Содержание
- 2. Overview: A Chemical Connection to Biology Biology is a multidisciplinary science Living organisms are subject to
- 3. Fig. 2-1
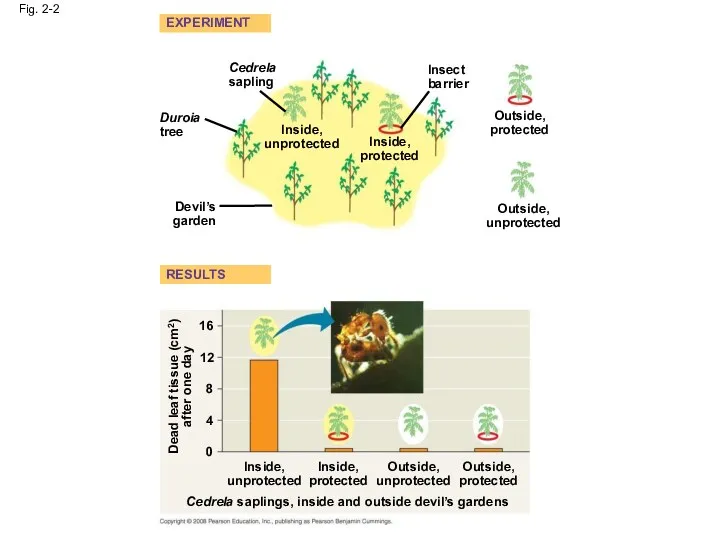
- 4. Fig. 2-2 EXPERIMENT RESULTS Cedrela sapling Duroia tree Inside, unprotected Inside, protected Devil’s garden Outside, unprotected
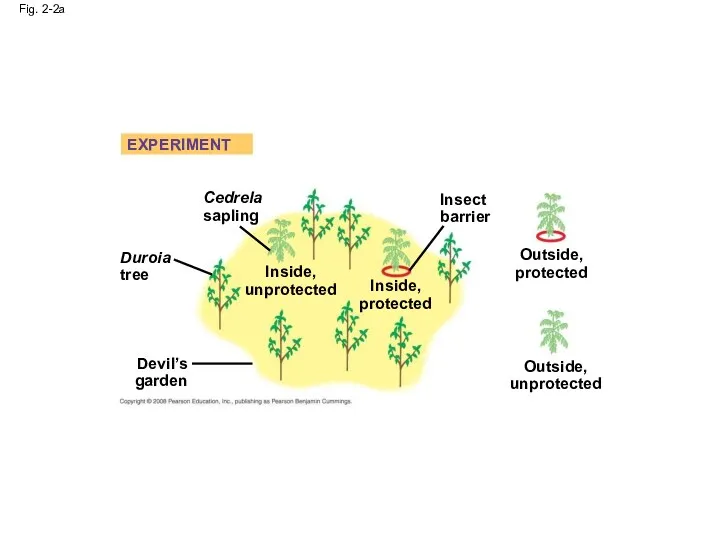
- 5. Fig. 2-2a Cedrela sapling Duroia tree Inside, unprotected Devil’s garden Inside, protected Insect barrier Outside, unprotected
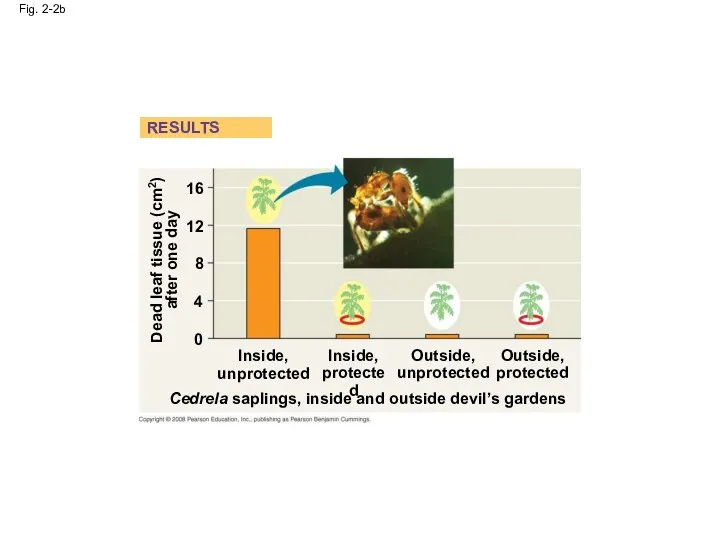
- 6. Fig. 2-2b Dead leaf tissue (cm2) after one day 16 12 8 4 0 Inside, unprotected
- 7. Concept 2.1: Matter consists of chemical elements in pure form and in combinations called compounds Organisms
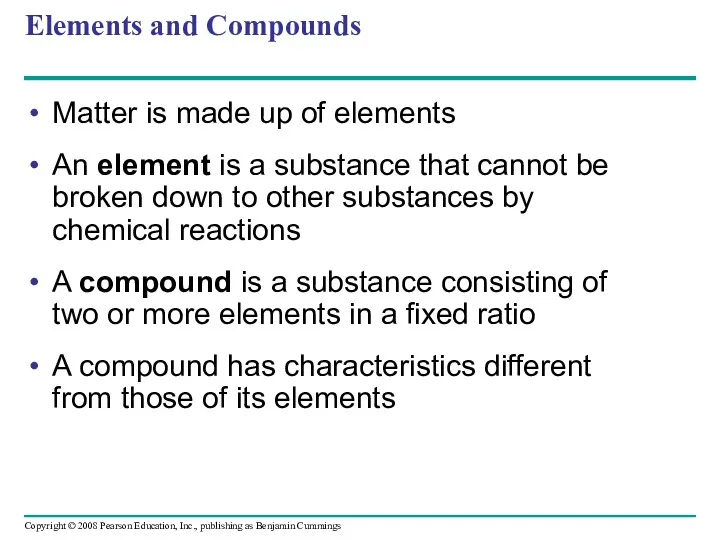
- 8. Elements and Compounds Matter is made up of elements An element is a substance that cannot
- 9. Fig. 2-3 Sodium Chlorine Sodium chloride
- 10. Fig. 2-3a Sodium
- 11. Fig. 2-3b Chlorine
- 12. Fig. 2-3c Sodium chloride
- 13. Essential Elements of Life About 25 of the 92 elements are essential to life Carbon, hydrogen,
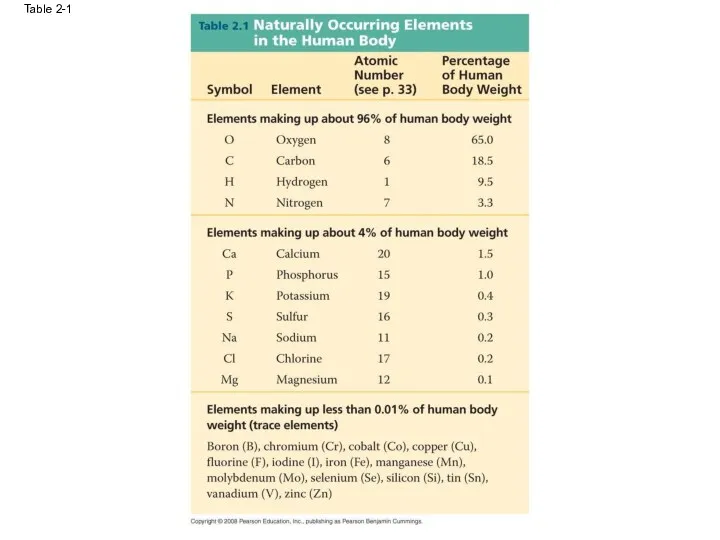
- 14. Table 2-1
- 15. (a) Nitrogen deficiency Fig. 2-4 (b) Iodine deficiency
- 16. Fig. 2-4a (a) Nitrogen deficiency
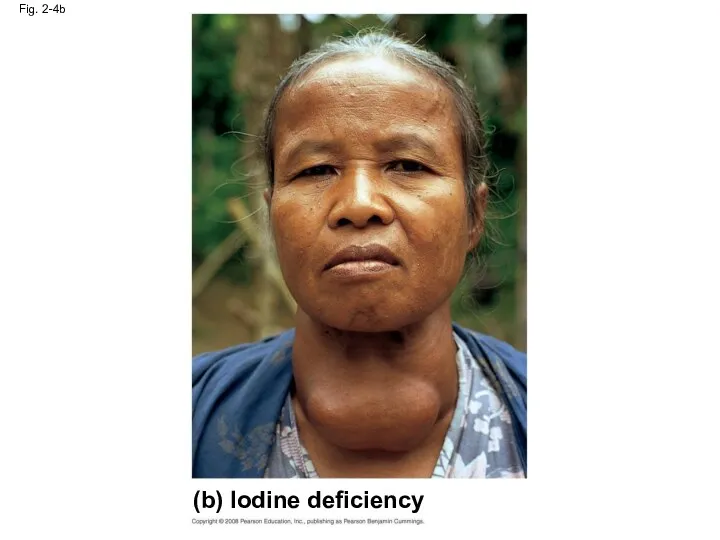
- 17. Fig. 2-4b (b) Iodine deficiency
- 18. Concept 2.2: An element’s properties depend on the structure of its atoms Each element consists of
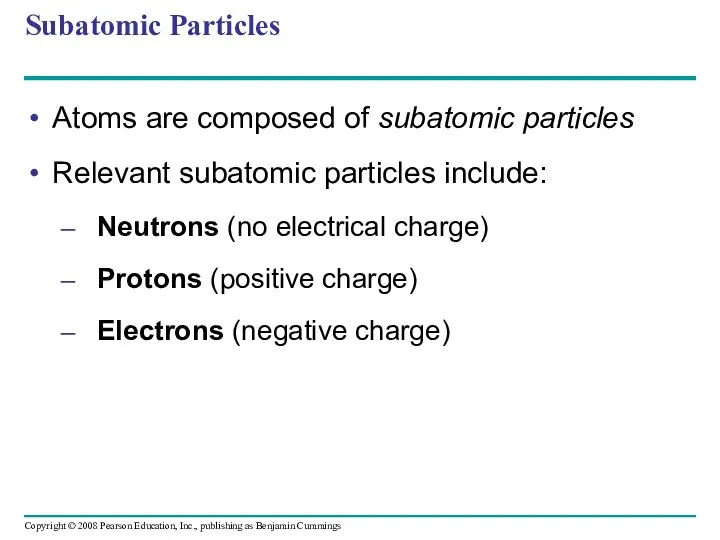
