Содержание
- 2. Aim the aim of this module is that you should understand the structure and function of
- 3. The respiratory system serves to ensure that all tissues receive the oxygen they need and can
- 4. Transport & exchange blood carries gases to and from tissues lungs exchange with atmosphere
- 5. Blood has the intrinsic capacity to pick up oxygen and lose CO2 if exposed to the
- 6. The Physics of gases the physiology is easy if you understand the physics
- 7. What is atmospheric pressure? Atmospheric pressure is the force per unit area exerted against a surface
- 8. Pressure is also expressed in mmHg (eg Blood Pressure of 120/80 mmHg). 1 kPa = 7.5
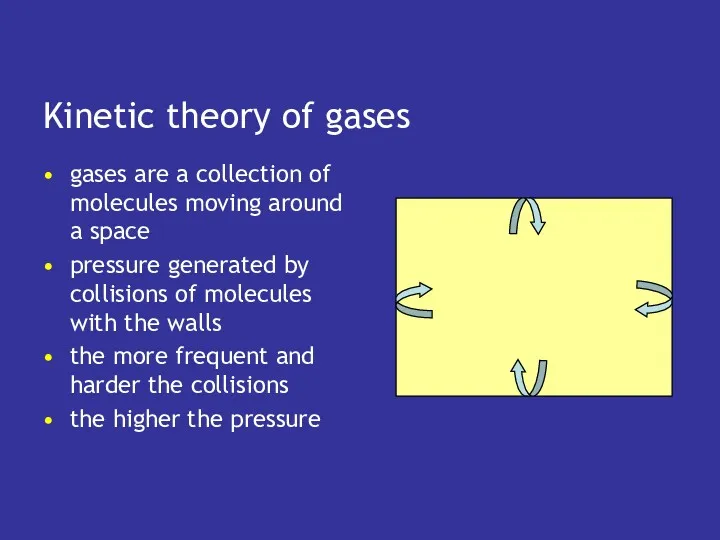
- 9. Kinetic theory of gases gases are a collection of molecules moving around a space pressure generated
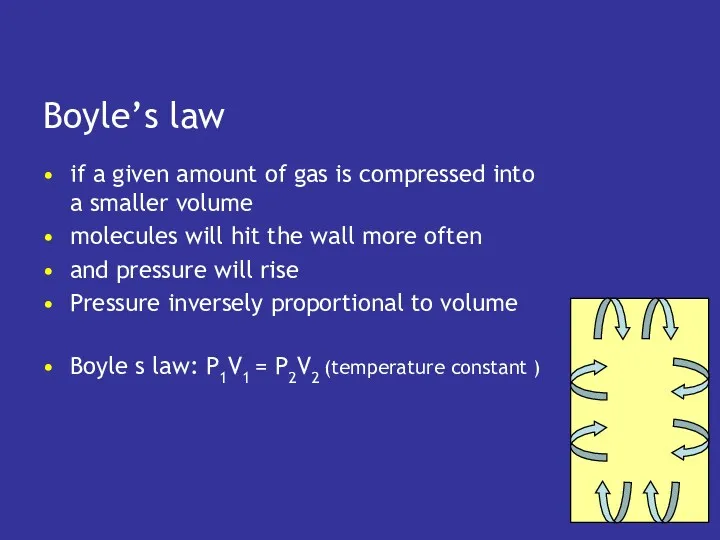
- 10. Boyle’s law if a given amount of gas is compressed into a smaller volume molecules will
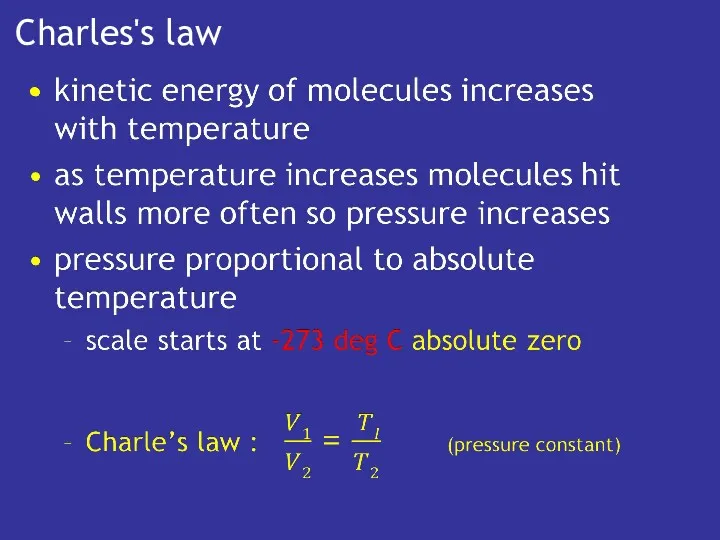
