Содержание
- 2. Biology course The chemicals of life. Water and its properties. Biological molecules: carbohydrates. Biomolecules. Lipids: cholesterol,
- 3. Molecular genetics. Trancription and translation Genetic technology. Gene cloning and protein expression. Agarose gel electrophoresis. PCR.
- 4. Biomolecules
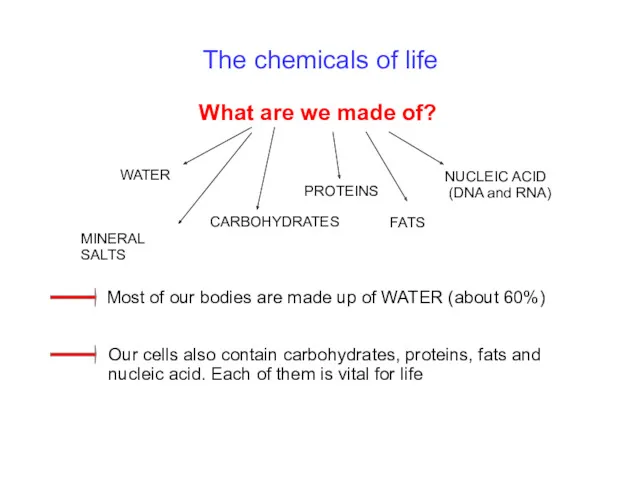
- 5. The chemicals of life What are we made of? WATER CARBOHYDRATES PROTEINS FATS NUCLEIC ACID (DNA
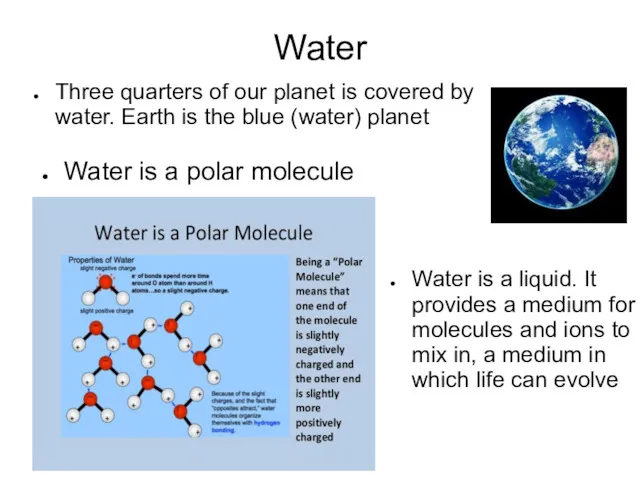
- 6. Water Three quarters of our planet is covered by water. Earth is the blue (water) planet
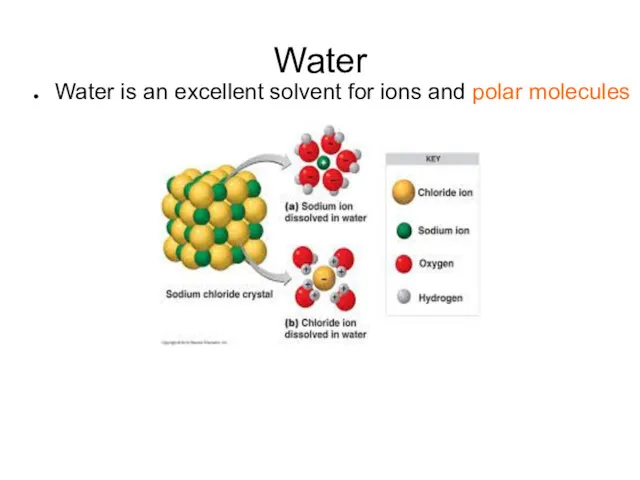
- 7. Water Water is an excellent solvent for ions and polar molecules
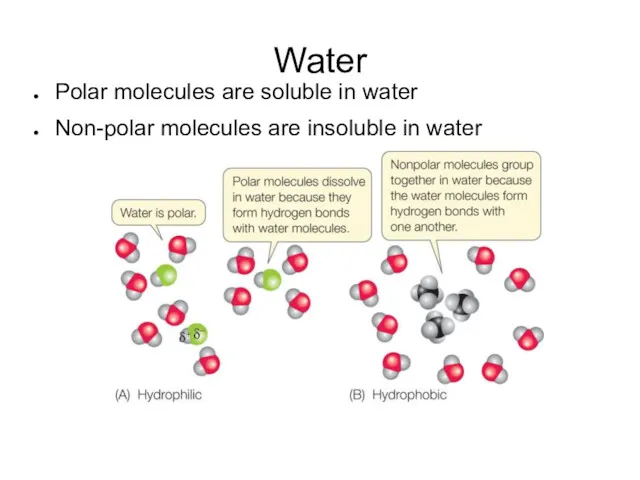
- 8. Water Polar molecules are soluble in water Non-polar molecules are insoluble in water
- 9. Inside every living organism metabolic reactions can only take place if the chemicals are dissolved in
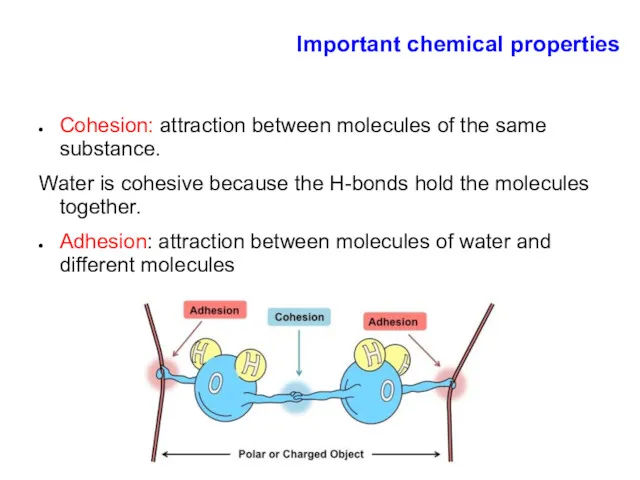
- 10. Important chemical properties Cohesion: attraction between molecules of the same substance. Water is cohesive because the
