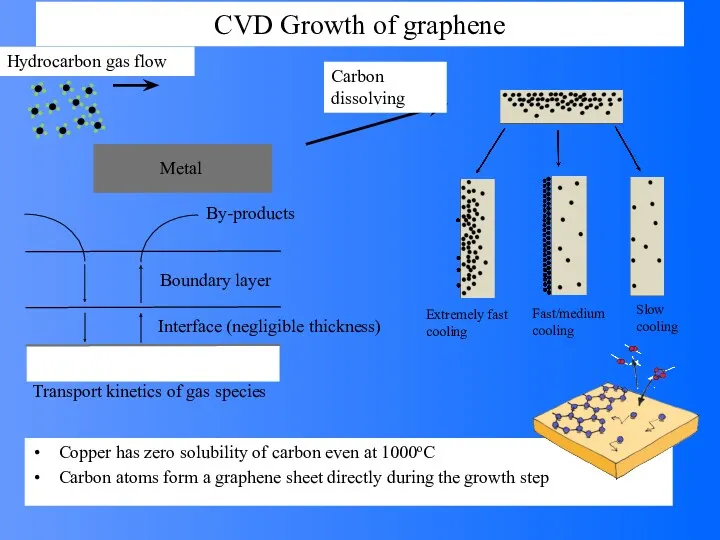
- 60. CVD Growth of graphene Hydrocarbon gas flow Carbon dissolving Metal Copper has zero solubility of carbon
- 61. Template assisted nanowire growth Create a template for nanowires to grow within Based on aluminum’s unique
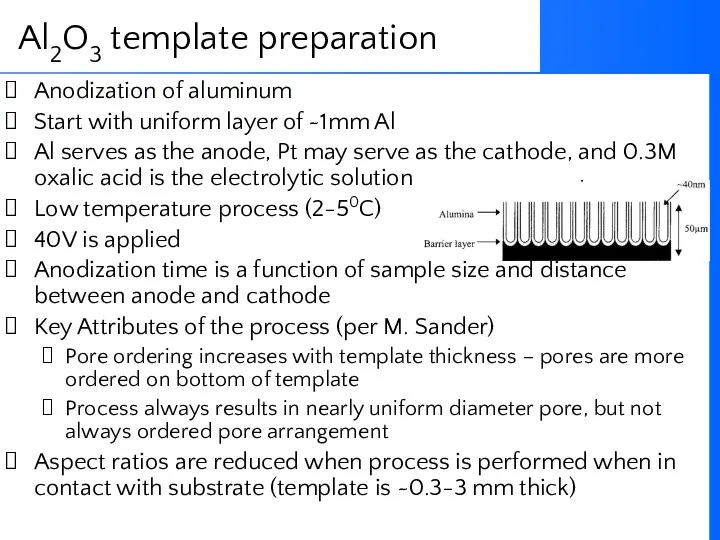
- 62. Anodization of aluminum Start with uniform layer of ~1mm Al Al serves as the anode, Pt
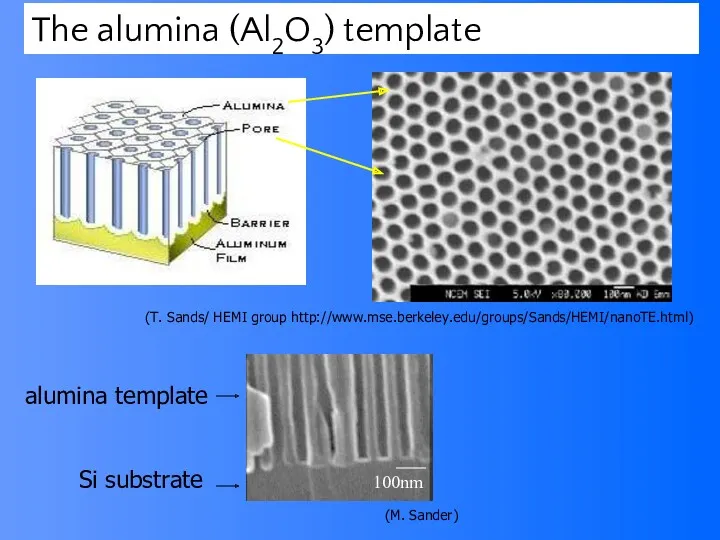
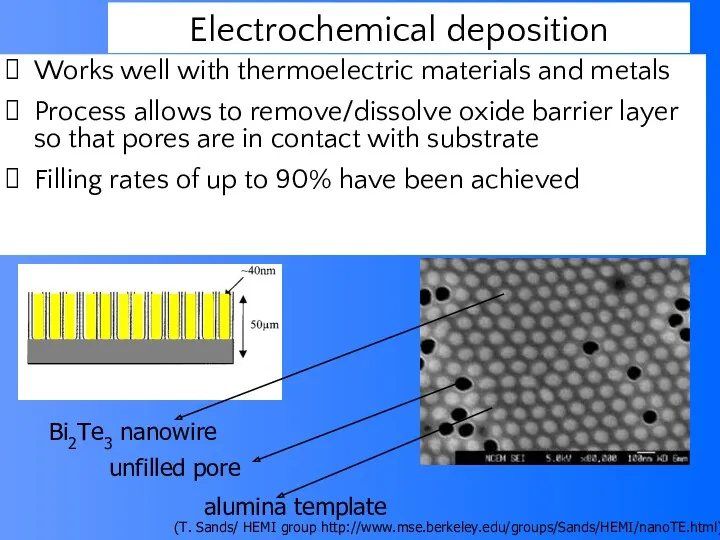
- 63. (T. Sands/ HEMI group http://www.mse.berkeley.edu/groups/Sands/HEMI/nanoTE.html) The alumina (Al2O3) template 100nm Si substrate alumina template (M. Sander)
- 64. Works well with thermoelectric materials and metals Process allows to remove/dissolve oxide barrier layer so that
- 66. Скачать презентацию














 Welcome to our Christmas (quiz)
Welcome to our Christmas (quiz) Modal verbs in school life
Modal verbs in school life How to fix grammar mistakes
How to fix grammar mistakes Present Perfect Tense
Present Perfect Tense Как написать письмо? Почтовая открытка: задание
Как написать письмо? Почтовая открытка: задание Система вправ для навчання іноземноі мови
Система вправ для навчання іноземноі мови The competition for English language Learn to win. MY IDOL