- 19. Subatomic Particles Atoms are composed of subatomic particles Relevant subatomic particles include: Neutrons (no electrical charge)
- 20. Neutrons and protons form the atomic nucleus Electrons form a cloud around the nucleus Neutron mass
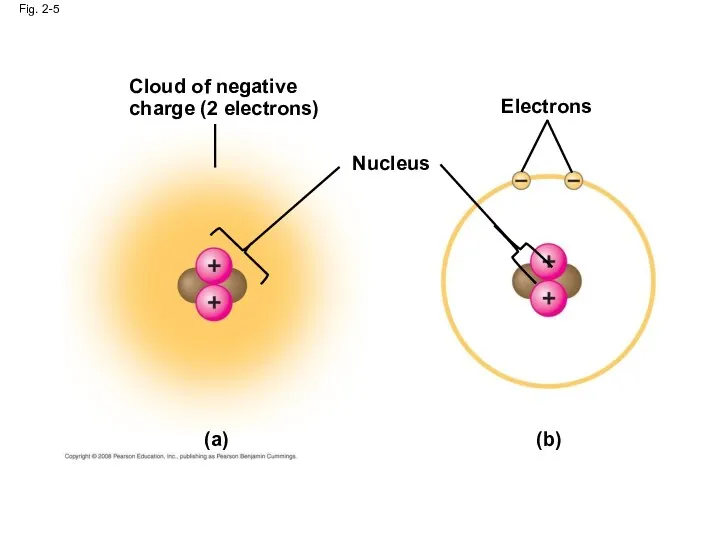
- 21. Cloud of negative charge (2 electrons) Fig. 2-5 Nucleus Electrons (b) (a)
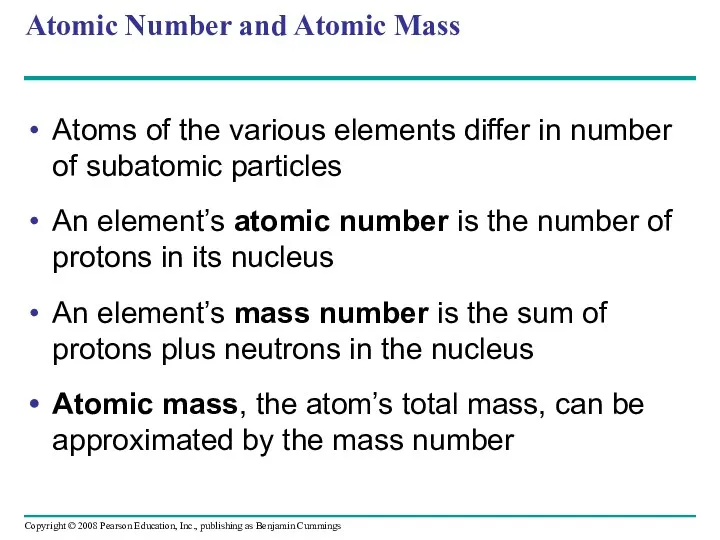
- 22. Atomic Number and Atomic Mass Atoms of the various elements differ in number of subatomic particles
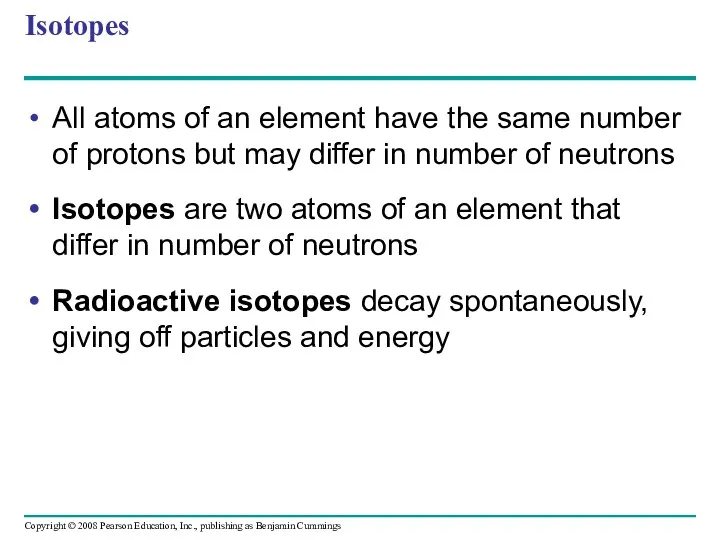
- 23. Isotopes All atoms of an element have the same number of protons but may differ in
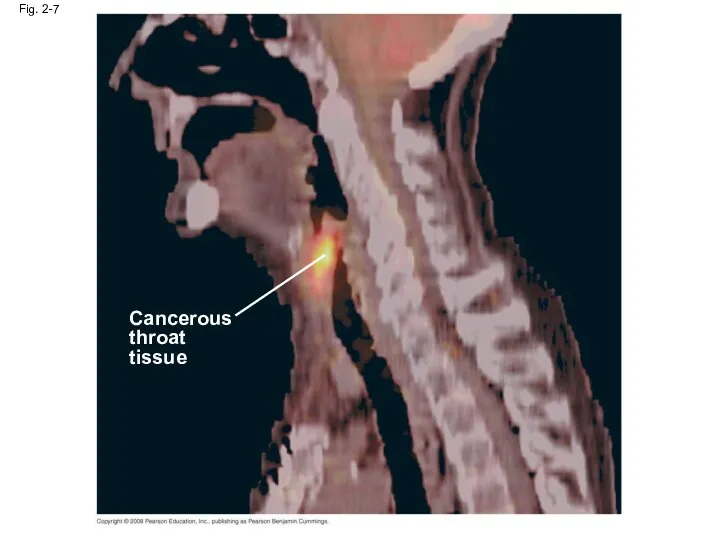
- 24. Some applications of radioactive isotopes in biological research are: Dating fossils Tracing atoms through metabolic processes
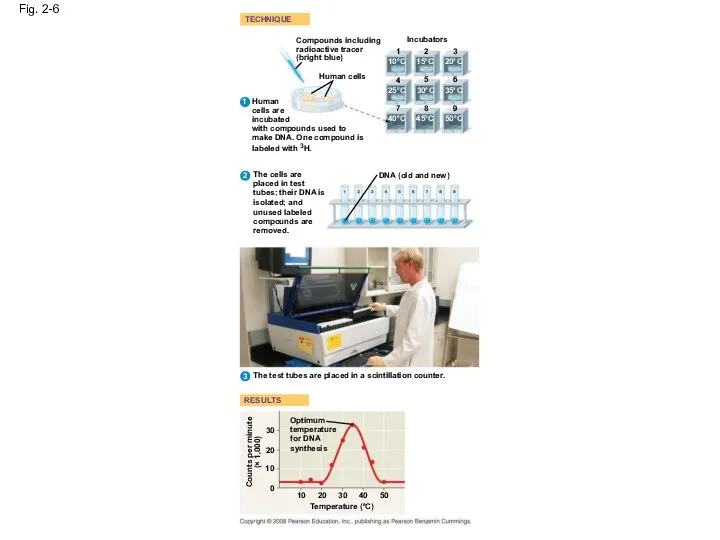
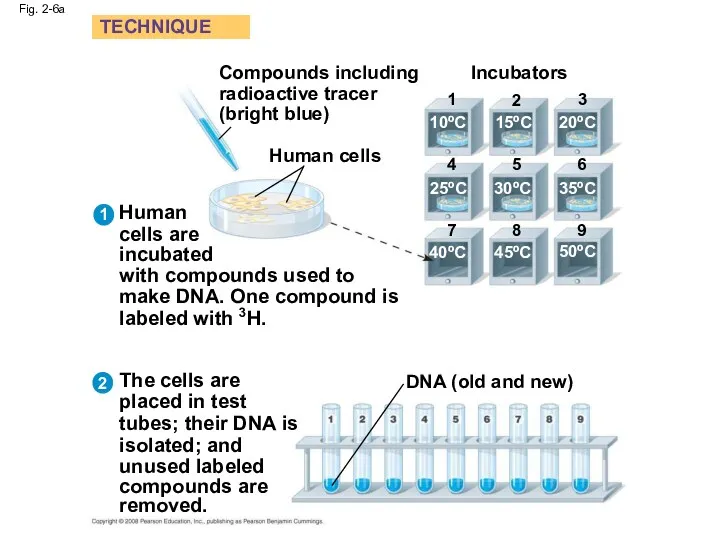
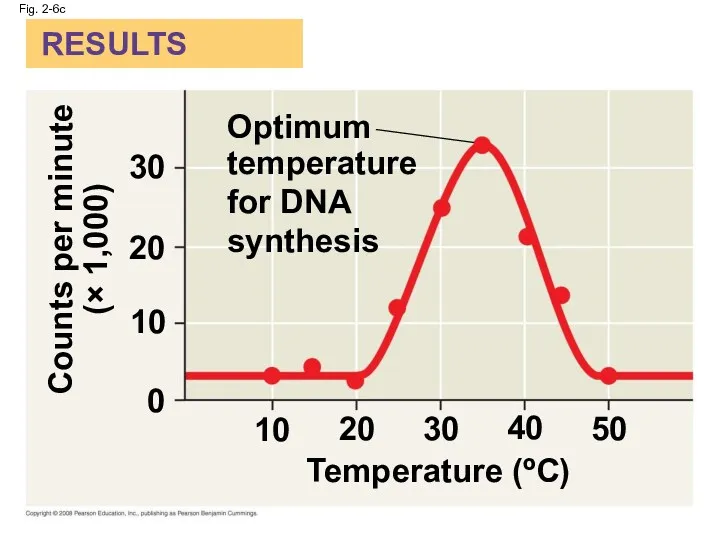
- 25. Fig. 2-6 TECHNIQUE RESULTS Compounds including radioactive tracer (bright blue) Incubators 1 2 3 4 5
- 26. Fig. 2-6a Compounds including radioactive tracer (bright blue) Human cells Incubators 1 2 3 4 5
- 27. Fig. 2-6b TECHNIQUE The test tubes are placed in a scintillation counter. 3
- 28. Fig. 2-6c RESULTS Counts per minute (× 1,000) 0 10 20 30 40 50 10 20
- 29. Fig. 2-7 Cancerous throat tissue
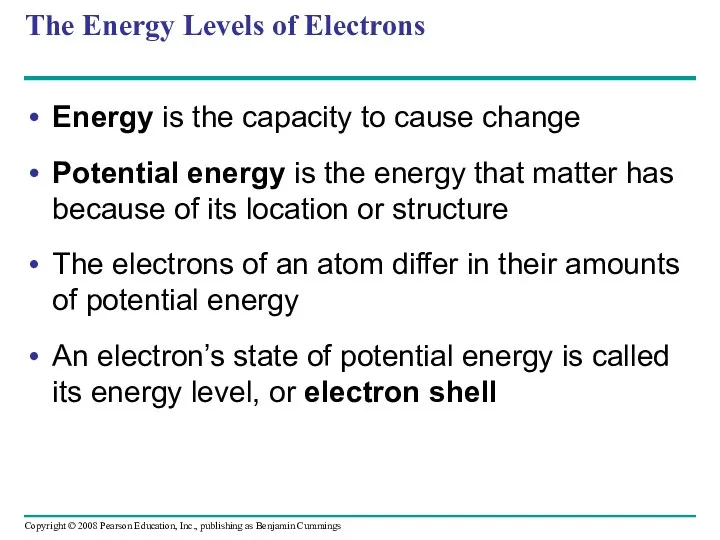
- 30. The Energy Levels of Electrons Energy is the capacity to cause change Potential energy is the