- 11. Charles's law
- 12. Universal gas law P.V=R.T allows calculation of how volume will change as pressure and temperature changes
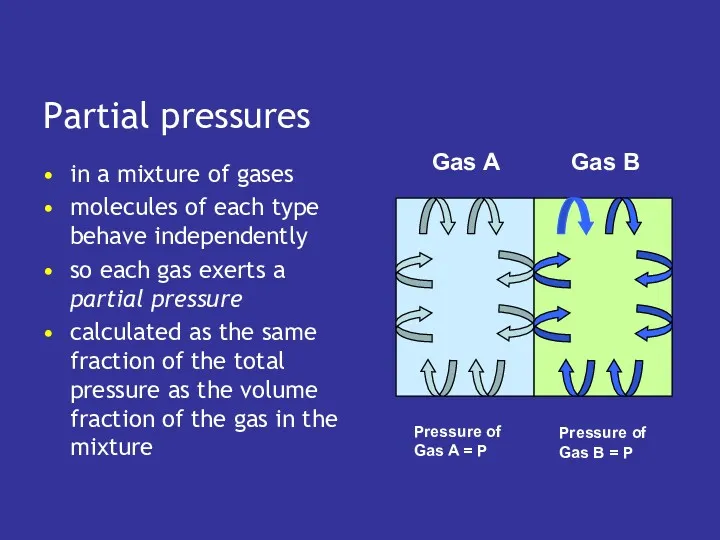
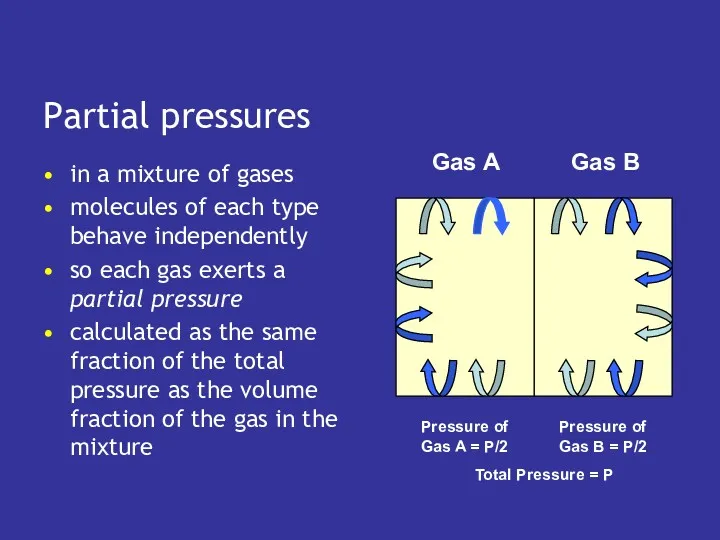
- 13. Partial pressures in a mixture of gases molecules of each type behave independently so each gas
- 14. Partial pressures in a mixture of gases molecules of each type behave independently so each gas
- 15. In respiratory physiology, one deals with mixtures of gases, mainly of O2, N2,and CO2. The rate
- 16. The concept of partial pressure can be explained as follows. Consider air, which has an approximate
- 17. Dalton’s law states that the partial pressure of a gas (x) in a gas mixture is
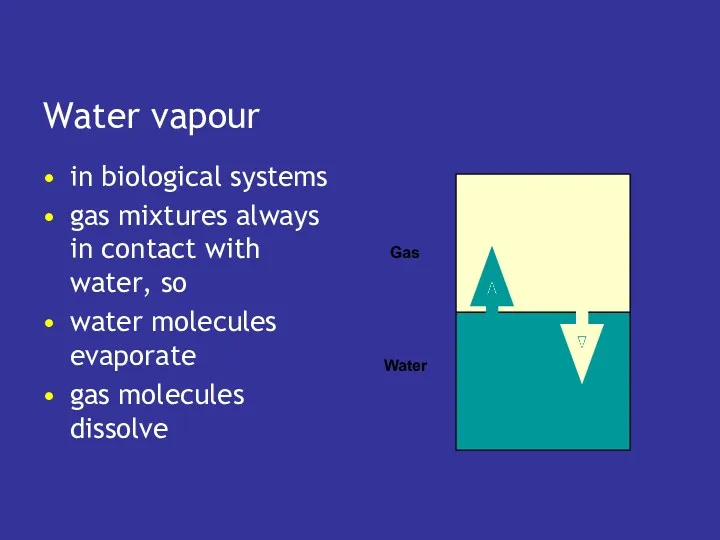
- 18. Water vapour in biological systems gas mixtures always in contact with water, so water molecules evaporate
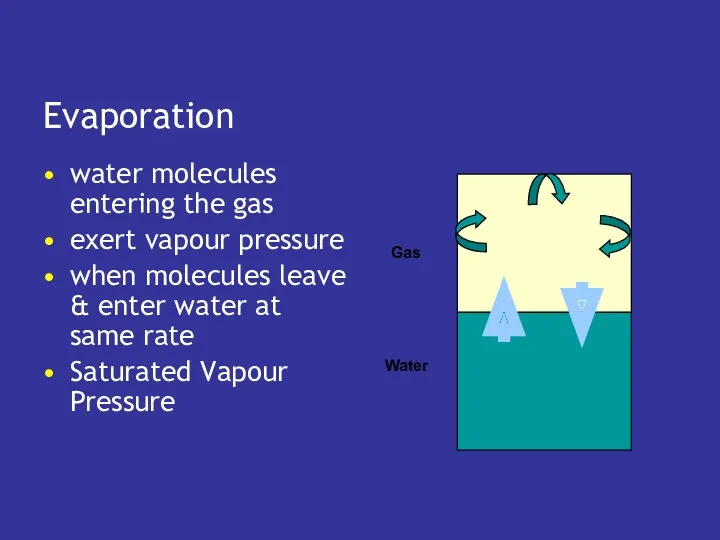
- 19. Evaporation water molecules entering the gas exert vapour pressure when molecules leave & enter water at