- 11. Cohesion results in Surface tension: a measure of the strength of water's surface Important chemical properties
- 12. Surface tension
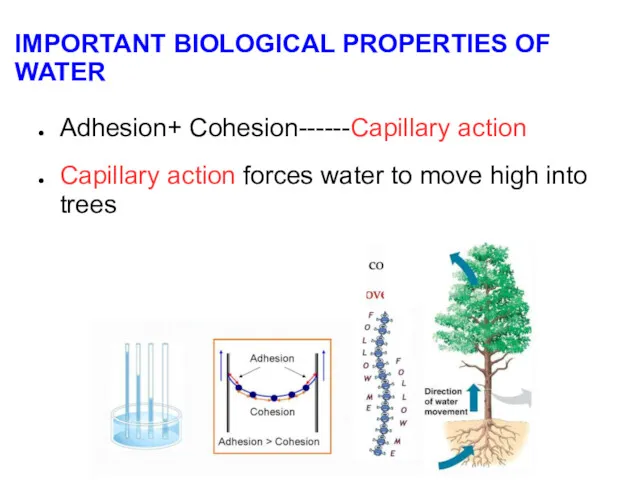
- 13. IMPORTANT BIOLOGICAL PROPERTIES OF WATER Adhesion+ Cohesion------Capillary action Capillary action forces water to move high into
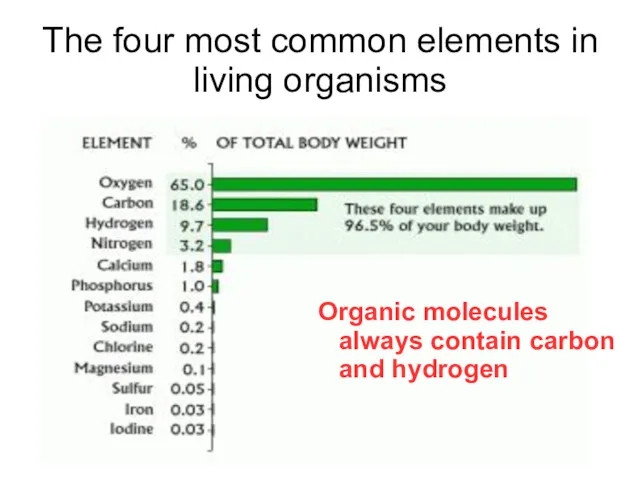
- 14. The four most common elements in living organisms Organic molecules always contain carbon and hydrogen
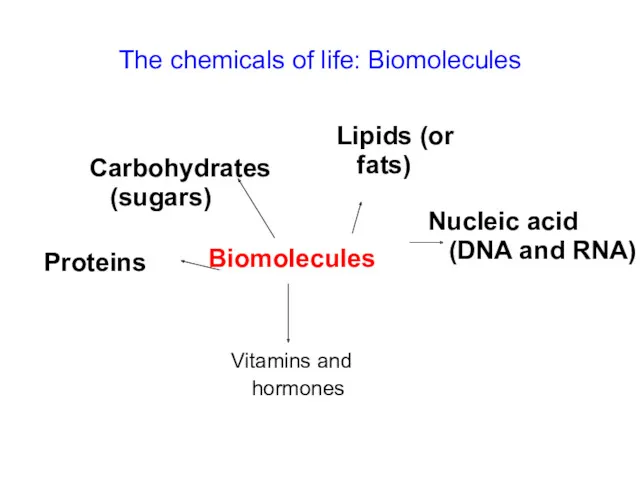
- 15. The chemicals of life: Biomolecules Biomolecules Carbohydrates (sugars) Vitamins and hormones Proteins Nucleic acid (DNA and
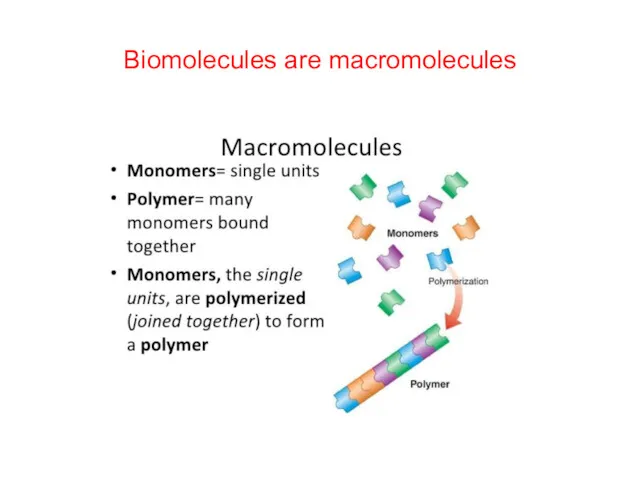
- 16. Biomolecules are macromolecules
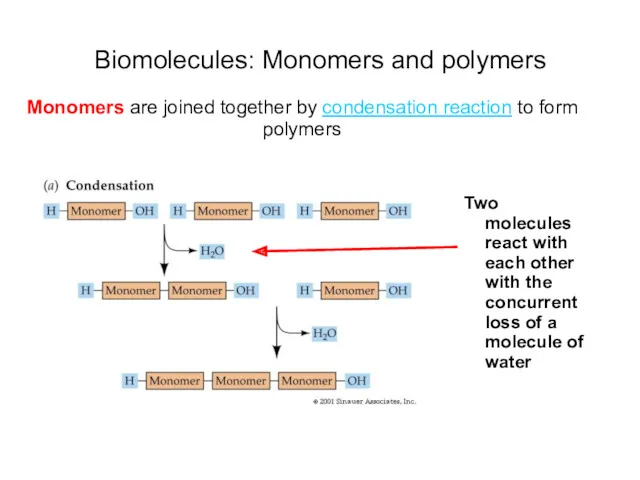
- 17. Biomolecules: Monomers and polymers Monomers are joined together by condensation reaction to form polymers Two molecules
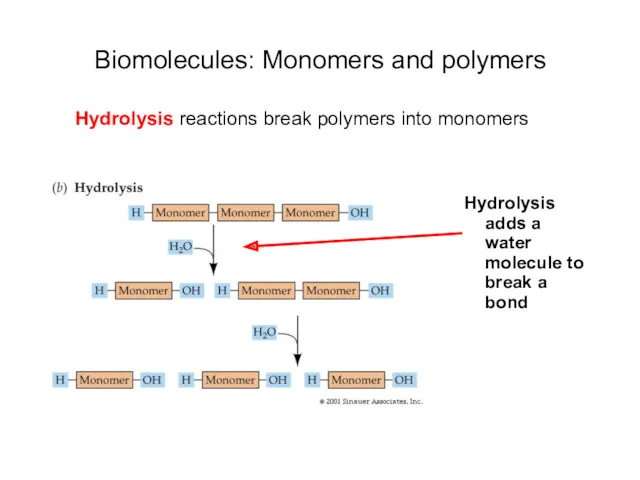
- 18. Biomolecules: Monomers and polymers Hydrolysis adds a water molecule to break a bond Hydrolysis reactions break
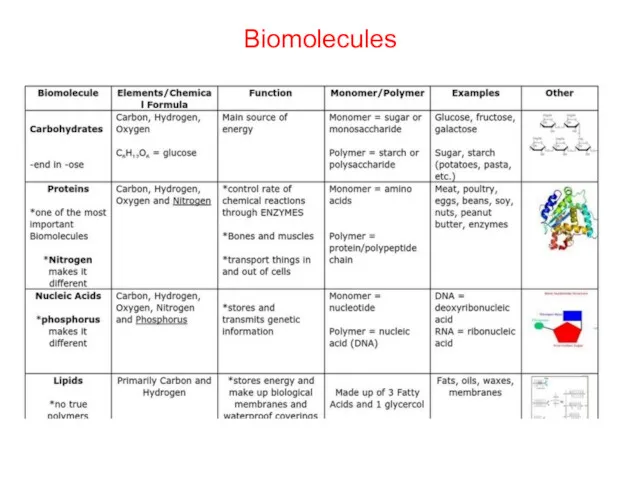
- 19. Biomolecules
- 20. Carbohydrates
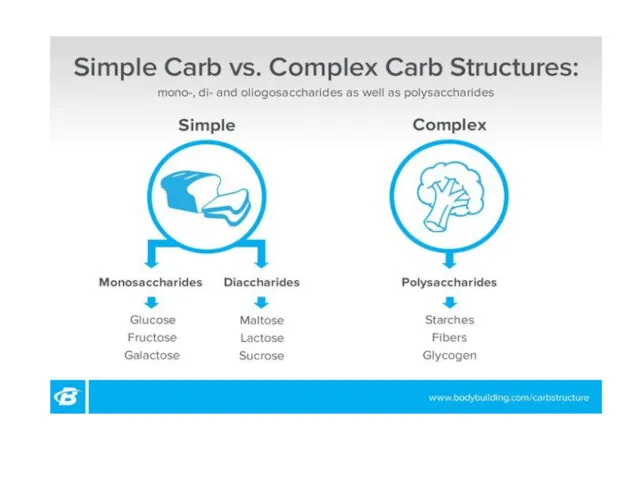
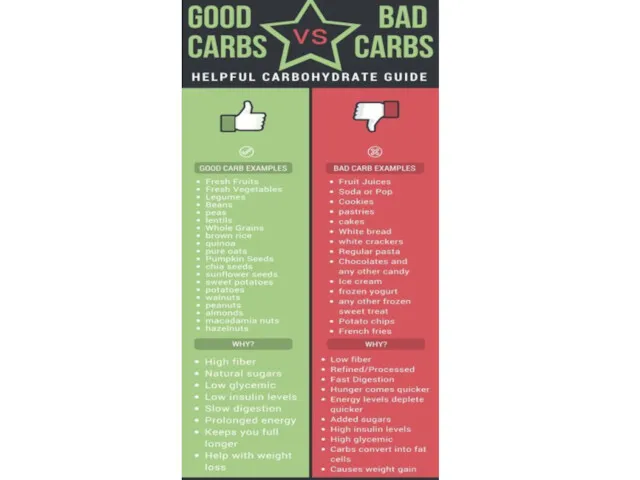
