Содержание
- 2. PLAN Denaturation Denaturation of Proteins Changing the Shape of a Protein Protein denaturation in food
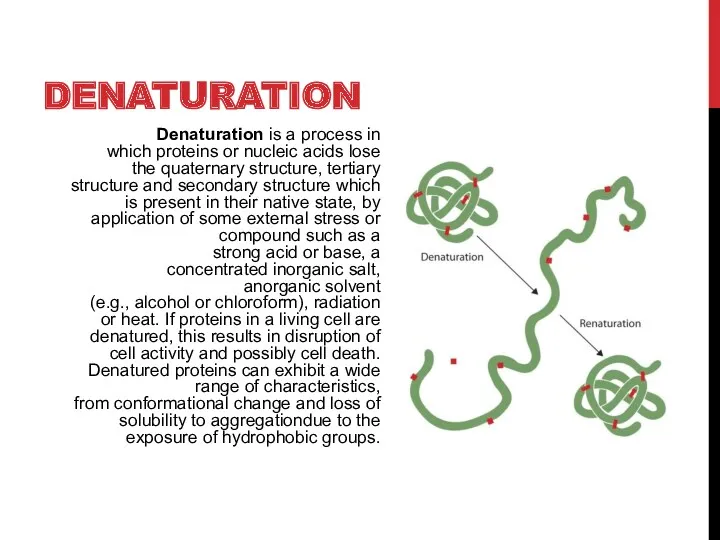
- 3. DENATURATION Denaturation is a process in which proteins or nucleic acids lose the quaternary structure, tertiary
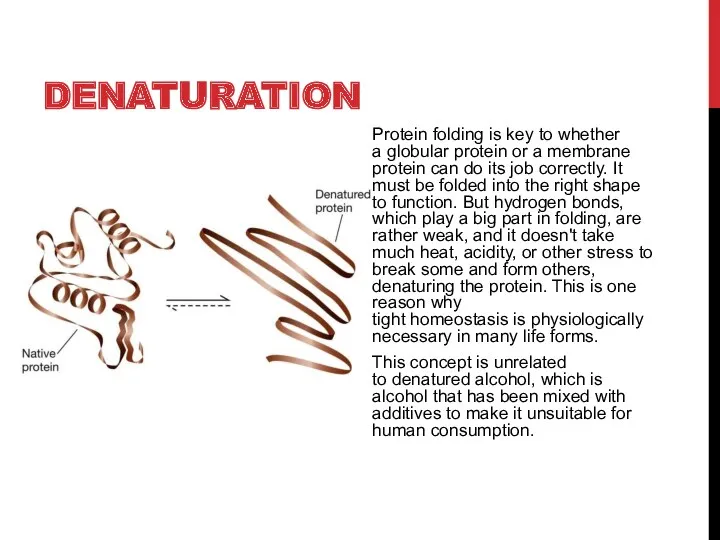
- 4. DENATURATION Protein folding is key to whether a globular protein or a membrane protein can do
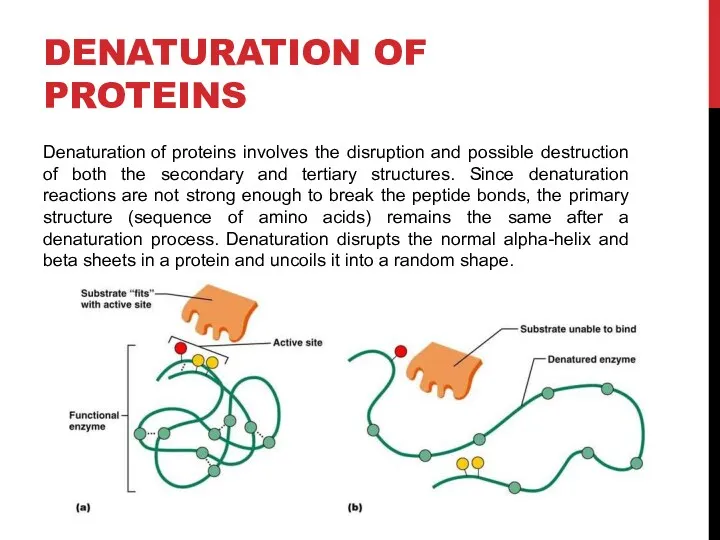
- 5. DENATURATION OF PROTEINS Denaturation of proteins involves the disruption and possible destruction of both the secondary
- 6. DENATURATION OF PROTEINS Denaturation occurs because the bonding interactions responsible for the secondary structure (hydrogen bonds
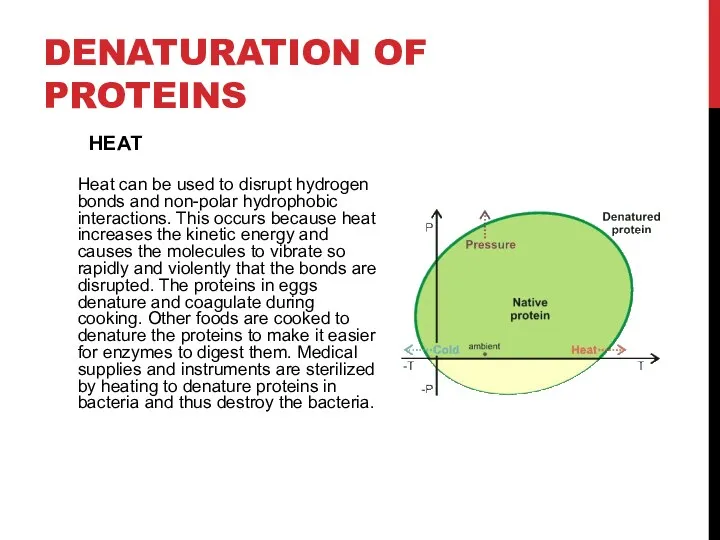
- 7. DENATURATION OF PROTEINS Heat can be used to disrupt hydrogen bonds and non-polar hydrophobic interactions. This
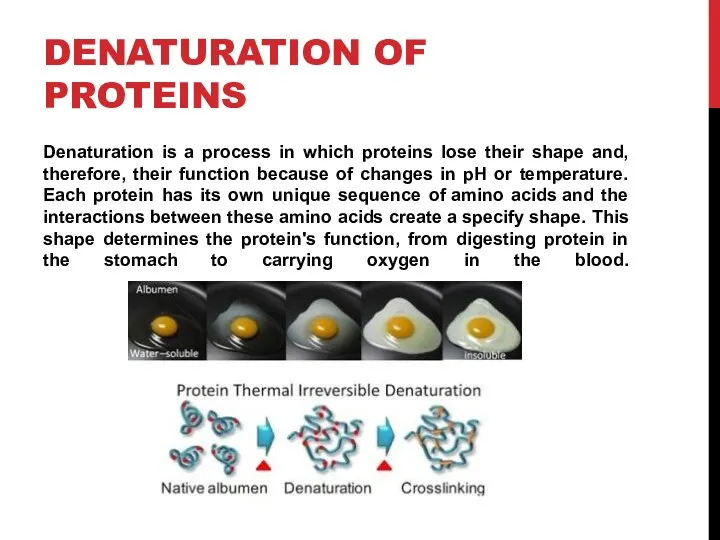
- 8. DENATURATION OF PROTEINS Denaturation is a process in which proteins lose their shape and, therefore, their
- 9. CHANGING THE SHAPE OF A PROTEIN If the protein is subject to changes in temperature, pH,