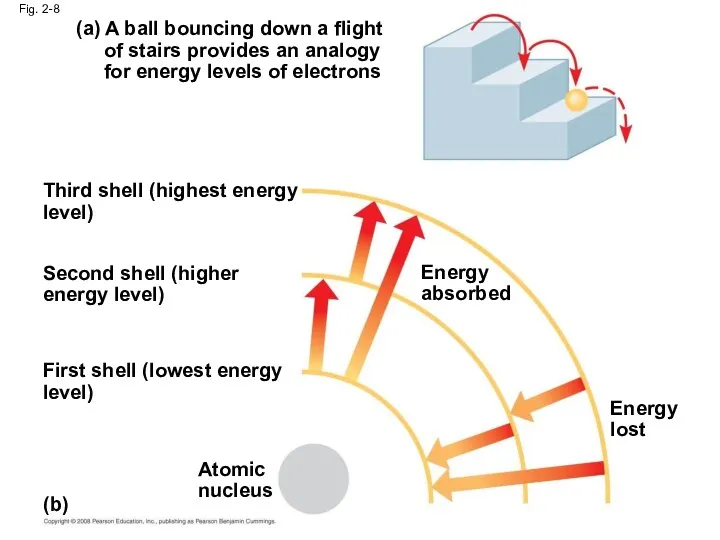
- 31. Fig. 2-8 (a) A ball bouncing down a flight of stairs provides an analogy for energy
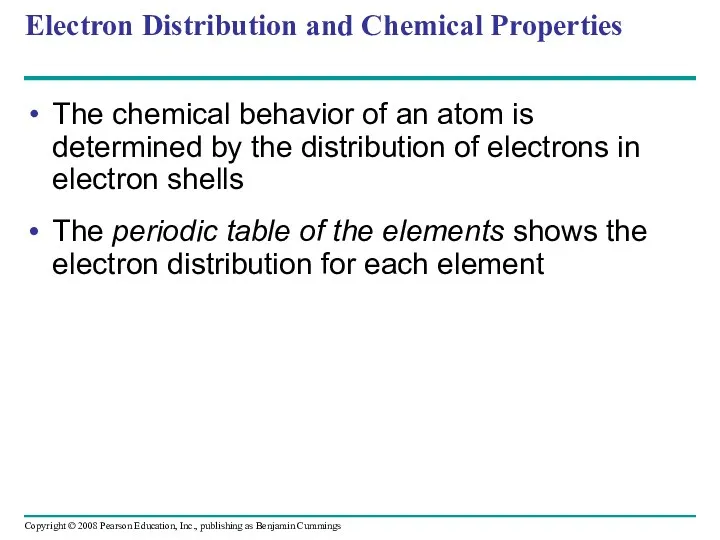
- 32. Electron Distribution and Chemical Properties The chemical behavior of an atom is determined by the distribution
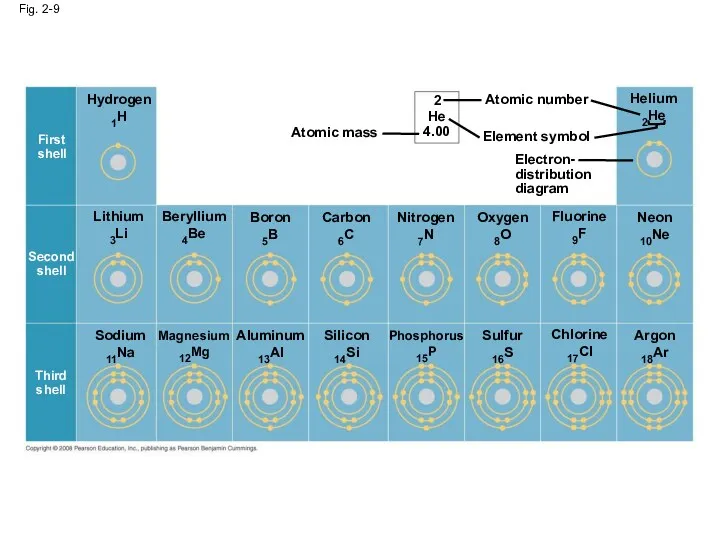
- 33. Fig. 2-9 Hydrogen 1H Lithium 3Li Beryllium 4Be Boron 5B Carbon 6C Nitrogen 7N Oxygen 8O
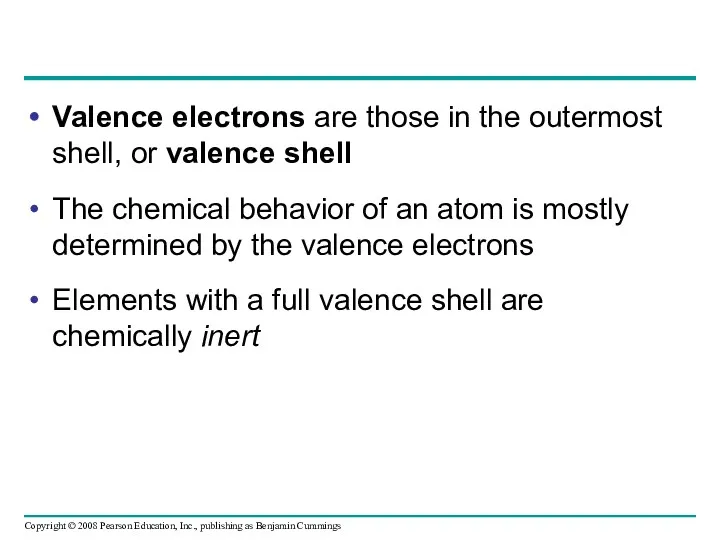
- 34. Valence electrons are those in the outermost shell, or valence shell The chemical behavior of an
- 35. Electron Orbitals An orbital is the three-dimensional space where an electron is found 90% of the
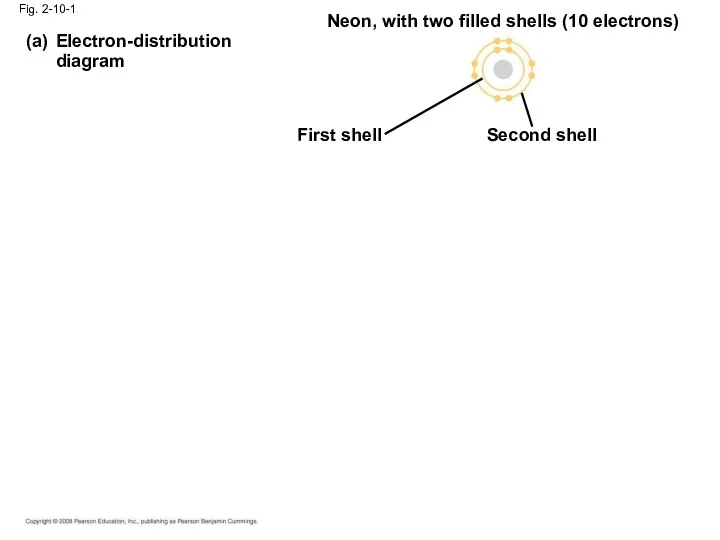
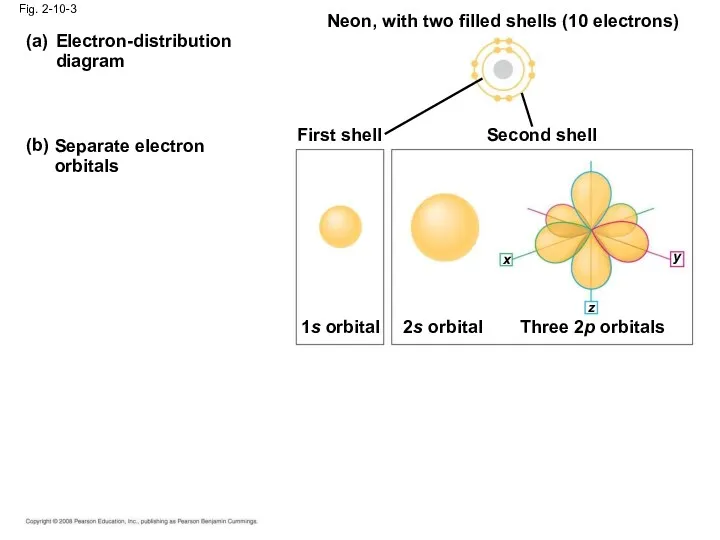
- 36. Fig. 2-10-1 Electron-distribution diagram (a) Neon, with two filled shells (10 electrons) First shell Second shell
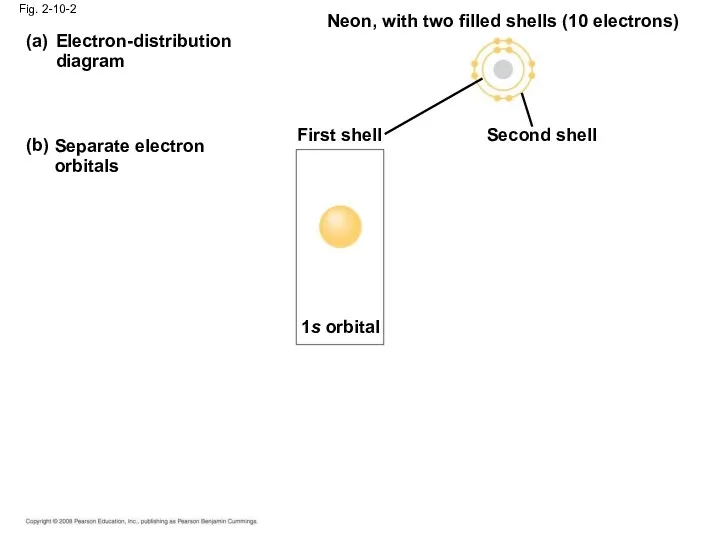
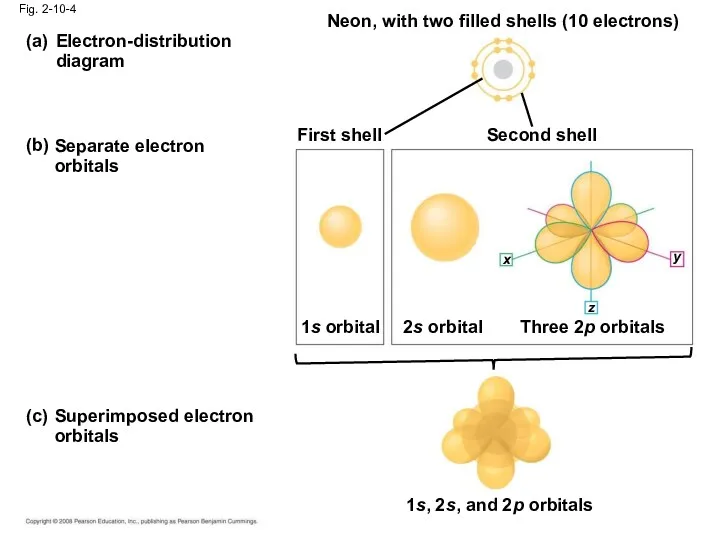
- 37. Electron-distribution diagram (a) (b) Separate electron orbitals Neon, with two filled shells (10 electrons) First shell
- 38. Electron-distribution diagram (a) (b) Separate electron orbitals Neon, with two filled shells (10 electrons) First shell
- 39. Electron-distribution diagram (a) (b) Separate electron orbitals Neon, with two filled shells (10 electrons) First shell
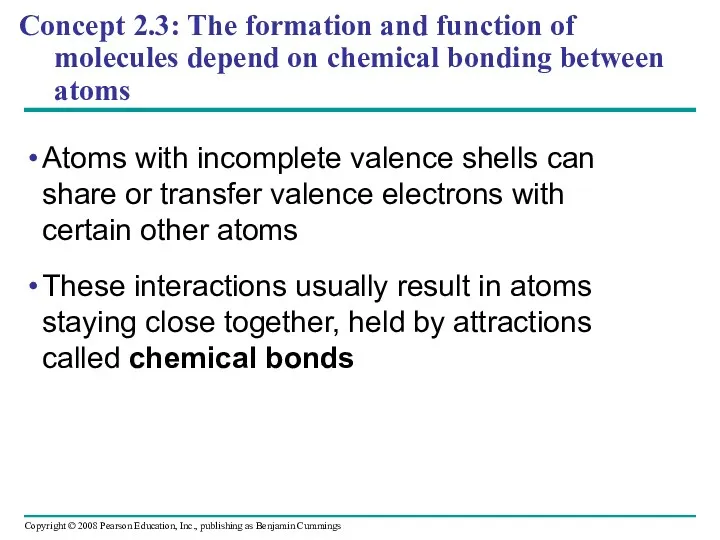
- 40. Concept 2.3: The formation and function of molecules depend on chemical bonding between atoms Atoms with
