Содержание
- 2. ‘Dosage compensation’ – a mechanism that is responsible for the equality of expression of X-linked genes
- 3. Dosage compensation: different modes During the course of evolution, an ancestor to the placental mammals must
- 4. Dosage compensation: different modes Selection will favor tight linkage between the sex determining locus and sexually
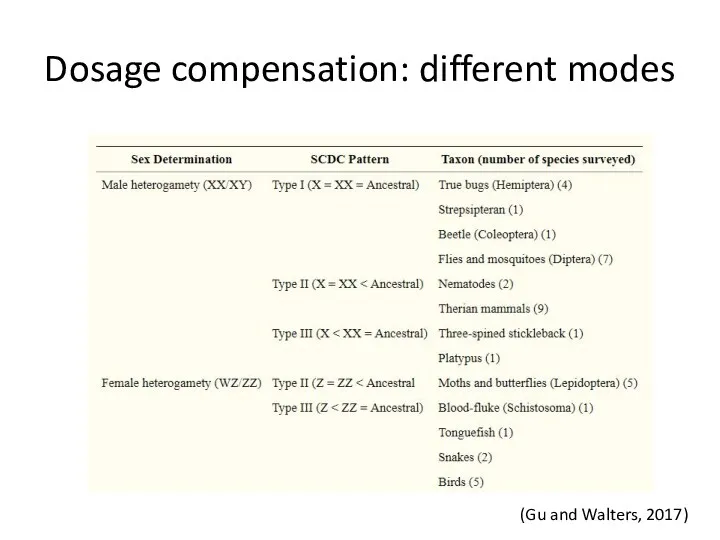
- 5. Dosage compensation: different modes (Gu and Walters, 2017)
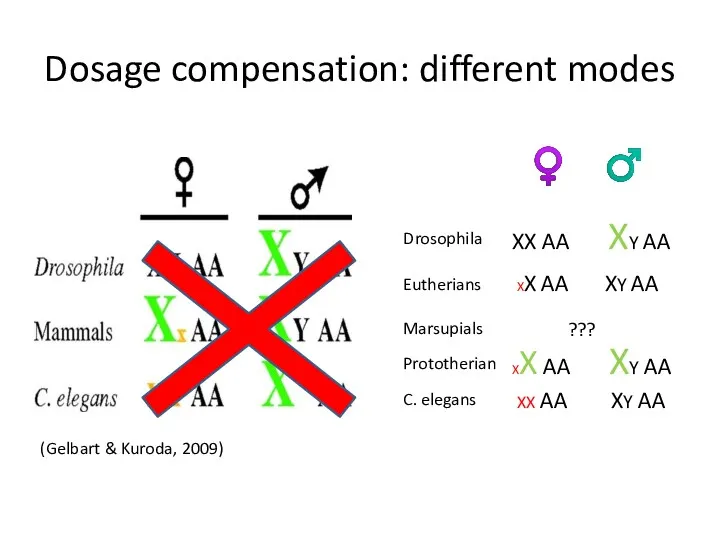
- 6. Dosage compensation: different modes Drosophila Eutherians Marsupials C. elegans ♀ ♂ XX AA XY AA XX
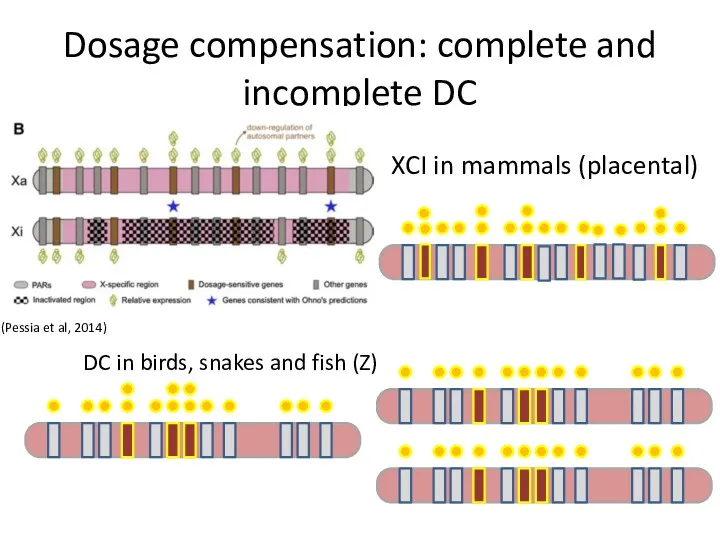