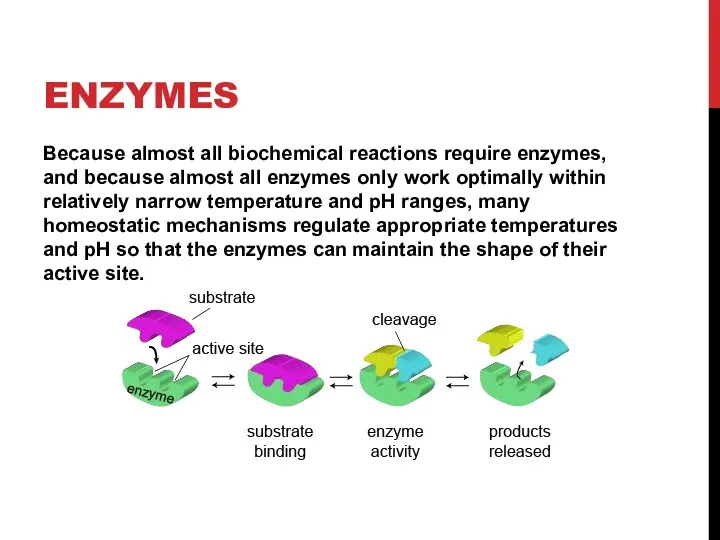
- 10. ENZYMES Because almost all biochemical reactions require enzymes, and because almost all enzymes only work optimally
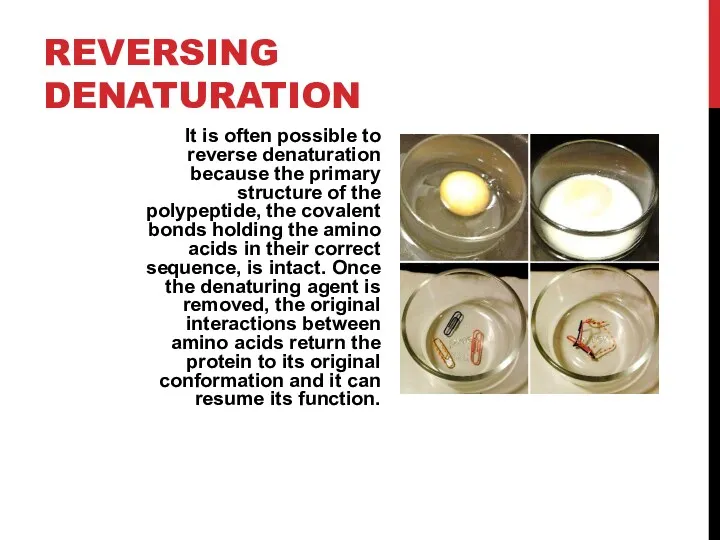
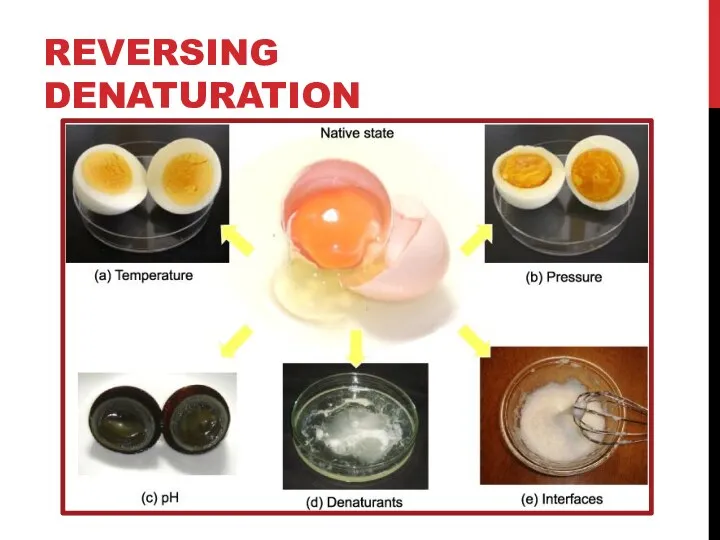
- 11. REVERSING DENATURATION It is often possible to reverse denaturation because the primary structure of the polypeptide,
- 12. REVERSING DENATURATION However, denaturation can be irreversible in extreme situations, like frying an egg. The heat
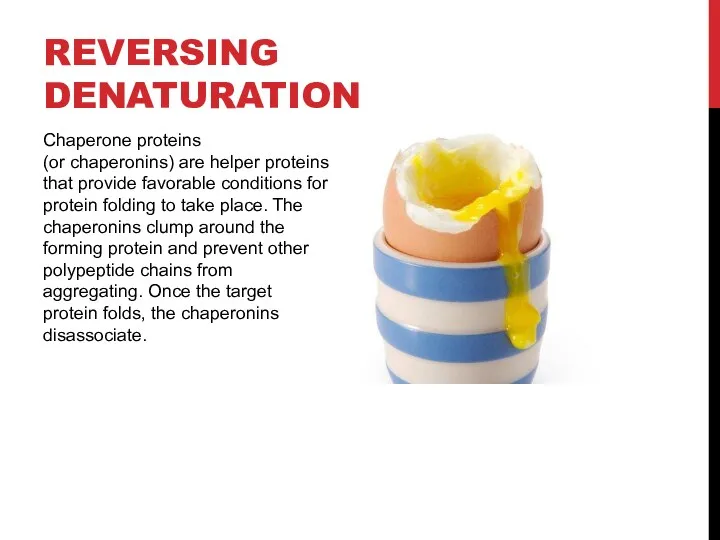
- 13. Chaperone proteins (or chaperonins) are helper proteins that provide favorable conditions for protein folding to take
- 14. REVERSING DENATURATION
- 15. PROTEIN DENATURATION IN FOOD In addition to having many vital functions within the body, proteins perform
- 16. PROTEIN DENATURATION IN FOOD Yogurt is another good example of proteins providing texture. Milk proteins called
- 17. PROTEIN DENATURATION IN FOOD When a cake is baked, the proteins are denatured. Denaturation refers to
- 18. PROTEIN DENATURATION IN FOOD Why do we cook many kinds of food before we eat them?