- 20. Saturated Vapour Pressure depends only on temperature water vapor pressure at 0°C is 5 mm Hg
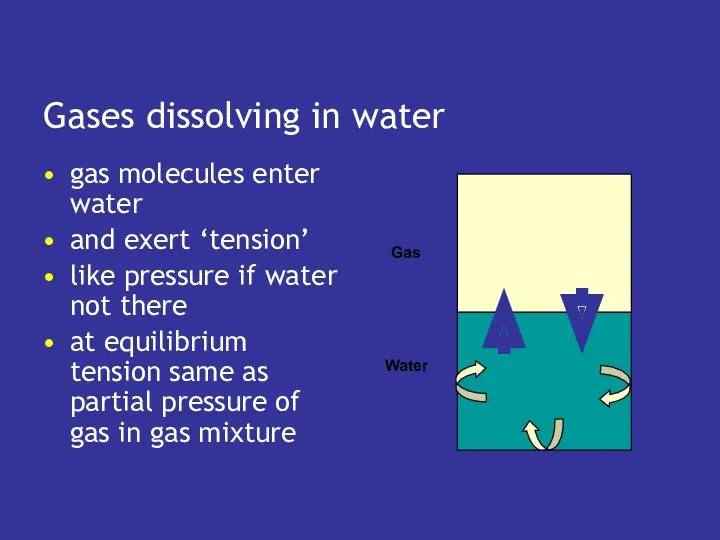
- 21. Gases dissolving in water gas molecules enter water and exert ‘tension’ like pressure if water not
- 22. Gas Tension in Liquids indicates how readily gas will leave the liquid not (at least directly)
- 23. Solubility the amount of gas which enters the liquid to establish a particular tension is determined
- 24. Solubility of Gases: The amount of a gas dissolved in plasma = solubility of that gas
- 25. Chemical reactions of gases with liquids if a gas reacts with a component of the liquid
- 26. Example plasma just dissolves oxygen at pO2 of 13.3 kPa (100 mmHg) content 0.13 mmol.l-1
- 27. whole blood contains Haemoglobin which reacts chemically with oxygen at 13.3 kPa Haemoglobin binds 8.8 mmol.l-1
- 28. Gas exchange in the lung at rest 5l of blood must pick up 12 mmol of