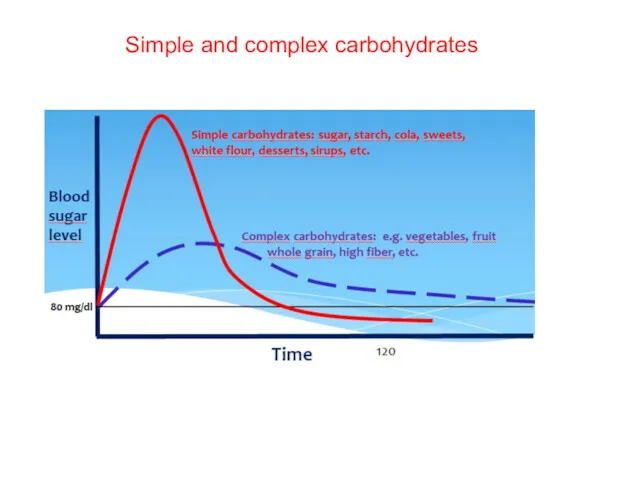
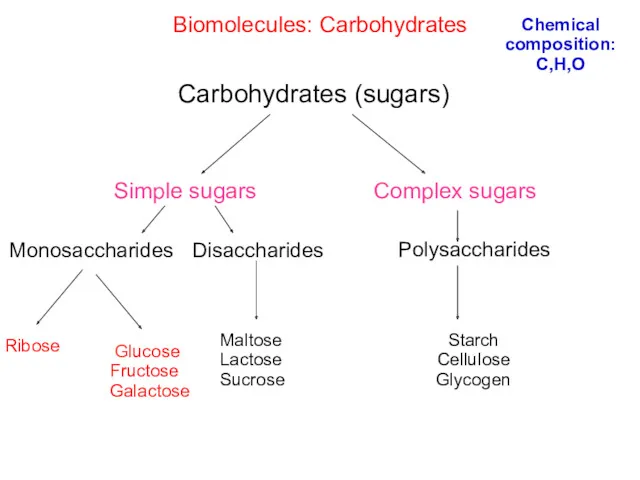
- 24. Simple and complex carbohydrates
- 25. Biomolecules: Carbohydrates Simple sugars Carbohydrates (sugars) Complex sugars Monosaccharides Starch Cellulose Glycogen Disaccharides Polysaccharides Ribose Maltose
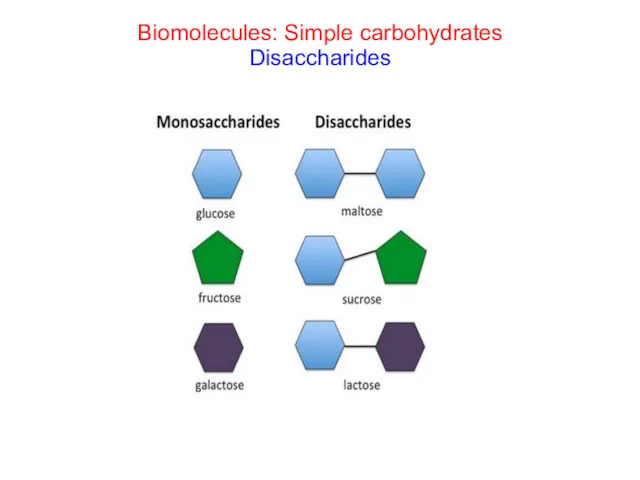
- 26. Biomolecules: Simple carbohydrates Disaccharides
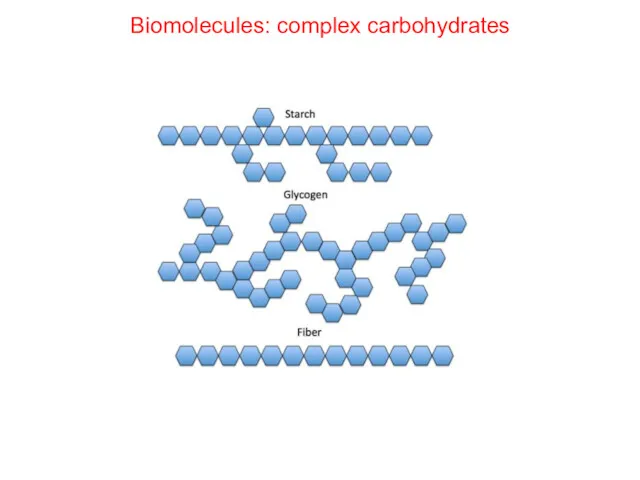
- 27. Biomolecules: complex carbohydrates
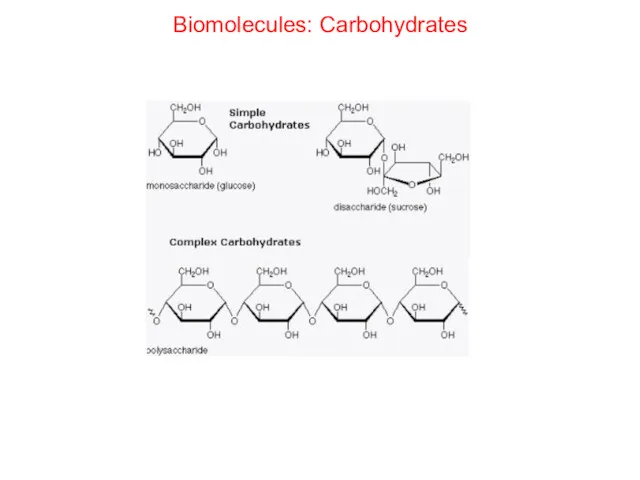
- 28. Biomolecules: Carbohydrates
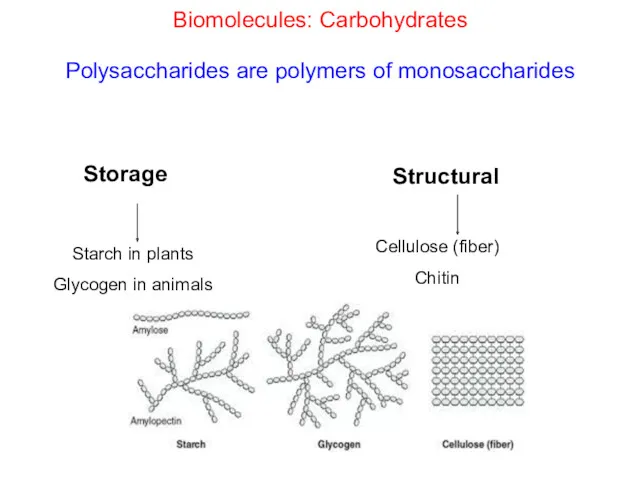
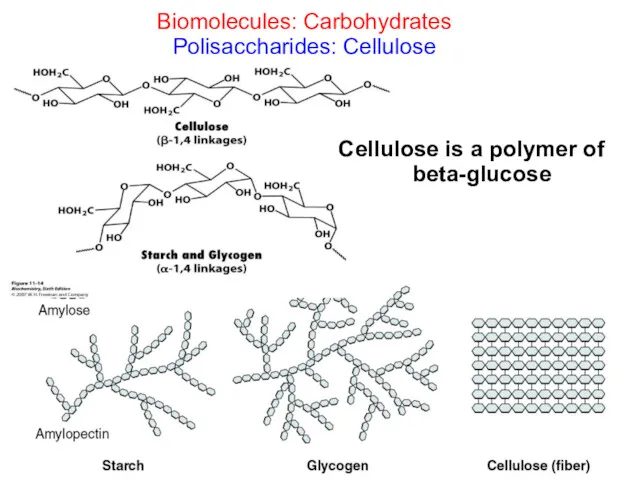
- 29. Biomolecules: Carbohydrates Polysaccharides are polymers of monosaccharides Structural Starch in plants Glycogen in animals Cellulose (fiber)
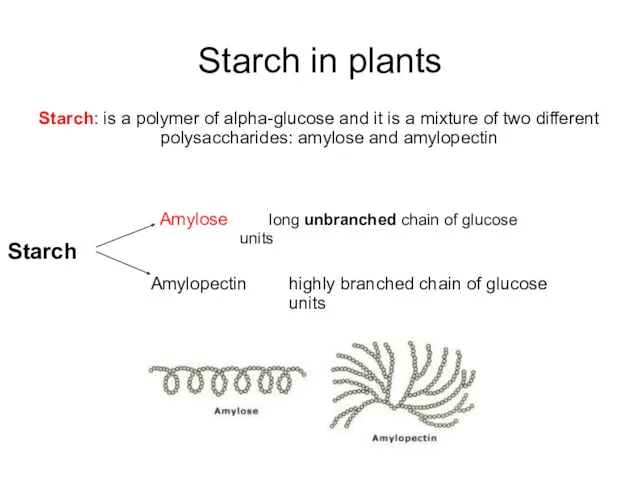
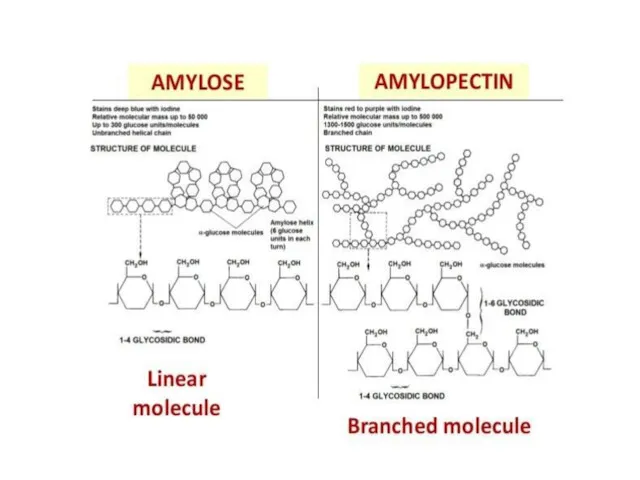
- 30. Starch in plants Starch: is a polymer of alpha-glucose and it is a mixture of two
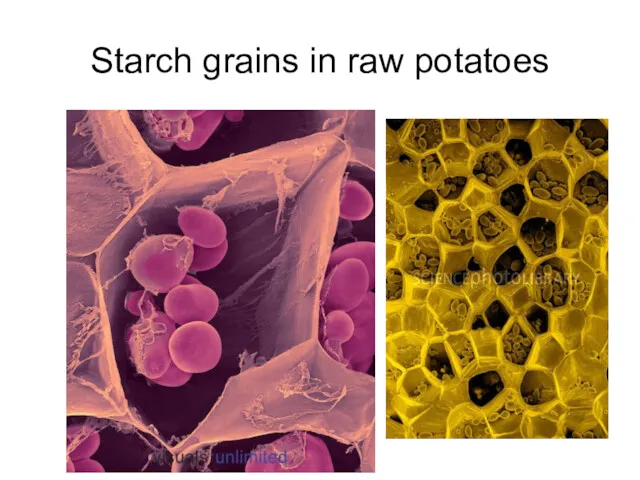
- 32. Starch grains in raw potatoes
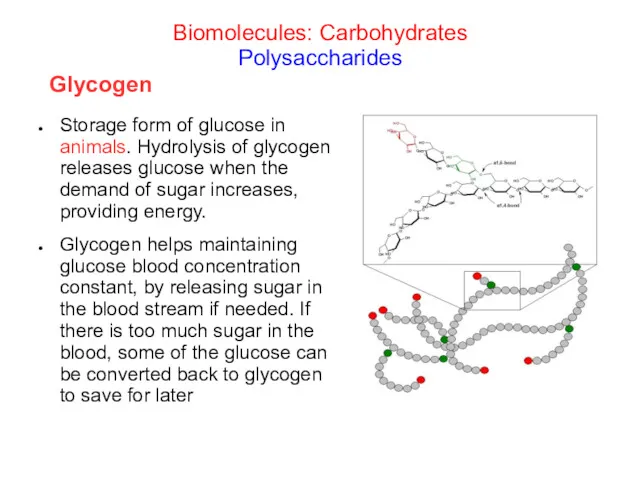
- 33. Storage form of glucose in animals. Hydrolysis of glycogen releases glucose when the demand of sugar