- 20. Скачать презентацию





 Обмен веществ и его роль в клетке. Энергетический обмен. Синтез АТФ
Обмен веществ и его роль в клетке. Энергетический обмен. Синтез АТФ Селекция растений, животных и микроорганизмов
Селекция растений, животных и микроорганизмов Кольчатые черви
Кольчатые черви Красоты Урала
Красоты Урала Скелет туловища, его развитие в фило- и онтогенезе
Скелет туловища, его развитие в фило- и онтогенезе Лимфоциты. Иммунная система
Лимфоциты. Иммунная система Влажные Экваториальные леса Африки
Влажные Экваториальные леса Африки Регуляція вісцеральних функцій організму. Фізіологія автономної нервової системи
Регуляція вісцеральних функцій організму. Фізіологія автономної нервової системи Желто-зеленые водоросли. Класс трибофициевые
Желто-зеленые водоросли. Класс трибофициевые Генетика. История развития генетики. Основные понятия
Генетика. История развития генетики. Основные понятия Как появляется бабочка
Как появляется бабочка Выращивание малины. Сорта малины
Выращивание малины. Сорта малины Отряд Зайцеобразные, Класса Млекопитающие
Отряд Зайцеобразные, Класса Млекопитающие Растения водоёмов
Растения водоёмов Внутреннее строение лягушки
Внутреннее строение лягушки Цель и задачи дисциплины. Значение биохимии. (Тема 1)
Цель и задачи дисциплины. Значение биохимии. (Тема 1) Периферический отдел нервной системы. Вегетативная нервная система
Периферический отдел нервной системы. Вегетативная нервная система Химический состав клетки
Химический состав клетки Овощеводство. Размножение овощных растений
Овощеводство. Размножение овощных растений Ядерный аппарат клетки (лекция 9, часть 2)
Ядерный аппарат клетки (лекция 9, часть 2) Тип членистоногие. Общая характеристика
Тип членистоногие. Общая характеристика Подготовка к ЕГЭ по биологии. 10 класс
Подготовка к ЕГЭ по биологии. 10 класс Division Angiospermae, Magnoliophyta. Class Dicotyledones
Division Angiospermae, Magnoliophyta. Class Dicotyledones Теломердің қартаю теориясы
Теломердің қартаю теориясы Презентация для учителей
Презентация для учителей Иммуноферментный анализ
Иммуноферментный анализ Анатомия и физиология гортани, трахеи и пищевода
Анатомия и физиология гортани, трахеи и пищевода Цікаві факти про гриби
Цікаві факти про гриби