- 7. Dosage compensation: complete and incomplete DC. DC≠XCI! The well-studied mammalian X chromosome inactivation system, to which
- 8. Dosage compensation: complete and incomplete DC XCI in mammals (placental) DC in birds, snakes and fish
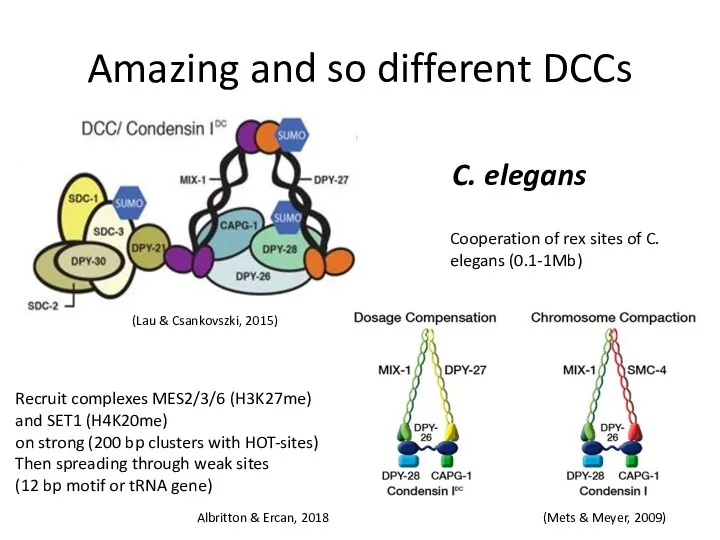
- 9. Amazing and so different DCCs C. elegans Recruit complexes MES2/3/6 (H3K27me) and SET1 (H4K20me) on strong
- 10. Questions from C. elegans What proteins recognize the 12-bp DNA sequence motif at the recruitment sites?
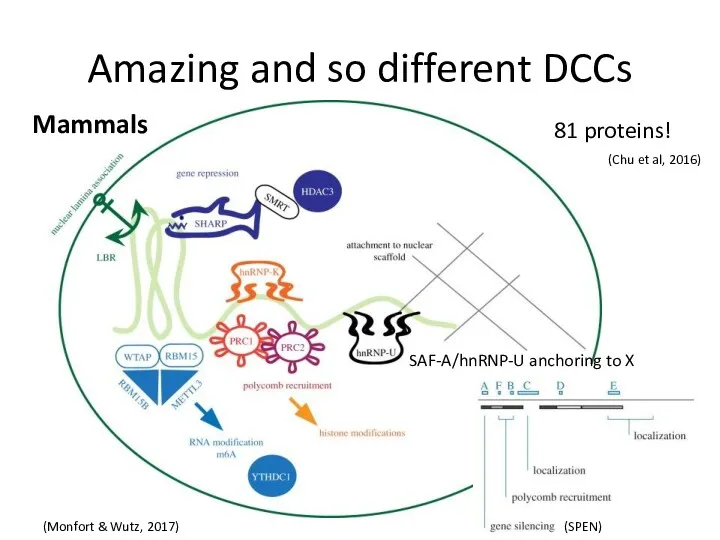
- 11. Amazing and so different DCCs (SPEN) 81 proteins! (Chu et al, 2016) SAF-A/hnRNP-U anchoring to X
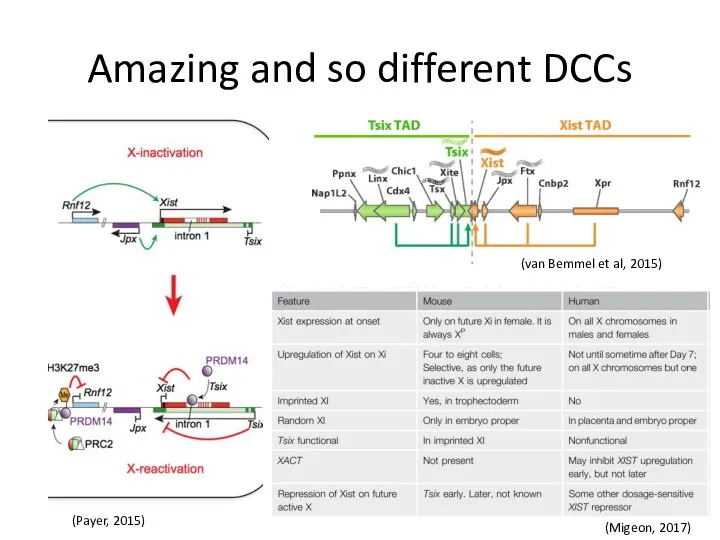