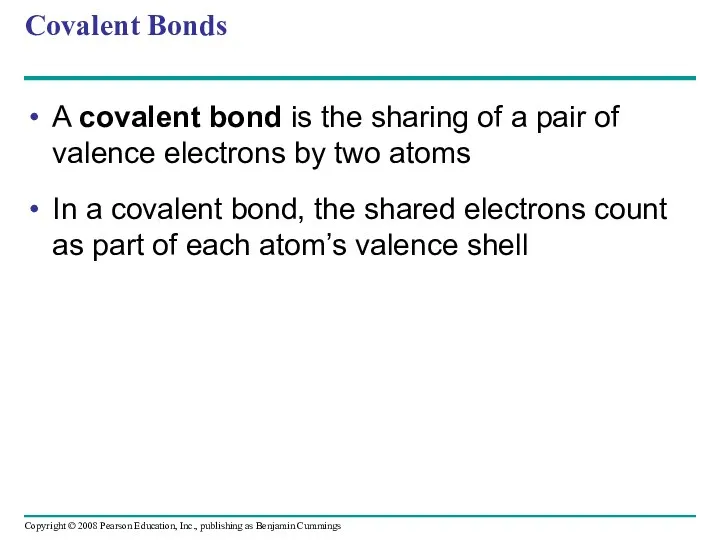
- 41. Covalent Bonds A covalent bond is the sharing of a pair of valence electrons by two
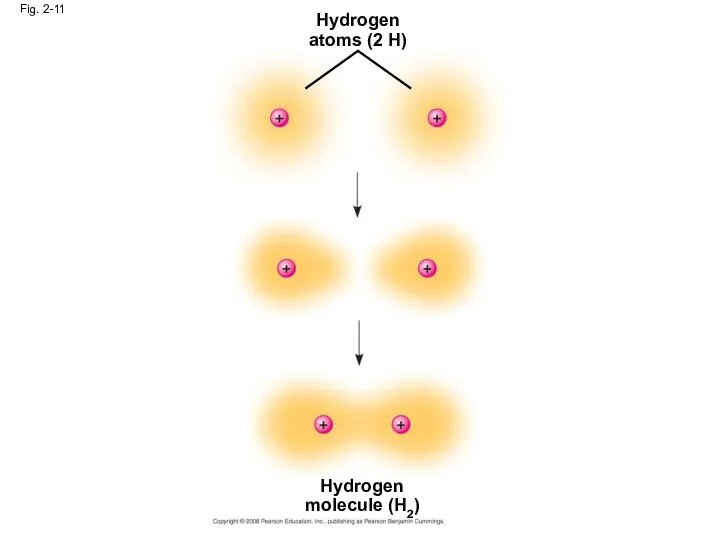
- 42. Fig. 2-11 Hydrogen atoms (2 H) Hydrogen molecule (H2)
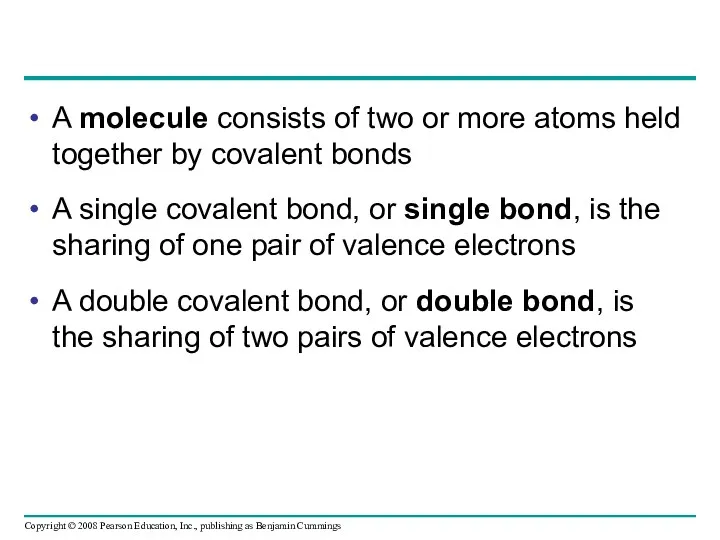
- 43. A molecule consists of two or more atoms held together by covalent bonds A single covalent
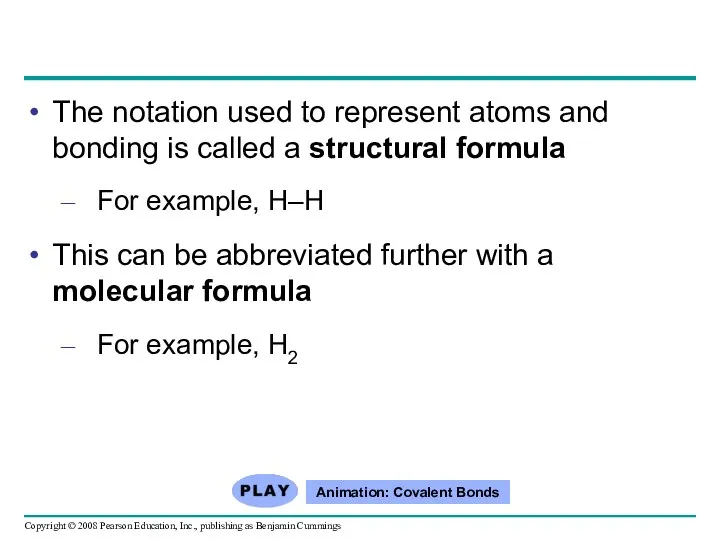
- 44. The notation used to represent atoms and bonding is called a structural formula For example, H–H
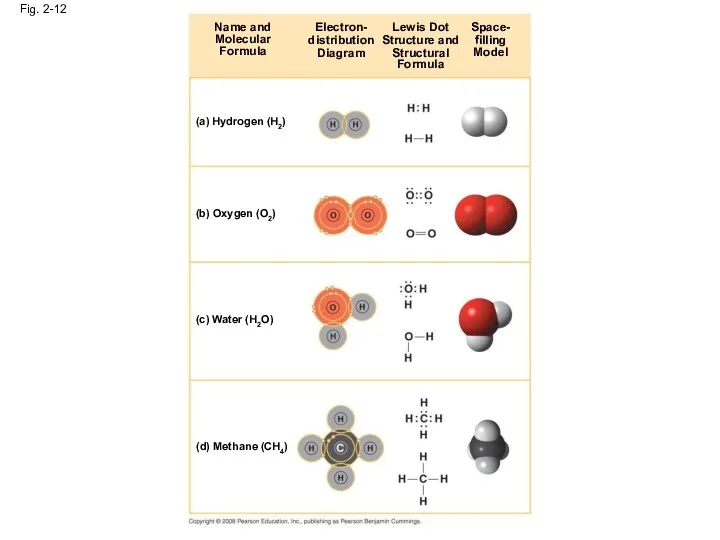
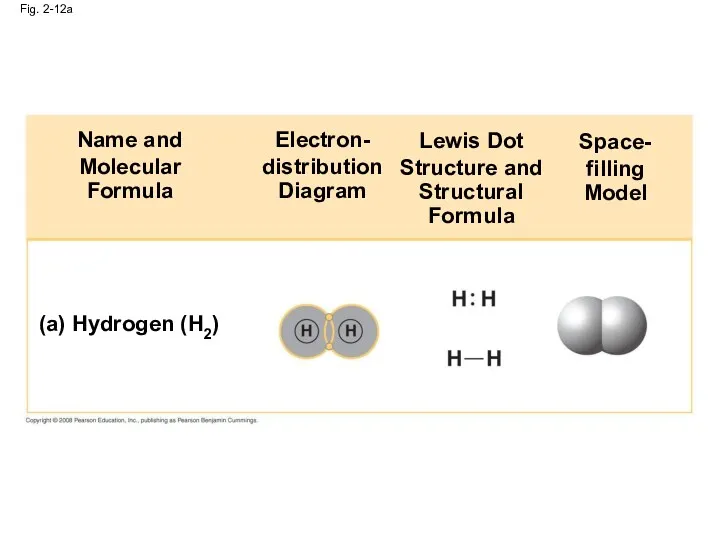
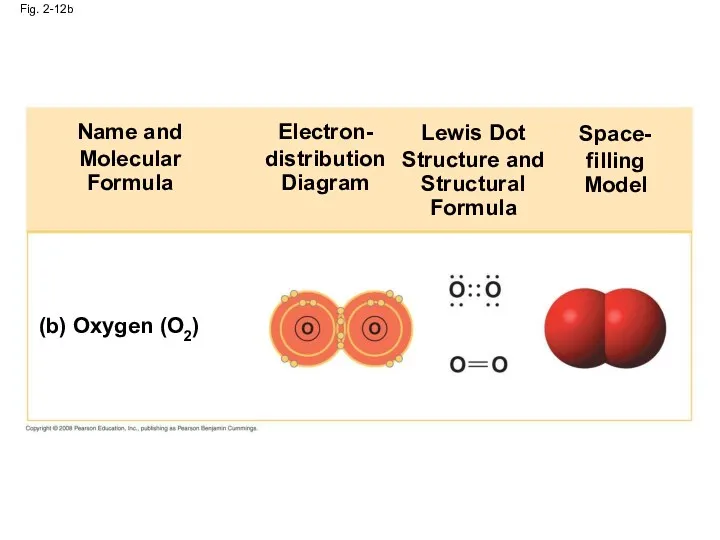
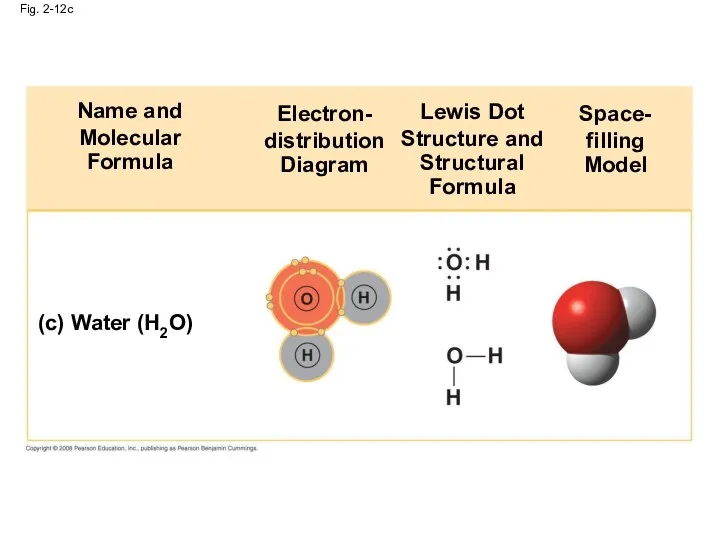
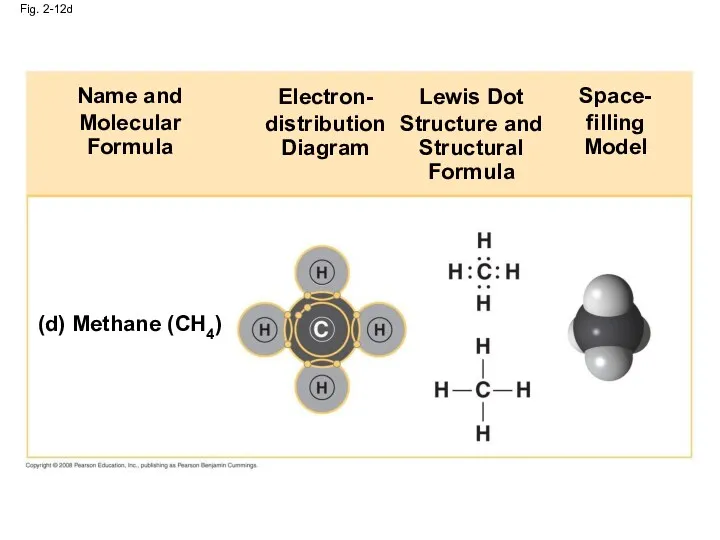
- 45. Fig. 2-12 Name and Molecular Formula Electron- distribution Diagram Lewis Dot Structure and Structural Formula Space-
- 46. Fig. 2-12a (a) Hydrogen (H2) Name and Molecular Formula Electron- distribution Diagram Lewis Dot Structure and
- 47. Fig. 2-12b (b) Oxygen (O2) Name and Molecular Formula Electron- distribution Diagram Lewis Dot Structure and
- 48. Fig. 2-12c (c) Water (H2O) Name and Molecular Formula Electron- distribution Diagram Lewis Dot Structure and
- 49. Fig. 2-12d (d) Methane (CH4) Name and Molecular Formula Electron- distribution Diagram Lewis Dot Structure and
- 50. Covalent bonds can form between atoms of the same element or atoms of different elements A
- 51. Electronegativity is an atom’s attraction for the electrons in a covalent bond The more electronegative an