- 34. Biomolecules: Carbohydrates Polisaccharides: Cellulose Cellulose is a polymer of beta-glucose
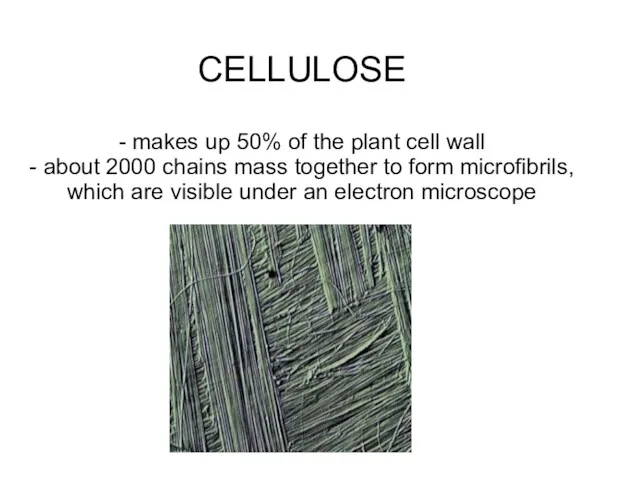
- 35. CELLULOSE - makes up 50% of the plant cell wall - about 2000 chains mass together
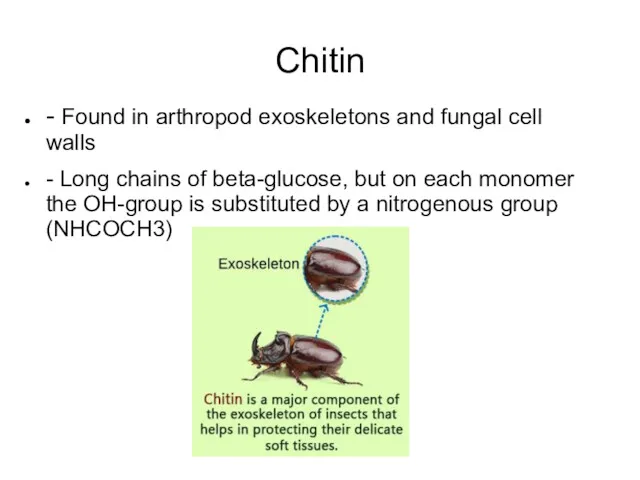
- 37. Chitin - Found in arthropod exoskeletons and fungal cell walls - Long chains of beta-glucose, but
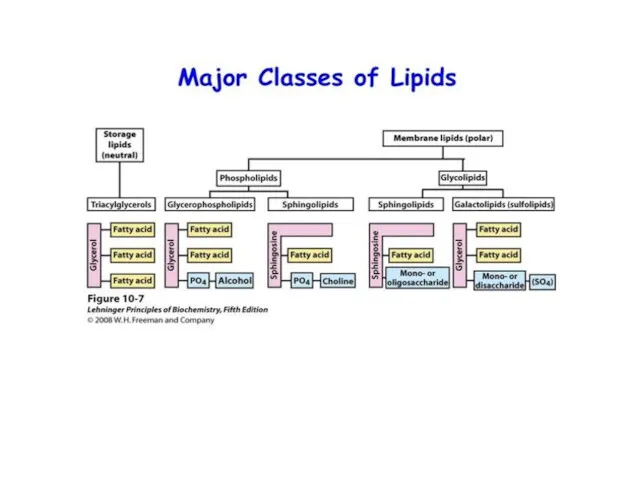
- 38. Lipids
- 39. Lipids Lipids are a very varied group of chemicals They are all organic molecules that are
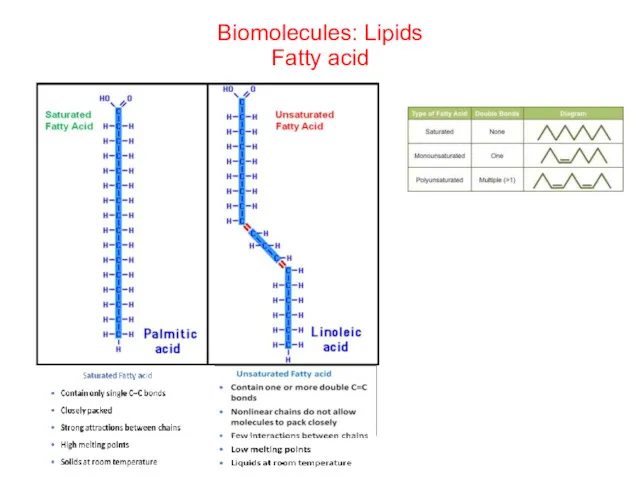
- 41. Biomolecules: Lipids Fatty acid
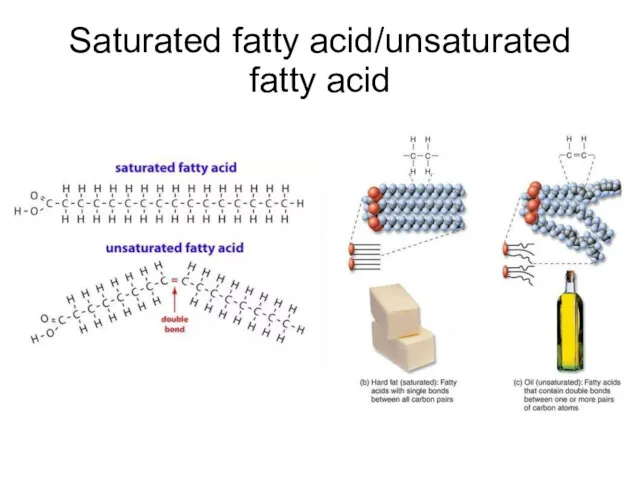
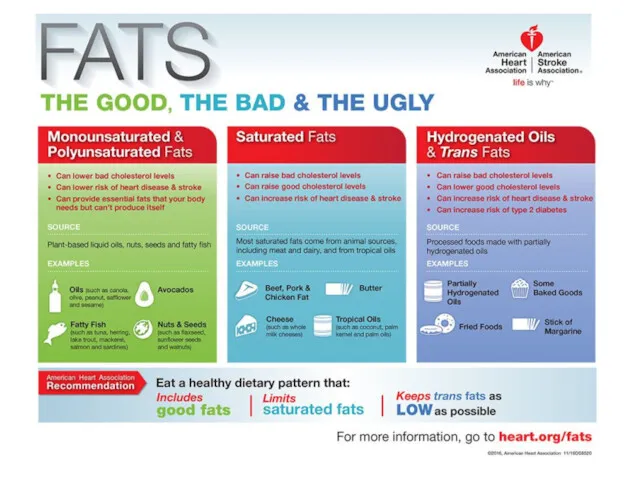
- 42. Saturated fatty acid/unsaturated fatty acid
- 43. Saturated fatty acid/unsaturated fatty acid
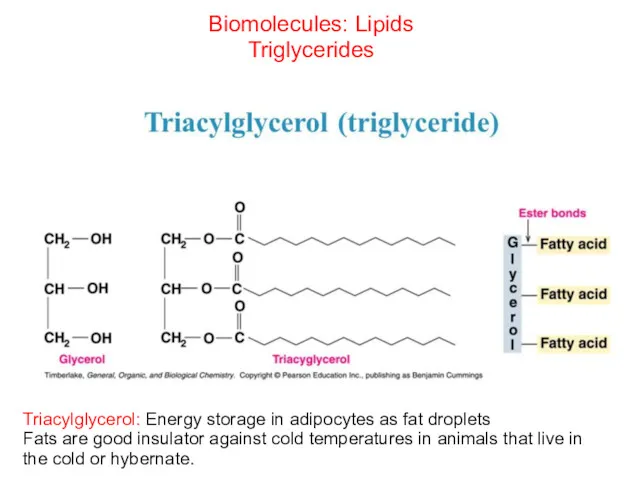
- 45. Biomolecules: Lipids Triglycerides Triacylglycerol: Energy storage in adipocytes as fat droplets Fats are good insulator against
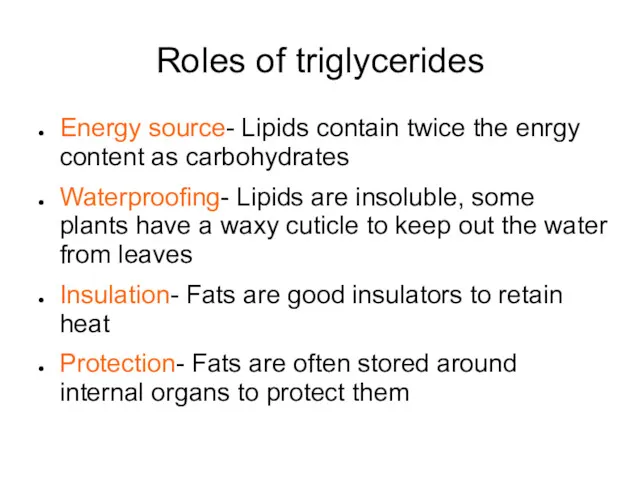
- 46. Roles of triglycerides Energy source- Lipids contain twice the enrgy content as carbohydrates Waterproofing- Lipids are
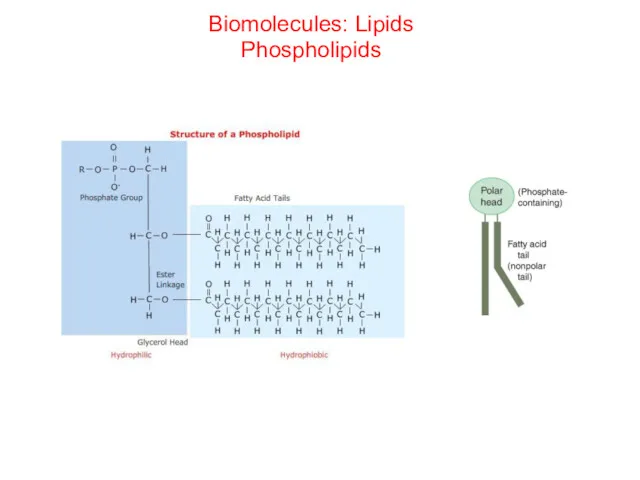