- 12. Amazing and so different DCCs (Migeon, 2017) (van Bemmel et al, 2015) (Payer, 2015)
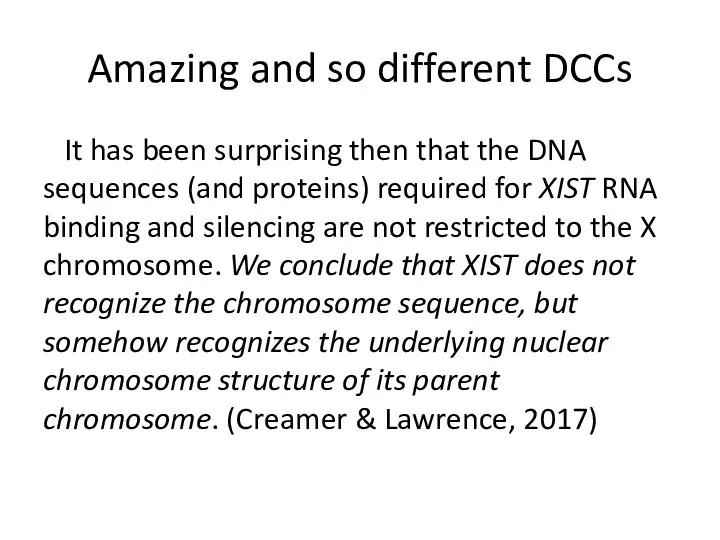
- 13. Amazing and so different DCCs It has been surprising then that the DNA sequences (and proteins)
- 14. Questions from mammals How does Xist propagate along X-chromosome? Why its propagation is confined? How does
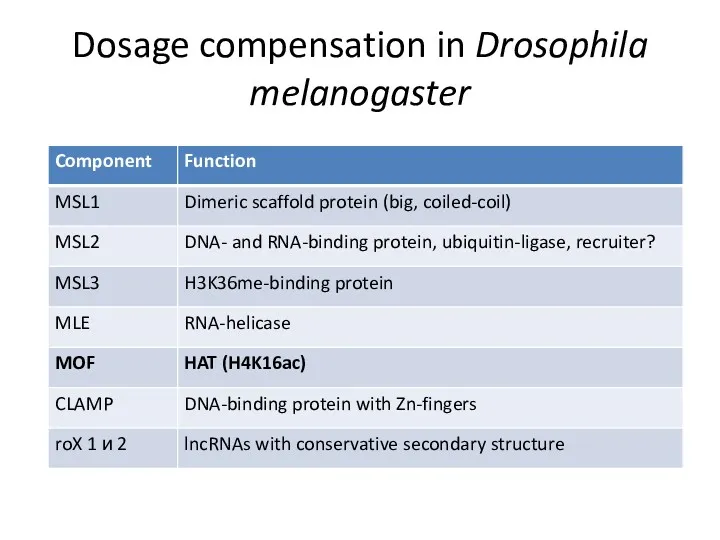
- 15. Dosage compensation in Drosophila melanogaster
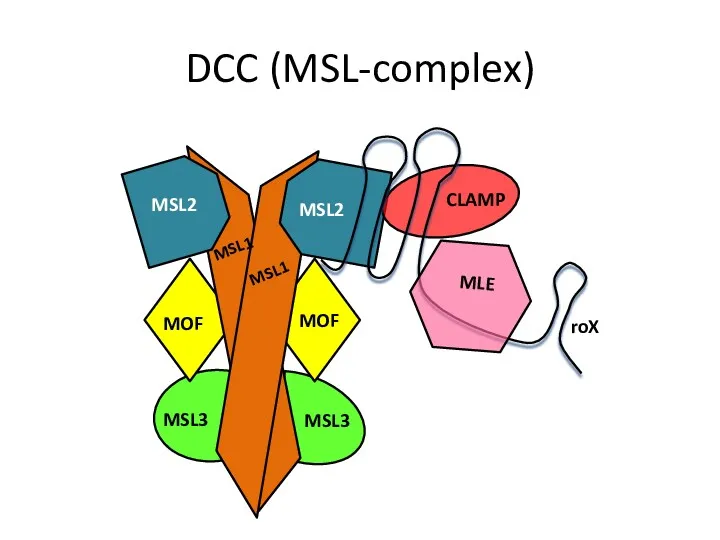
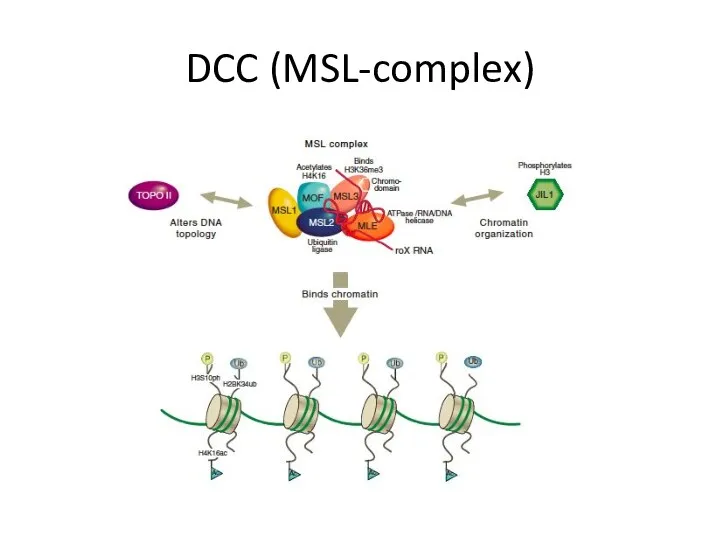
- 16. DCC (MSL-complex)
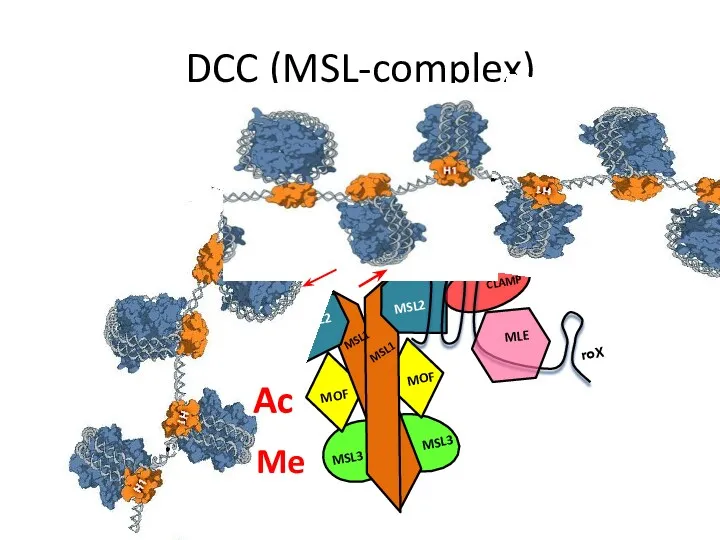
- 17. DCC (MSL-complex)
- 18. DCC (MSL-complex)
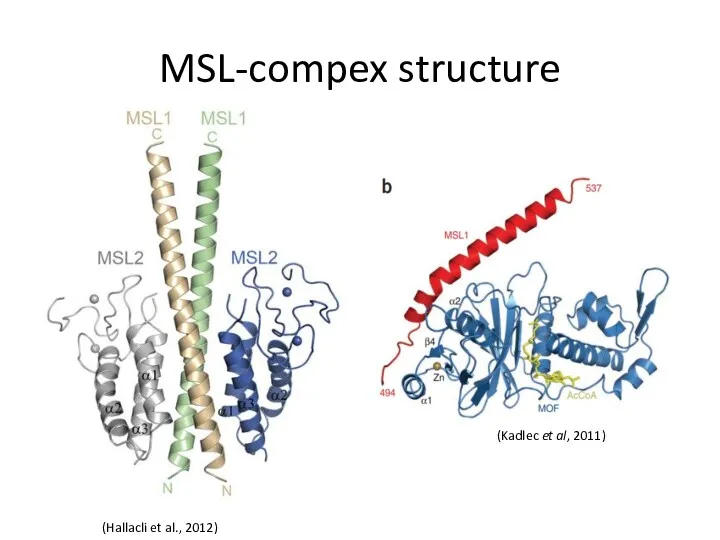
- 19. MSL-compex structure (Hallacli et al., 2012) (Kadlec et al, 2011)