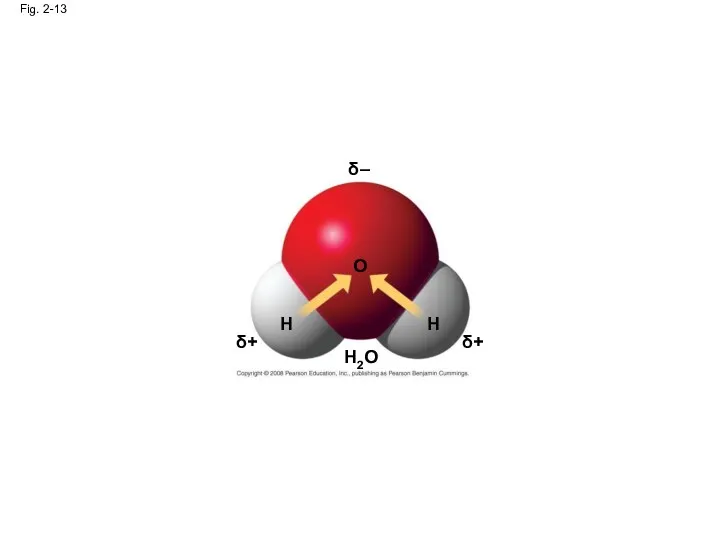
- 52. In a nonpolar covalent bond, the atoms share the electron equally In a polar covalent bond,
- 53. Fig. 2-13 δ – δ+ δ+ H H O H2O
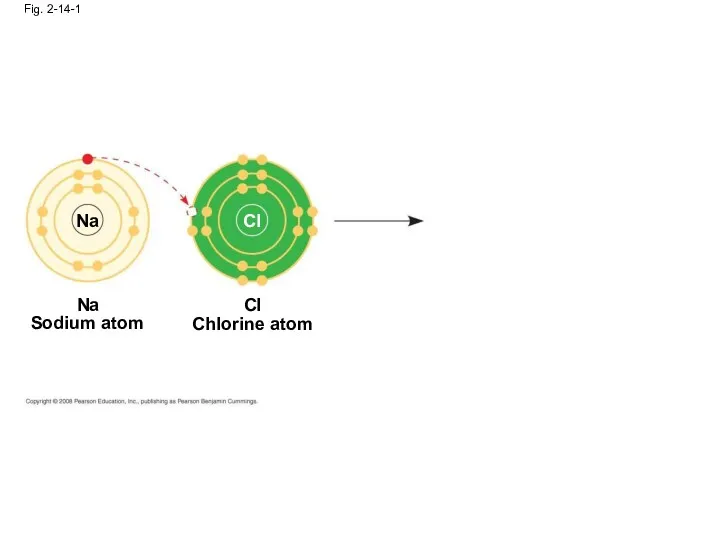
- 54. Ionic Bonds Atoms sometimes strip electrons from their bonding partners An example is the transfer of
- 55. Fig. 2-14-1 Na Cl Na Sodium atom Chlorine atom Cl
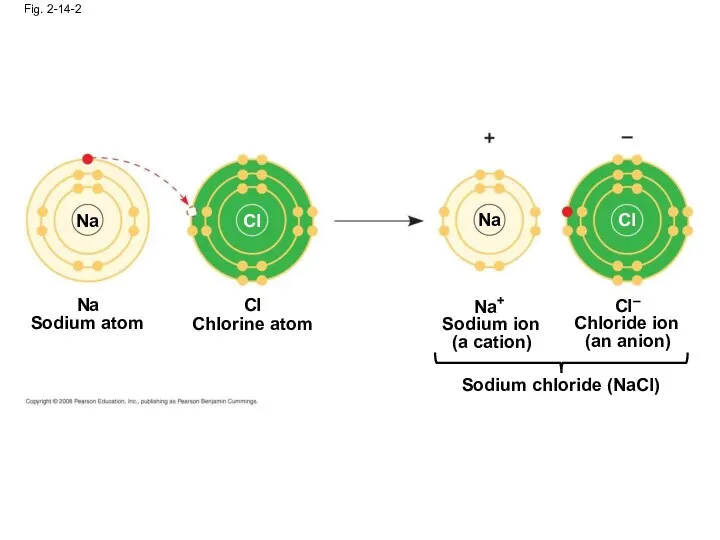
- 56. Fig. 2-14-2 Na Cl Na Cl Na Sodium atom Chlorine atom Cl Na+ Sodium ion (a
- 57. A cation is a positively charged ion An anion is a negatively charged ion An ionic
- 58. Compounds formed by ionic bonds are called ionic compounds, or salts Salts, such as sodium chloride
- 59. Fig. 2-15 Na+ Cl–
- 60. Weak Chemical Bonds Most of the strongest bonds in organisms are covalent bonds that form a
- 61. Hydrogen Bonds A hydrogen bond forms when a hydrogen atom covalently bonded to one electronegative atom
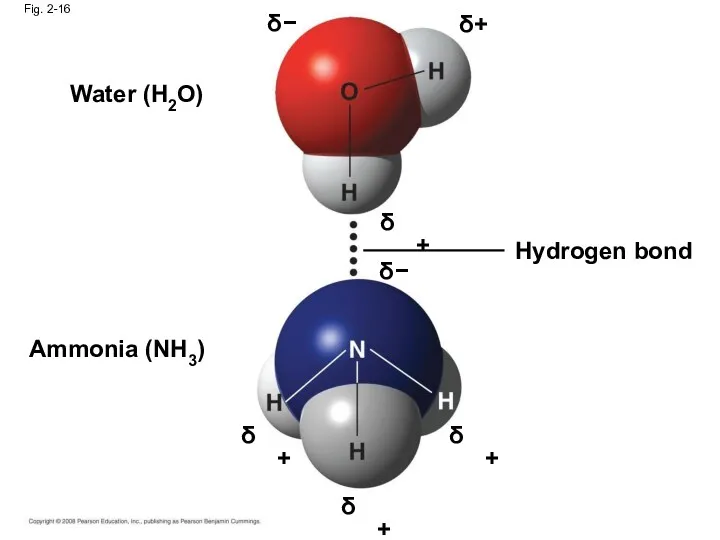
- 62. Fig. 2-16 δ − δ+ δ+ δ − δ+ δ+ δ+ Water (H2O) Ammonia (NH3) Hydrogen
- 63. Van der Waals Interactions If electrons are distributed asymmetrically in molecules or atoms, they can result
- 64. Collectively, such interactions can be strong, as between molecules of a gecko’s toe hairs and a
- 65. Fig. 2-UN1
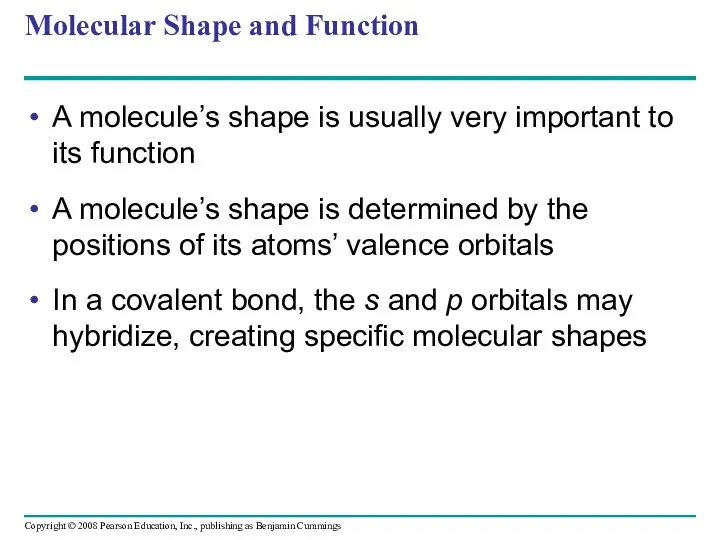
- 66. Molecular Shape and Function A molecule’s shape is usually very important to its function A molecule’s
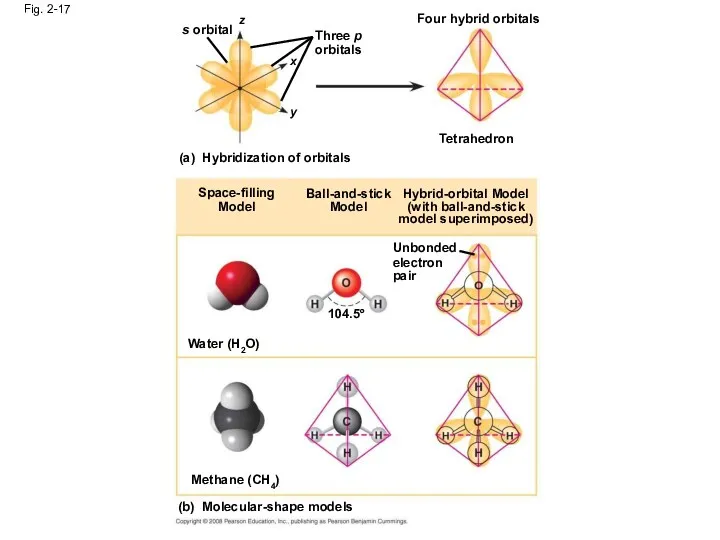