- 29. Getting a tennis court into the thorax need a very large number of very small compartments
- 30. Airways air reaches the alveoli via a complex tree of airways over 20 divisions
- 31. Airways Trachea branches to main bronchi main bronchi to lobar bronchi 3 on right 2 on
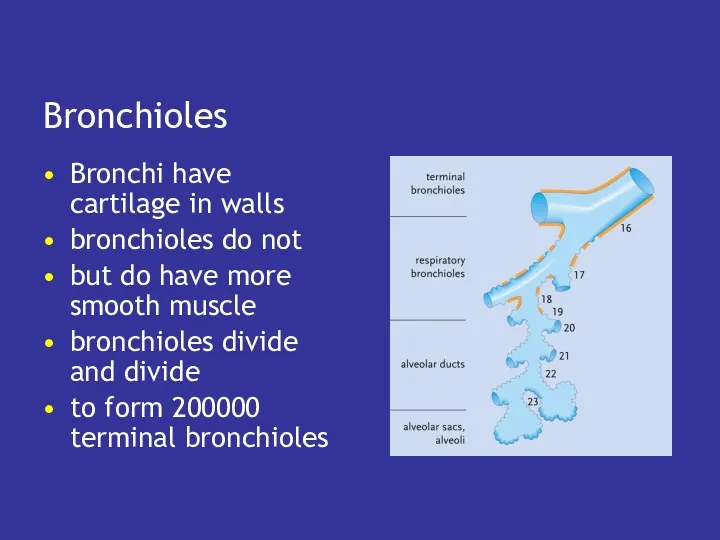
- 32. Bronchioles Bronchi have cartilage in walls bronchioles do not but do have more smooth muscle bronchioles
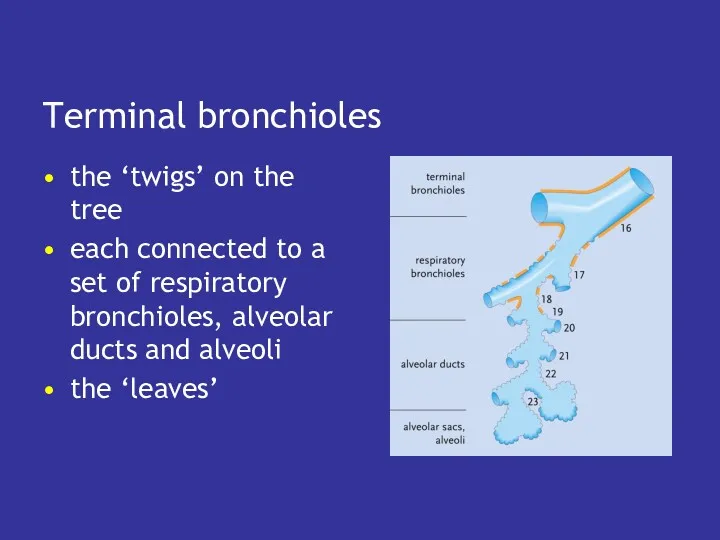
- 33. Terminal bronchioles the ‘twigs’ on the tree each connected to a set of respiratory bronchioles, alveolar
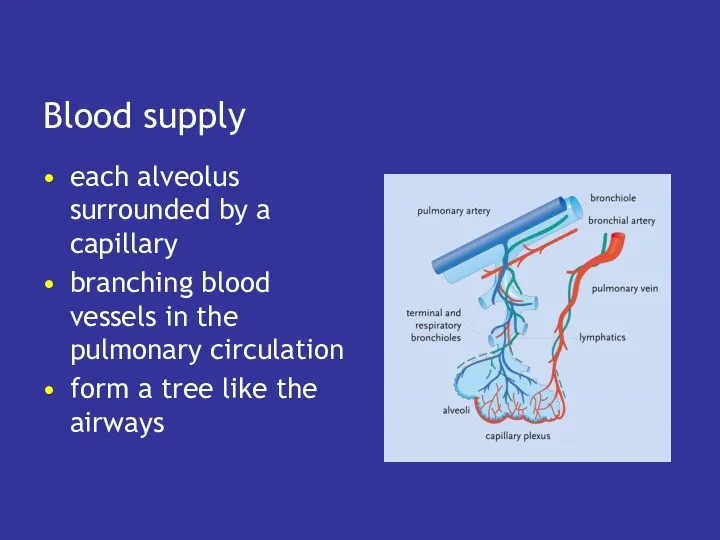
- 34. Blood supply each alveolus surrounded by a capillary branching blood vessels in the pulmonary circulation form
- 35. The lungs are a means of getting air to one side and blood to the other
- 36. The pulmonary circulation low resistance low pressure receives entire cardiac output
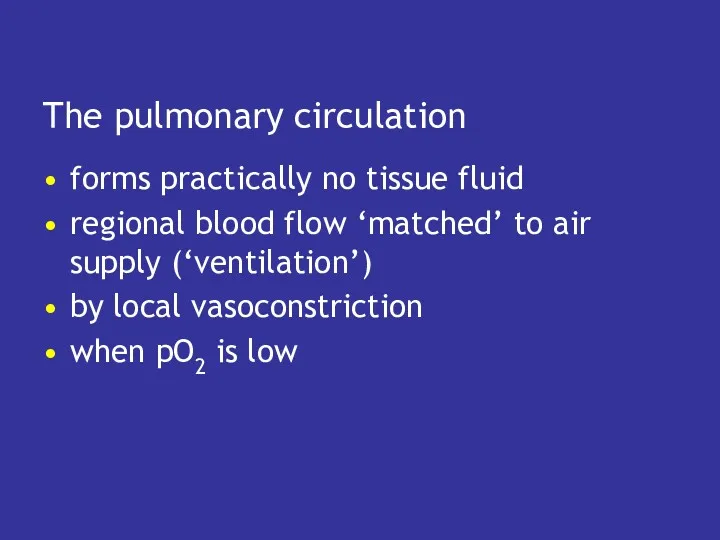
- 37. The pulmonary circulation forms practically no tissue fluid regional blood flow ‘matched’ to air supply (‘ventilation’)