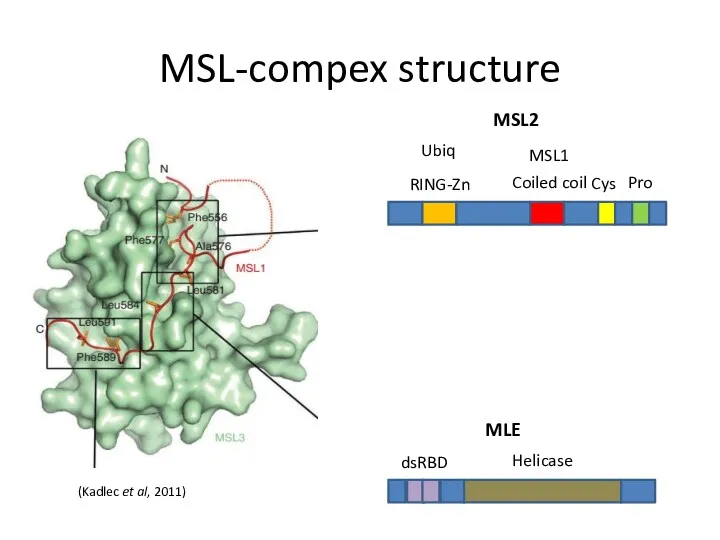
- 20. MSL-compex structure RING-Zn Coiled coil Cys Pro Ubiq MSL1 (Kadlec et al, 2011) MSL2 dsRBD Helicase
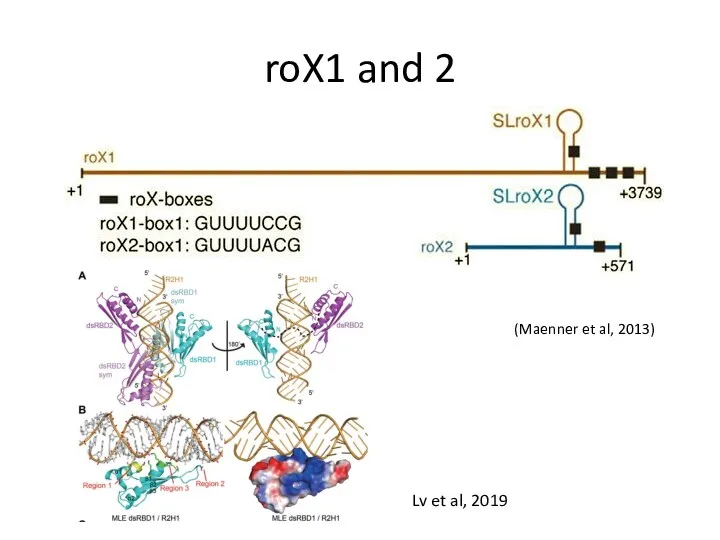
- 21. roX1 and 2 (Maenner et al, 2013) Lv et al, 2019
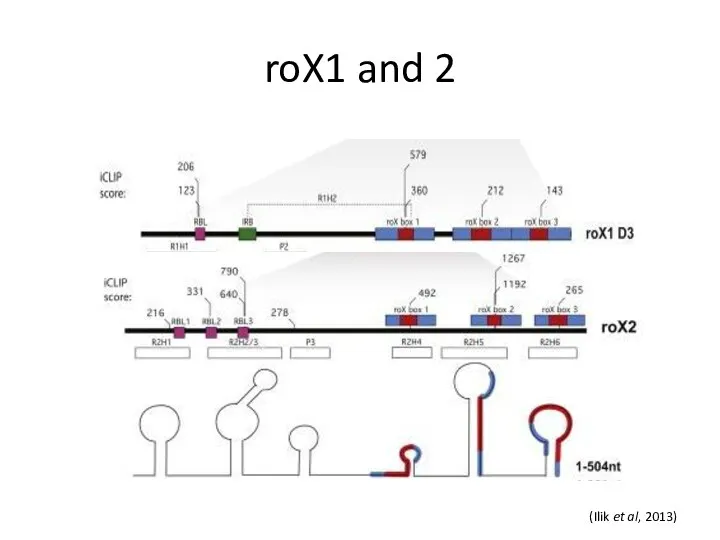
- 22. roX1 and 2 (Ilik et al, 2013)
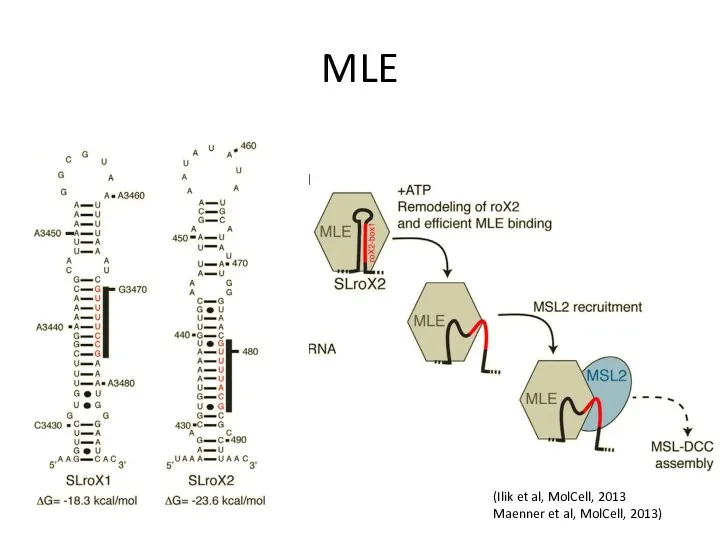
- 23. MLE (Ilik et al, MolCell, 2013 Maenner et al, MolCell, 2013)
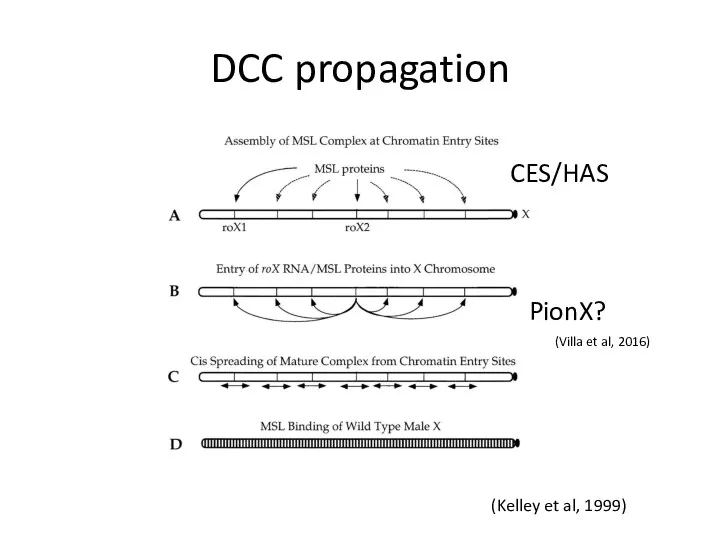
- 24. DCC propagation (Kelley et al, 1999) CES/HAS PionX? (Villa et al, 2016)
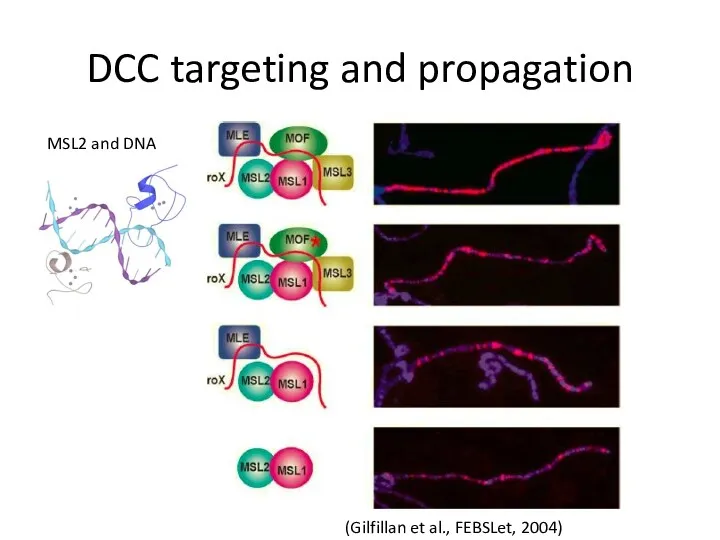
- 25. DCC targeting and propagation (Gilfillan et al., FEBSLet, 2004) MSL2 and DNA