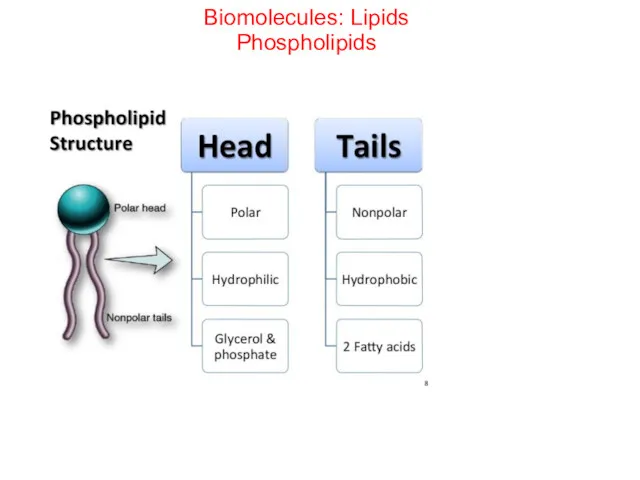
- 47. Biomolecules: Lipids Phospholipids
- 48. Biomolecules: Lipids Phospholipids
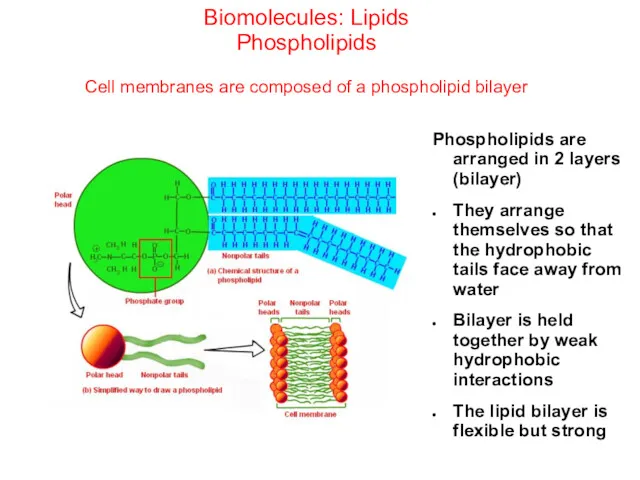
- 49. Biomolecules: Lipids Phospholipids Cell membranes are composed of a phospholipid bilayer Phospholipids are arranged in 2
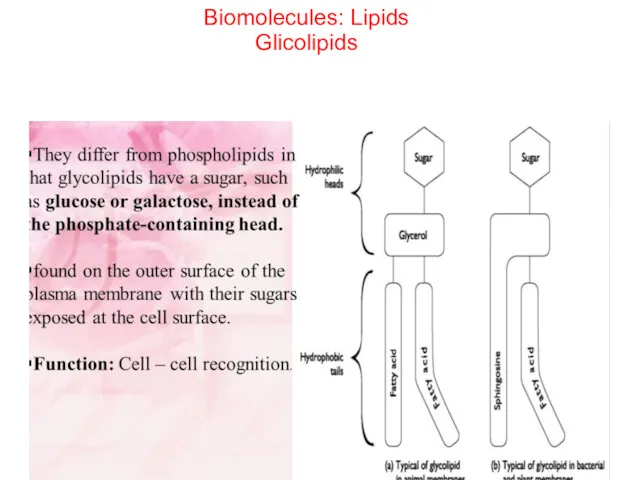
- 50. Biomolecules: Lipids Glicolipids
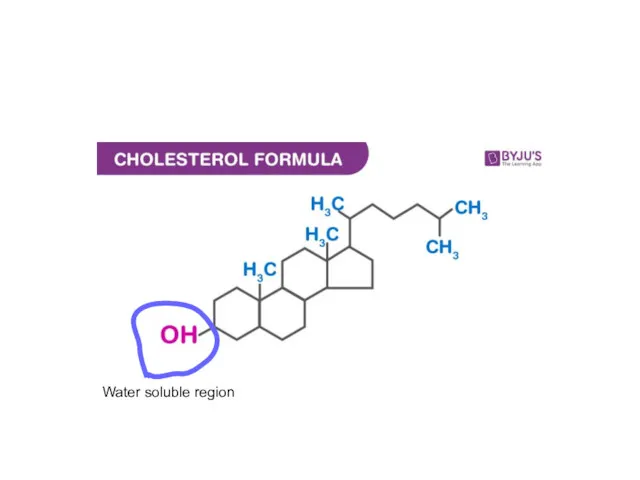
- 51. Biomolecules: Lipids Wax and steroids
- 52. Water soluble region
- 53. Proteins
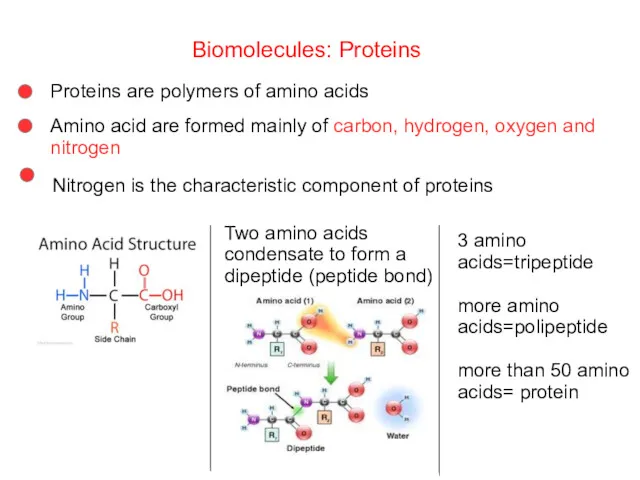
- 54. Biomolecules: Proteins Proteins are polymers of amino acids Amino acid are formed mainly of carbon, hydrogen,
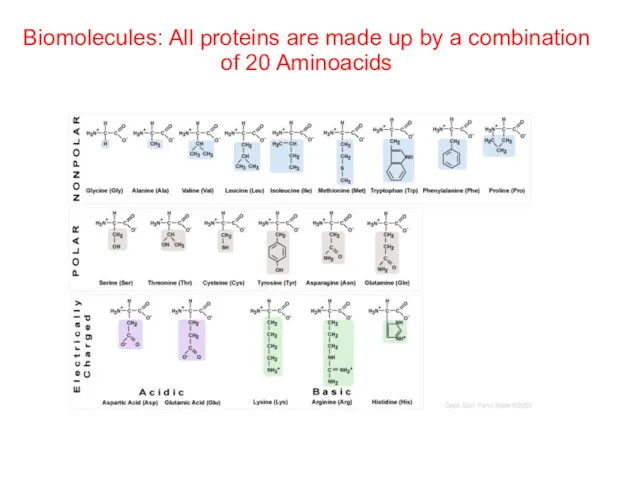
- 55. Biomolecules: All proteins are made up by a combination of 20 Aminoacids
- 56. Biomolecules: Essential aminoacids Arginine and Histidine are semi-essential. They can be synthesized by adults but not
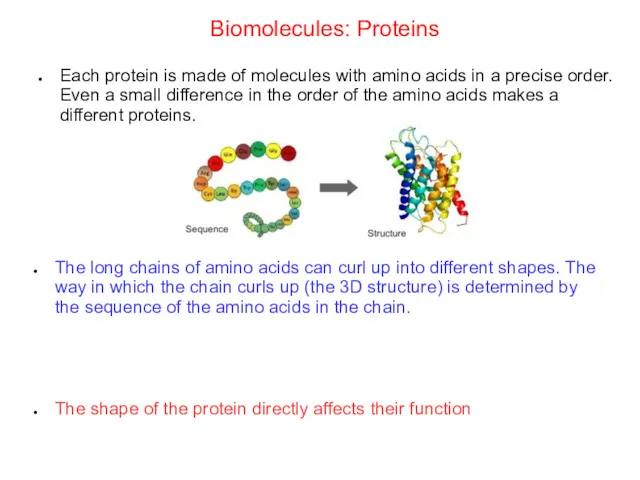
- 57. Biomolecules: Proteins Each protein is made of molecules with amino acids in a precise order. Even
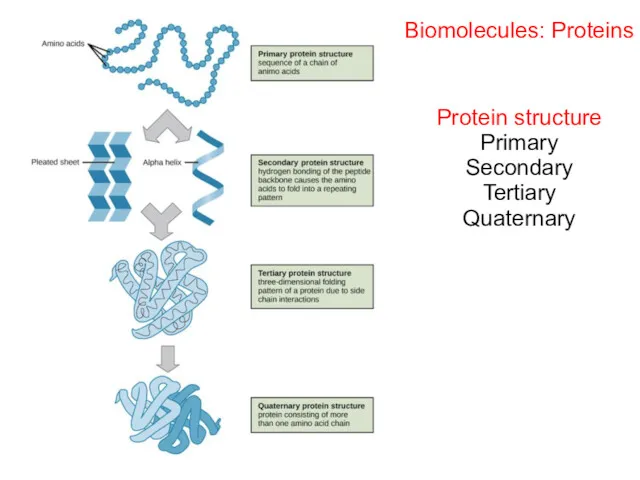
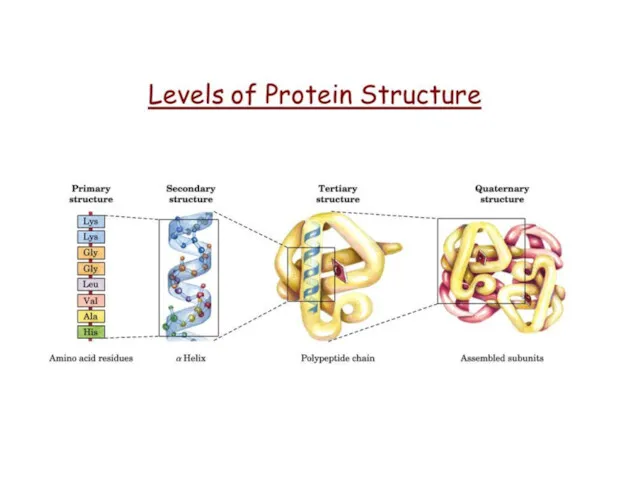
- 58. Biomolecules: Proteins Protein structure Primary Secondary Tertiary Quaternary