- 67. Fig. 2-17 s orbital Three p orbitals (a) Hybridization of orbitals Tetrahedron Four hybrid orbitals Space-filling
- 68. Fig. 2-17a s orbital z x y Three p orbitals Hybridization of orbitals Four hybrid orbitals
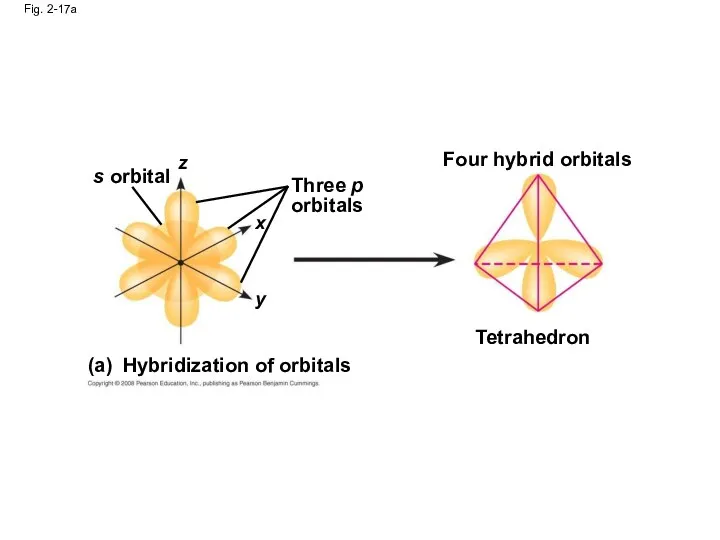
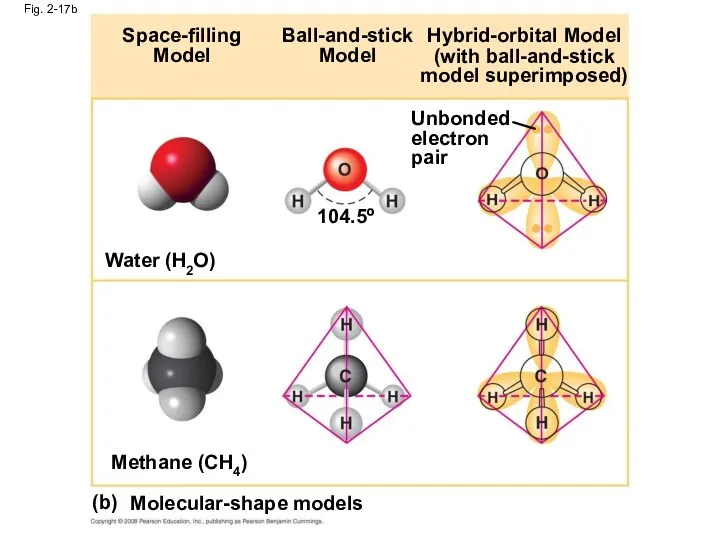
- 69. Fig. 2-17b Space-filling Model Ball-and-stick Model Hybrid-orbital Model (with ball-and-stick model superimposed) Unbonded electron pair 104.5º
- 70. Biological molecules recognize and interact with each other with a specificity based on molecular shape Molecules
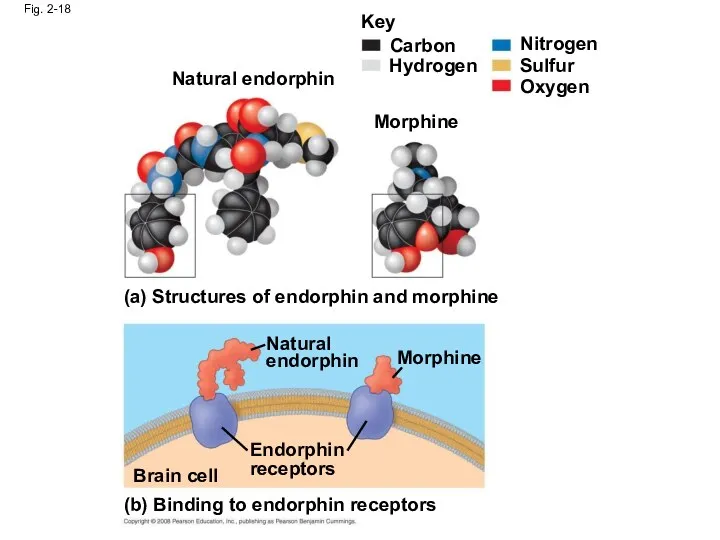
- 71. Fig. 2-18 (a) Structures of endorphin and morphine (b) Binding to endorphin receptors Natural endorphin Endorphin
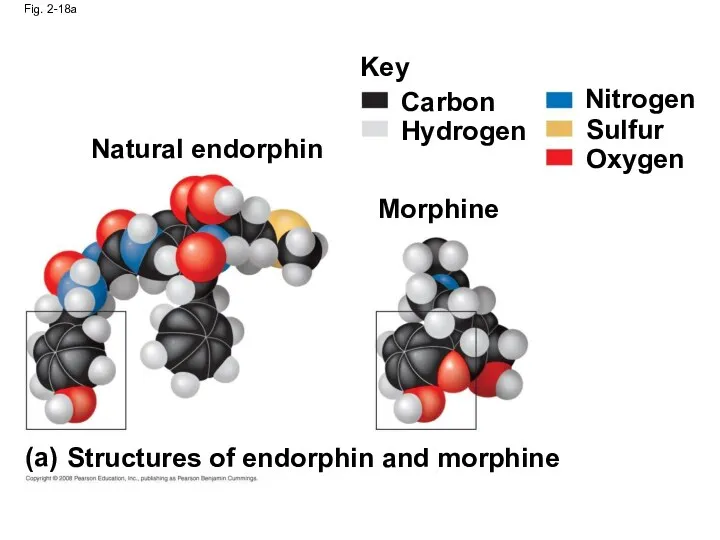
- 72. Fig. 2-18a Natural endorphin Morphine Key Carbon Hydrogen Nitrogen Sulfur Oxygen Structures of endorphin and morphine
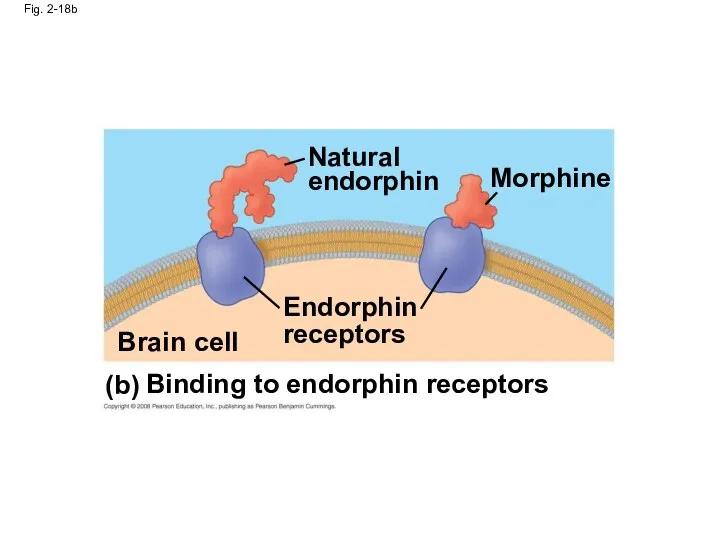
- 73. Fig. 2-18b Natural endorphin Endorphin receptors Brain cell Binding to endorphin receptors Morphine (b)
- 74. Concept 2.4: Chemical reactions make and break chemical bonds Chemical reactions are the making and breaking
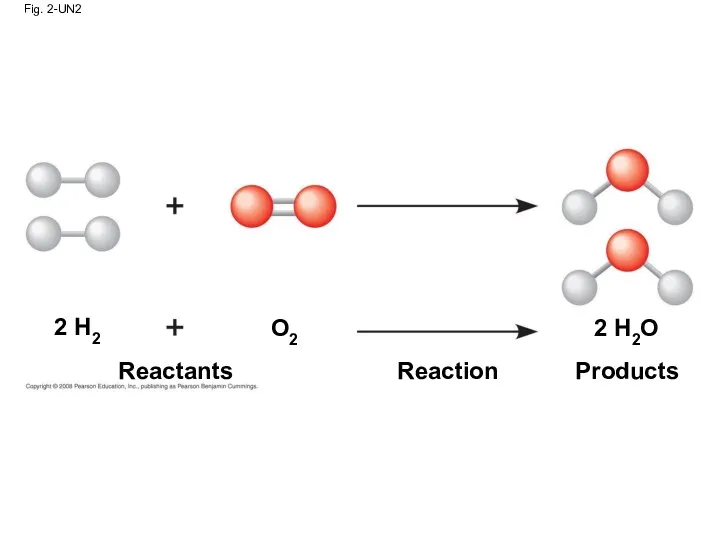
- 75. Fig. 2-UN2 Reactants Reaction Products 2 H2 O2 2 H2O
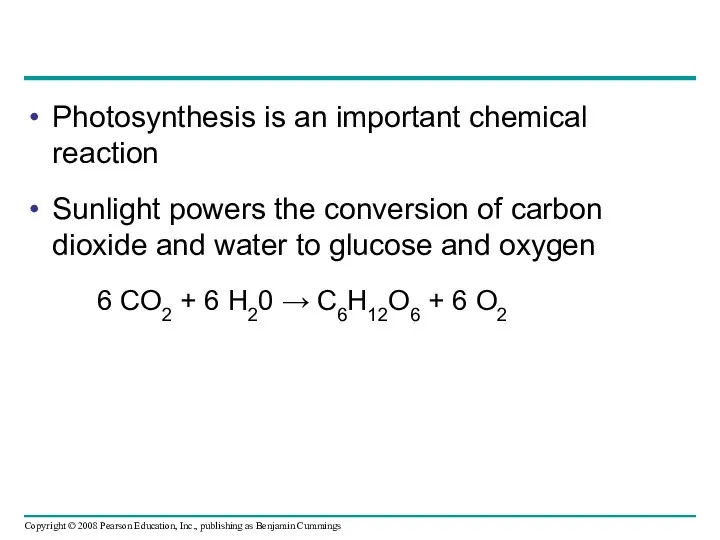
- 76. Photosynthesis is an important chemical reaction Sunlight powers the conversion of carbon dioxide and water to
- 77. Fig. 2-19