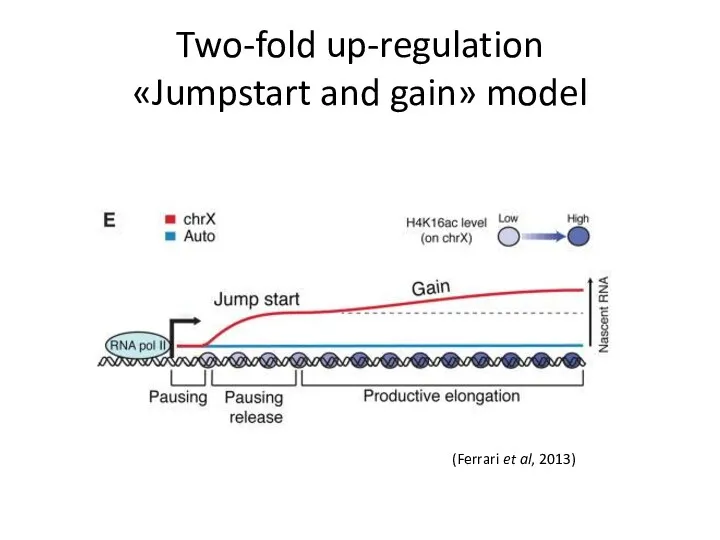
- 26. Two-fold up-regulation «Jumpstart and gain» model (Ferrari et al, 2013)
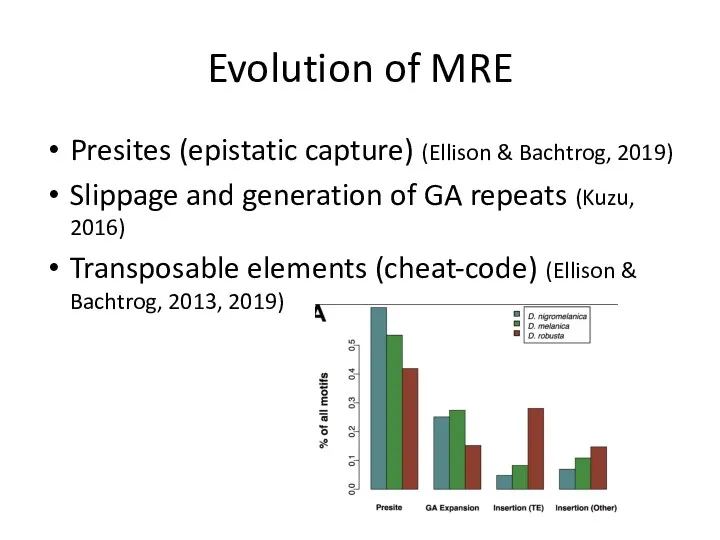
- 27. Evolution of MRE Presites (epistatic capture) (Ellison & Bachtrog, 2019) Slippage and generation of GA repeats
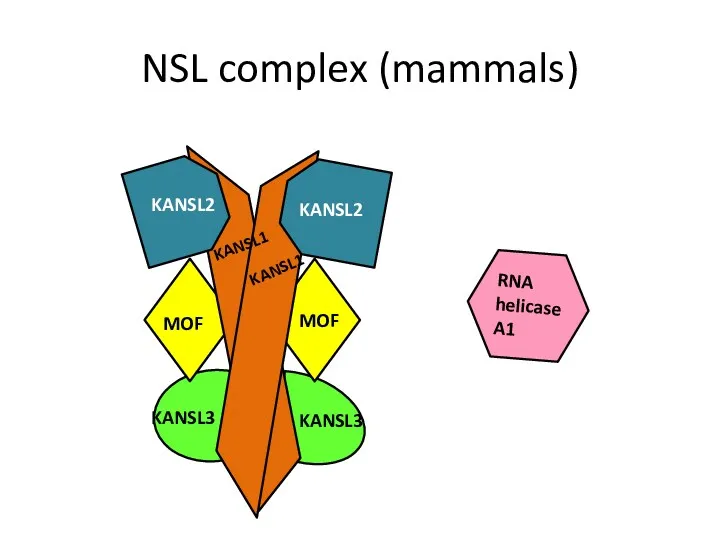
- 28. NSL complex (mammals)
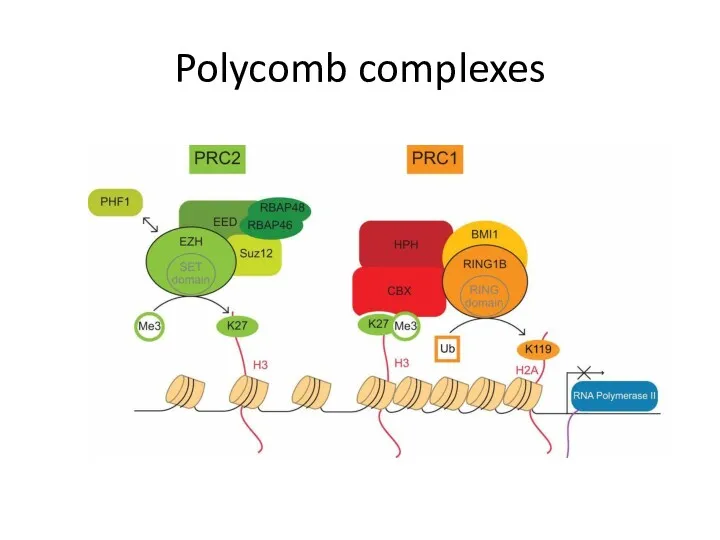
- 29. Polycomb complexes
- 30. DC regulation in males and females
- 32. Скачать презентацию





 Классификация и морфология микроорганизмов
Классификация и морфология микроорганизмов Погонофоралар типі - pogonopora
Погонофоралар типі - pogonopora Мир динозавров
Мир динозавров Химический состав клетки