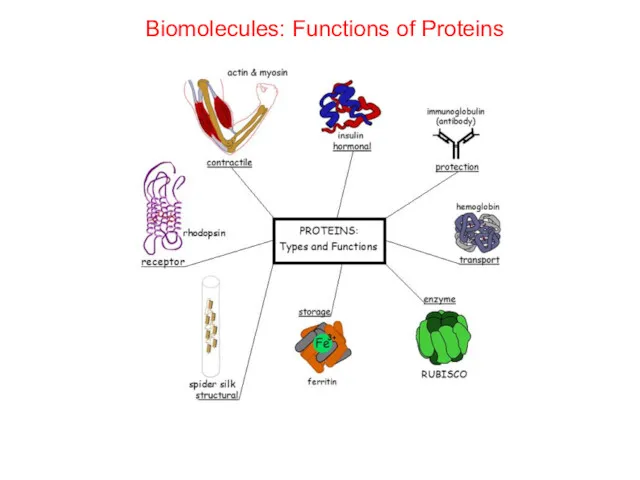
- 59. Biomolecules: Functions of Proteins
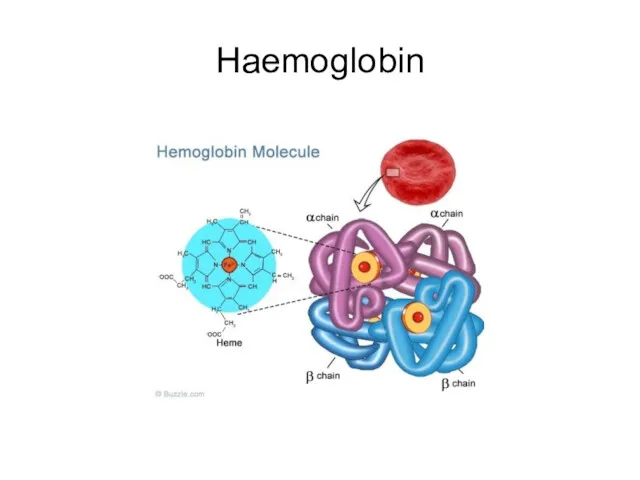
- 61. Haemoglobin
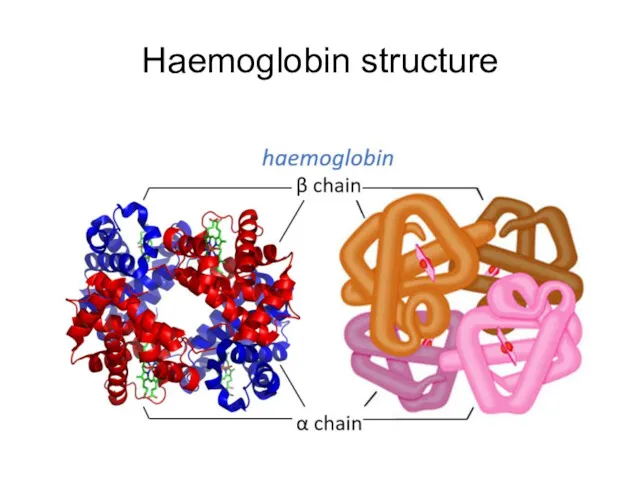
- 62. Haemoglobin structure
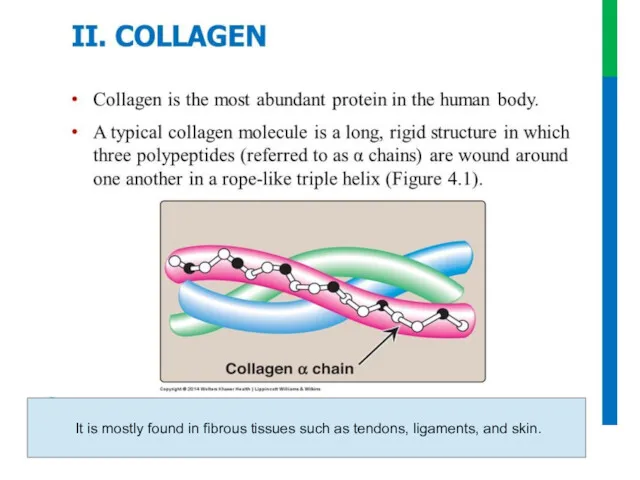
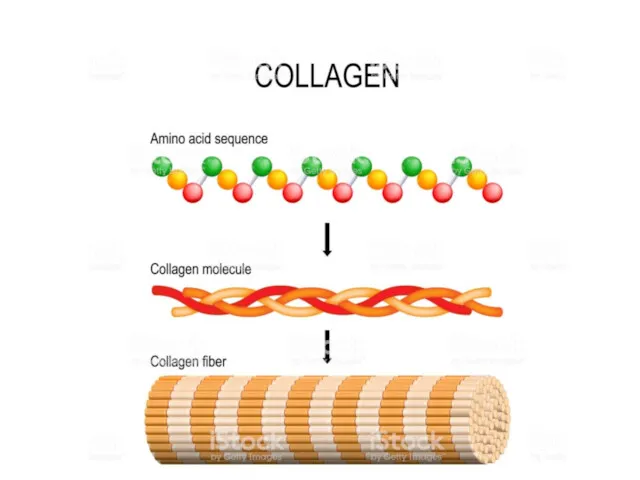
- 63. It is mostly found in fibrous tissues such as tendons, ligaments, and skin.
- 64. Collagen
- 65. Nucleic acids
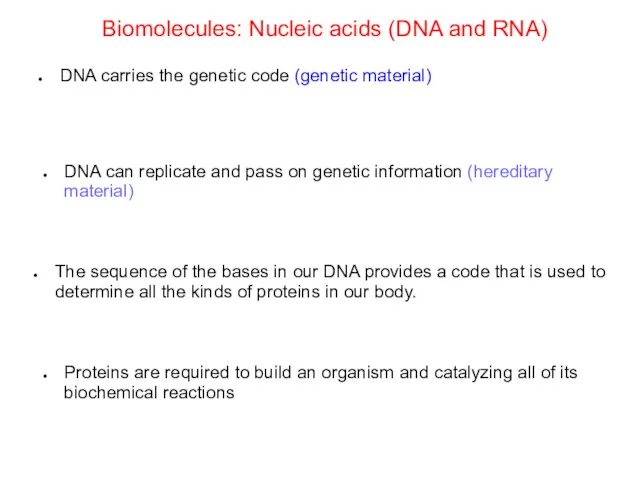
- 66. Biomolecules: Nucleic acids (DNA and RNA) DNA carries the genetic code (genetic material) DNA can replicate
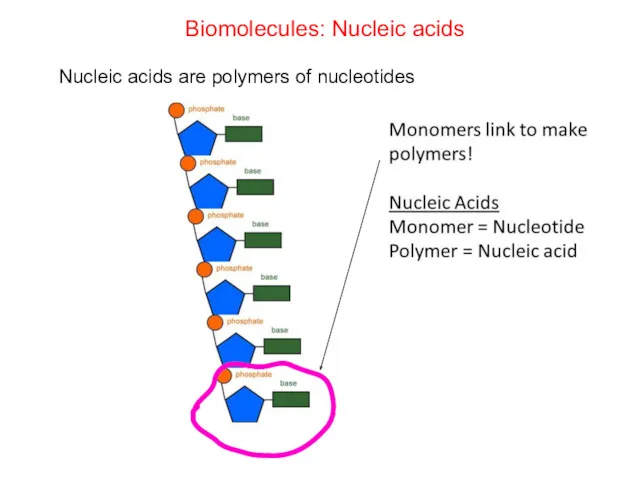
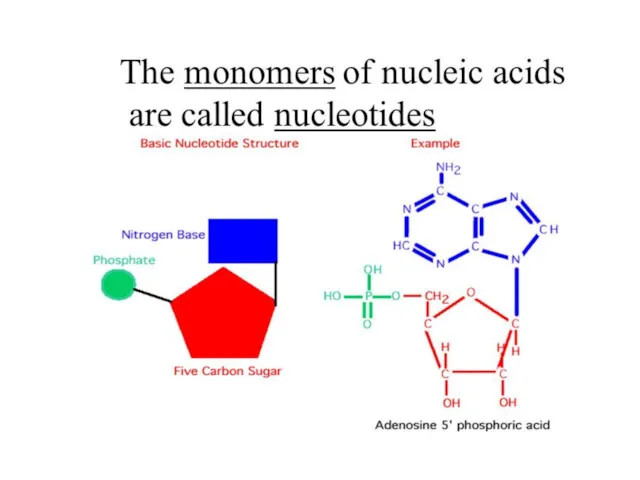
- 67. Biomolecules: Nucleic acids Nucleic acids are polymers of nucleotides
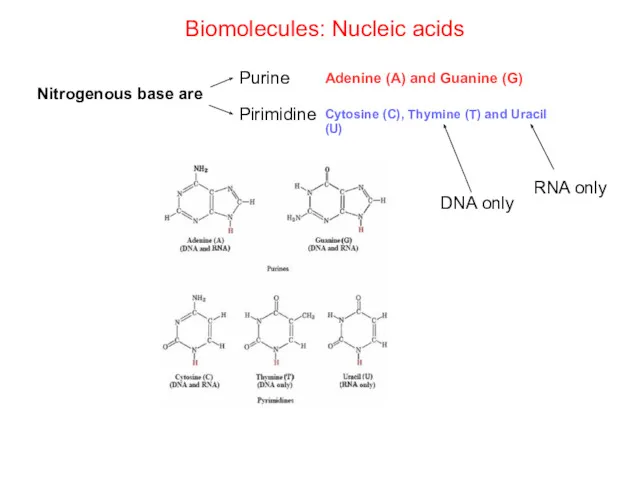
- 69. Biomolecules: Nucleic acids Nitrogenous base are Purine Adenine (A) and Guanine (G) Pirimidine Cytosine (C), Thymine
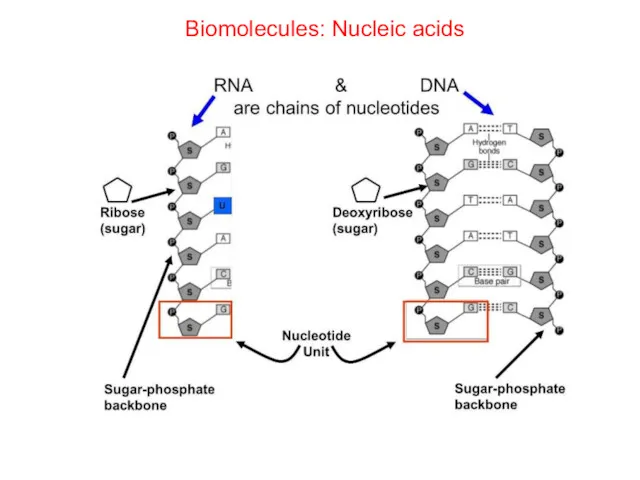
- 70. Biomolecules: Nucleic acids
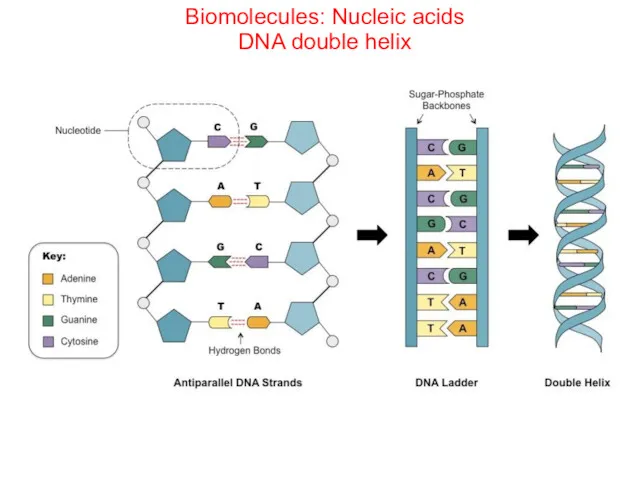
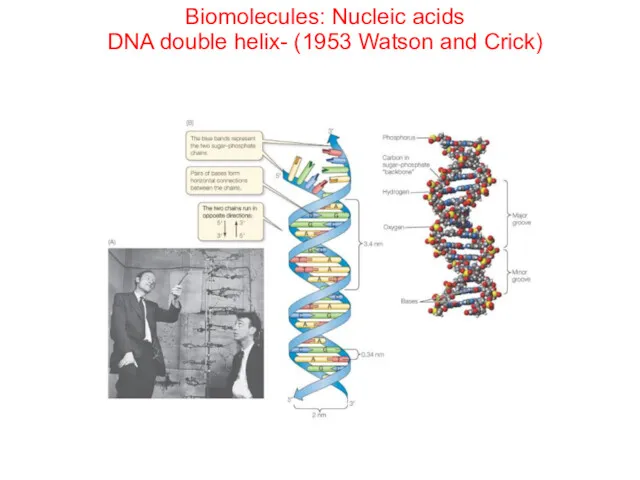
- 71. Biomolecules: Nucleic acids DNA double helix
- 72. Biomolecules: Nucleic acids DNA double helix- (1953 Watson and Crick)