The competition for English language Learn to win. MY IDOL Guess these colour idioms 2
Guess these colour idioms 2 Сравнение полицейских
Сравнение полицейских Глагол TO HAVE
Глагол TO HAVE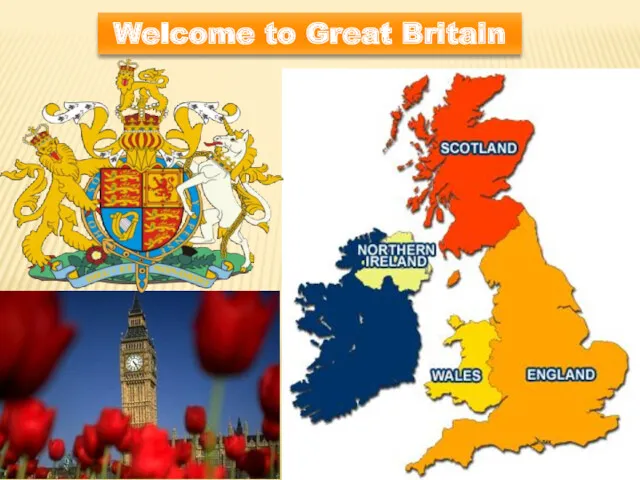 Welcome to Great Britain
Welcome to Great Britain Look outside. How’s the weather?
Look outside. How’s the weather? Сложное дополнение
Сложное дополнение Famous people of Great Britain
Famous people of Great Britain Environmental Protection ( Охрана окружающей среды). 7 класс
Environmental Protection ( Охрана окружающей среды). 7 класс Marking Punctuation
Marking Punctuation My favorite holiday
My favorite holiday Plan for today
Plan for today How to shop propertly
How to shop propertly St. Petersburg architecture
St. Petersburg architecture Content and language integrated learning
Content and language integrated learning Повторение по английскому языку
Повторение по английскому языку Согласование времен в английском языке
Согласование времен в английском языке Условные предложения. Conditionals
Условные предложения. Conditionals He loves jelly (lesson 20)
He loves jelly (lesson 20) Food. Еда
Food. Еда Irregular plurals
Irregular plurals Chestnut
Chestnut