Химический состав клетки Насекомые. Вредители культурных растений
Насекомые. Вредители культурных растений Doom and Boom on a Resilient Reef: Climate Change, Algal Overgrowth and Coral Recovery
Doom and Boom on a Resilient Reef: Climate Change, Algal Overgrowth and Coral Recovery Социально-биологические основы физической культуры
Социально-биологические основы физической культуры Магний и его роль в организме
Магний и его роль в организме Строение и функции мочевыделительной системы
Строение и функции мочевыделительной системы Внутренняя среда. Значение крови и ее состав. 8 класс
Внутренняя среда. Значение крови и ее состав. 8 класс презентация по проверке знаний в 8 классе
презентация по проверке знаний в 8 классе Общий путь катаболизма
Общий путь катаболизма Мендель Грегор Иоган (1822–1884) – основоположник генетики
Мендель Грегор Иоган (1822–1884) – основоположник генетики Проект учащихся 5 класса ВЛИЯНИЕ СВЕТА НА ПРОРАСТАНИЕ СЕМЯН ПОДСОЛНЕЧНИКА
Проект учащихся 5 класса ВЛИЯНИЕ СВЕТА НА ПРОРАСТАНИЕ СЕМЯН ПОДСОЛНЕЧНИКА Семейство Кошачьи
Семейство Кошачьи Законы Менделя
Законы Менделя Пингвины
Пингвины Биоэтика
Биоэтика Главные направления эволюции органического мира
Главные направления эволюции органического мира Роль биологических и социальных факторов в эволюции человека