- 78. Some chemical reactions go to completion: all reactants are converted to products All chemical reactions are
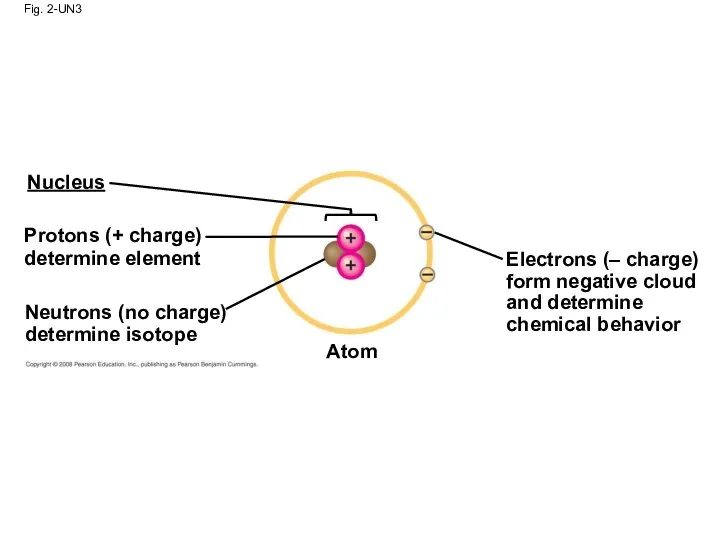
- 79. Fig. 2-UN3 Nucleus Protons (+ charge) determine element Neutrons (no charge) determine isotope Atom Electrons (–
- 80. Fig. 2-UN4
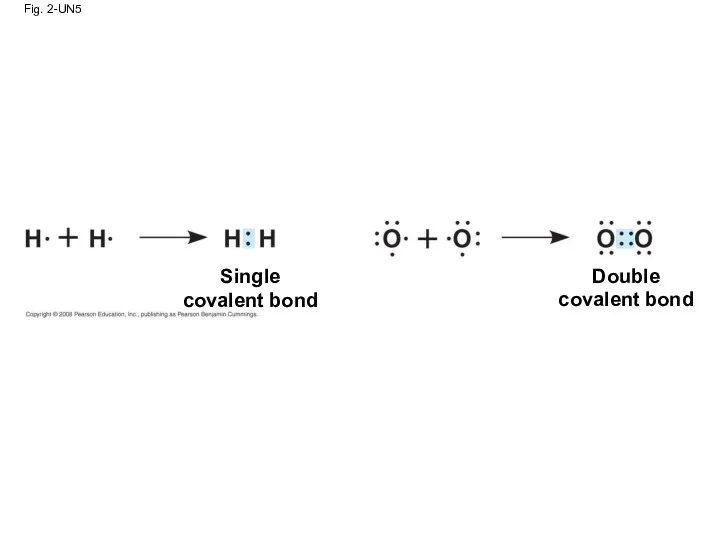
- 81. Fig. 2-UN5 Single covalent bond Double covalent bond
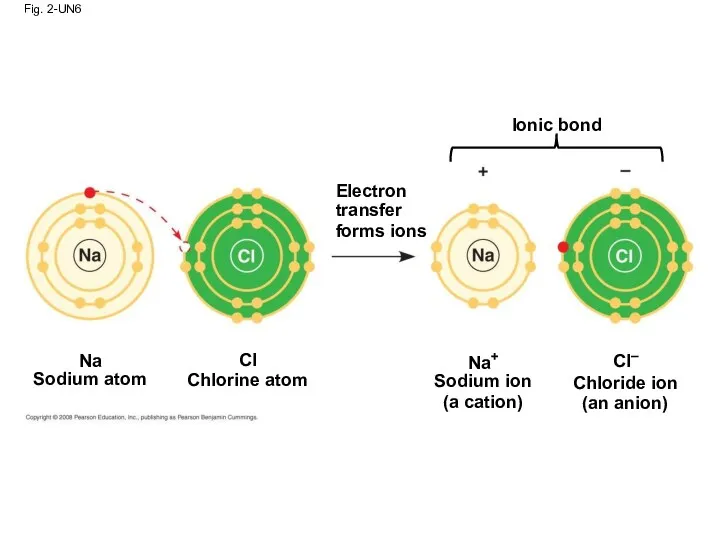
- 82. Fig. 2-UN6 Ionic bond Electron transfer forms ions Na Sodium atom Cl Chlorine atom Na+ Sodium
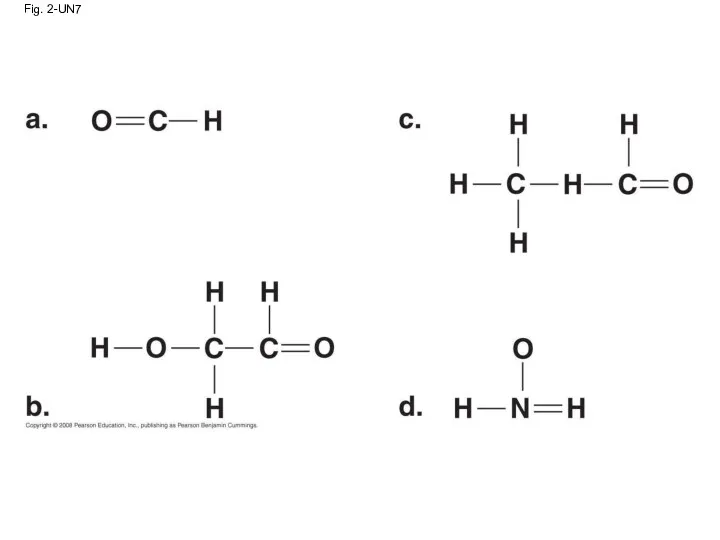
- 83. Fig. 2-UN7
- 84. Fig. 2-UN8
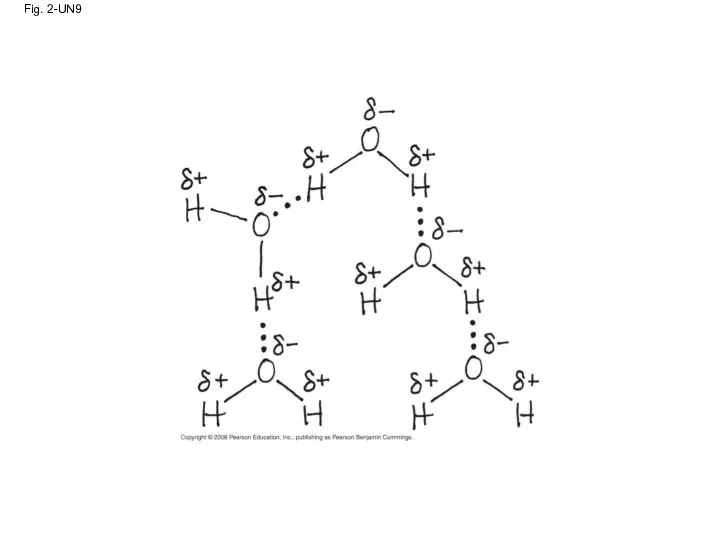
- 85. Fig. 2-UN9
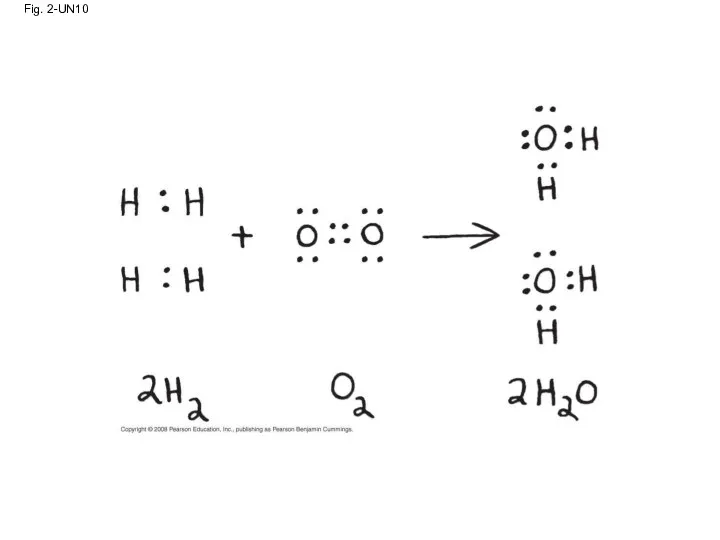
- 86. Fig. 2-UN10
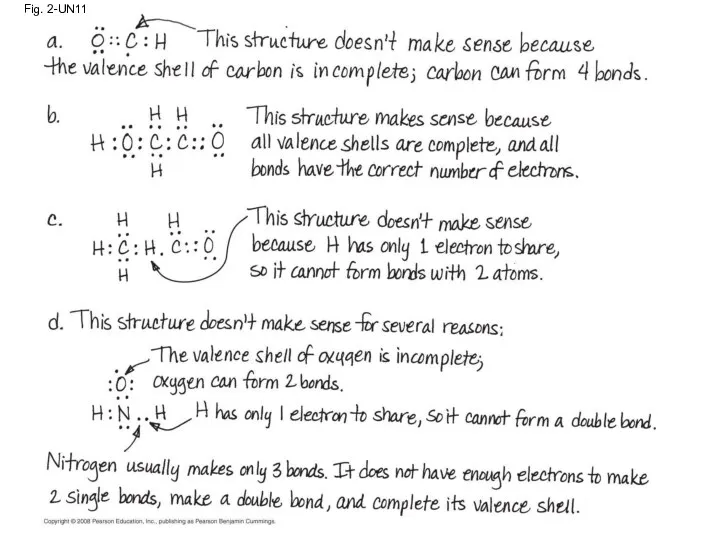
- 87. Fig. 2-UN11
- 89. Скачать презентацию















 Тип Членистоногие (Arthropoda). Класс Скрыточелюстные насекомые (Insecta - Entognatha)
Тип Членистоногие (Arthropoda). Класс Скрыточелюстные насекомые (Insecta - Entognatha) Эволюционное учение (урок обобщающего повторения для 10 класса)
Эволюционное учение (урок обобщающего повторения для 10 класса) Цветковые растения
Цветковые растения История развития биологических знаний. Предмет, задачи и методы биологии. Основные концепции современной биологии
История развития биологических знаний. Предмет, задачи и методы биологии. Основные концепции современной биологии Гідрологія. Показники якості води
Гідрологія. Показники якості води Сердечно-сосудистая система. Сердце. Кровеносные сосуды. Лимфатическая система