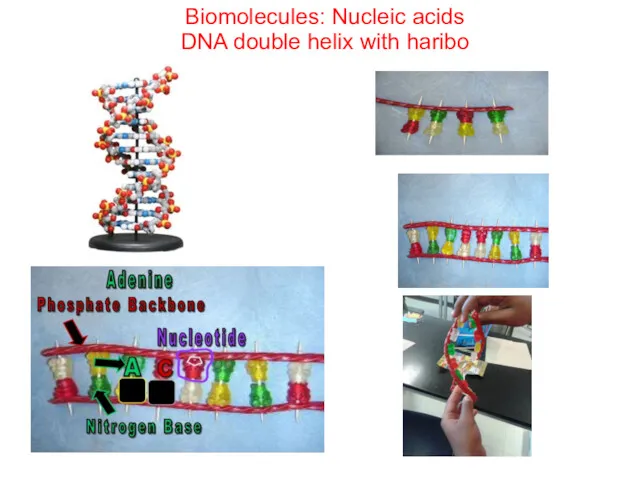
- 73. Biomolecules: Nucleic acids DNA double helix with haribo
- 75. Скачать презентацию














 Красная книга Казахстана
Красная книга Казахстана Бионика. Бионическая архитектура
Бионика. Бионическая архитектура Семейство сельдевых, тресковых, камбаловых и ставридовых рыб
Семейство сельдевых, тресковых, камбаловых и ставридовых рыб Бауырдың биохимиясы
Бауырдың биохимиясы Кісткові і Хрящові риби. 7 клас
Кісткові і Хрящові риби. 7 клас Лікарські рослини, які містять полісахариди
Лікарські рослини, які містять полісахариди Сезонные изменения в природе и жизнедеятельности организмов
Сезонные изменения в природе и жизнедеятельности организмов презентация по биологии 7 класс Плоские черви
презентация по биологии 7 класс Плоские черви Объекты биотехнологии. Биообъекты, применяемые на практике
Объекты биотехнологии. Биообъекты, применяемые на практике Лисичка желтая
Лисичка желтая Семейство розоцветных.
Семейство розоцветных. Эндокринная система
Эндокринная система Физиология сенсорных систем
Физиология сенсорных систем Биоэнергетика. Биологическое окисление. Биологические виды энергии
Биоэнергетика. Биологическое окисление. Биологические виды энергии Реакции электрофильного замещения в ароматическом и гетероциклическом рядах SE. (Лекция 5)
Реакции электрофильного замещения в ароматическом и гетероциклическом рядах SE. (Лекция 5) Exotic animals
Exotic animals Строение семян
Строение семян Определение соотношения размеров хвоинок и шишек хвойных деревьев. Выявление наиболее полезных хвойных деревьев
Определение соотношения размеров хвоинок и шишек хвойных деревьев. Выявление наиболее полезных хвойных деревьев Carbohydrates
Carbohydrates Метод селекции породы собак сиба-ину
Метод селекции породы собак сиба-ину Строение. Функции. Значение кожи
Строение. Функции. Значение кожи Царство Растения
Царство Растения Молекулярный уровень
Молекулярный уровень Основные компоненты клетки
Основные компоненты клетки Ознаки живих організмів
Ознаки живих організмів Моногибридное скрещивание
Моногибридное скрещивание Состояние и перспективы развития пчеловодства в Республике Башкортостан. История развития, ведущие ученые
Состояние и перспективы развития пчеловодства в Республике Башкортостан. История развития, ведущие ученые о тиграх
о тиграх