Роль биологических и социальных факторов в эволюции человека Методы микробиологической диагностики вирусных инфекций. Профилактика вирусных инфекций
Методы микробиологической диагностики вирусных инфекций. Профилактика вирусных инфекций Химический состав клетки. Неорганические вещества
Химический состав клетки. Неорганические вещества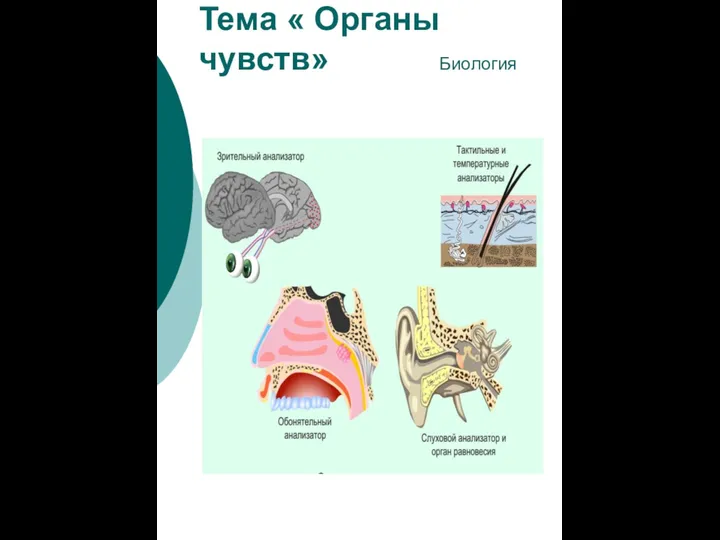 Органы чувств
Органы чувств Адаптация - приспособления организмов к среде обитания
Адаптация - приспособления организмов к среде обитания Иммунитет. Факторы врожденного иммунитета
Иммунитет. Факторы врожденного иммунитета Доказательства эволюции
Доказательства эволюции Формы крон декоративных растений
Формы крон декоративных растений презентация Вред курения
презентация Вред курения