Сердечно-сосудистая система. Сердце. Кровеносные сосуды. Лимфатическая система Презентация к уроку биологии Царство: грибы 6 класс Часть 2 Диск
Презентация к уроку биологии Царство: грибы 6 класс Часть 2 Диск Цитоскелет
Цитоскелет Физиология ЦНС. Промежуточный мозг и ретикулярная формация
Физиология ЦНС. Промежуточный мозг и ретикулярная формация Урок-презентация по теме :Бабочки.
Урок-презентация по теме :Бабочки. Происхождение человека (антропогенез)
Происхождение человека (антропогенез) Розовый слон
Розовый слон Гепард
Гепард Презентация по биологии 6 класс Мхи
Презентация по биологии 6 класс Мхи Генетика человека
Генетика человека Живые организмы весной
Живые организмы весной Видоизменения побегов
Видоизменения побегов Cellular Respiration
Cellular Respiration 20230330_genetika_obnov_wecompress.com_
20230330_genetika_obnov_wecompress.com_ Применение проектной технологии на уроках биологии и во внеурочной деятельности
Применение проектной технологии на уроках биологии и во внеурочной деятельности Зоология позвоночных. Надкласс четвероногие. (Лекция 7)
Зоология позвоночных. Надкласс четвероногие. (Лекция 7) Гортензия метельчатая Bombshell
Гортензия метельчатая Bombshell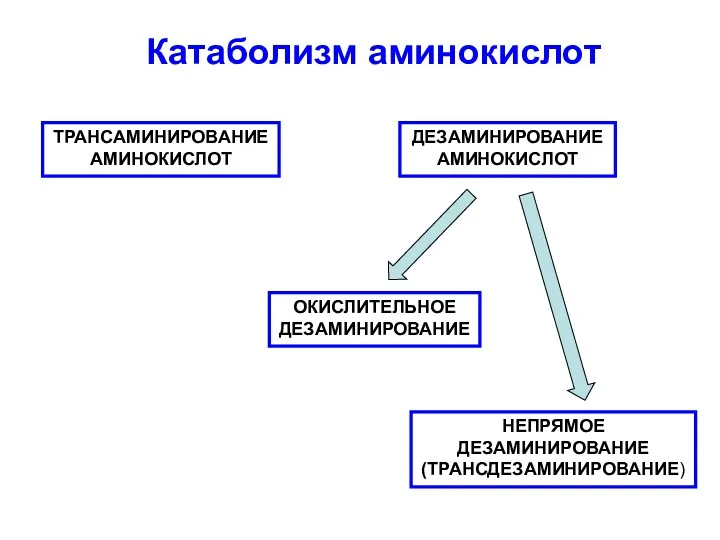 Катаболизм аминокислот
Катаболизм аминокислот Разнообразие рептилий
Разнообразие рептилий Digestion
Digestion Перелетные птицы
Перелетные птицы Популяция. Свойства популяций
Популяция. Свойства популяций Красная книга Крыма
Красная книга Крыма