- 38. Ventilation perfusion matching is vital and often disturbed by disease
- 39. Ventilation air drawn into lungs by increasing volume of terminal and respiratory bronchioles as lungs expand
- 41. Скачать презентацию

























 Исследование полезных свойств видов рода прострел и создание фиточаев. Проект
Исследование полезных свойств видов рода прострел и создание фиточаев. Проект Роль биологии в космических исследованиях
Роль биологии в космических исследованиях Генетика пола. Наследование,сцепленное с полом
Генетика пола. Наследование,сцепленное с полом Животные жарких стран. Для дошкольников
Животные жарких стран. Для дошкольников Трихинеллез. Устойчивость возбудителя. Лабораторная диагностика
Трихинеллез. Устойчивость возбудителя. Лабораторная диагностика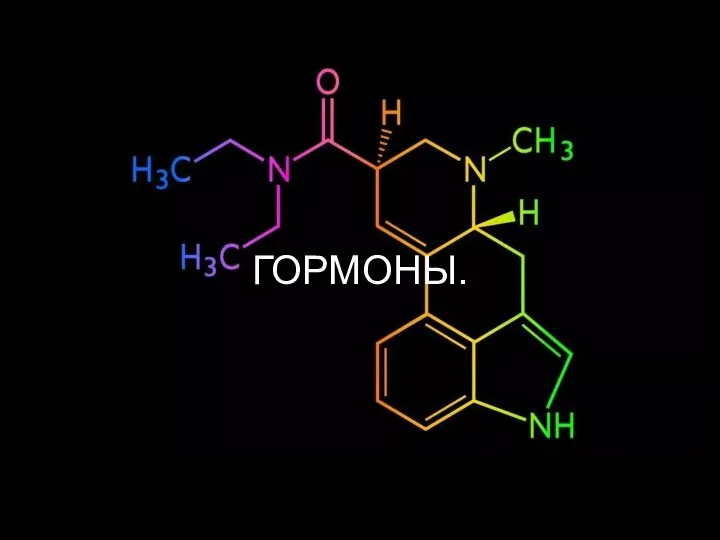 Гормоны. Виды гормонов и их классификация
Гормоны. Виды гормонов и их классификация Ткани растений
Ткани растений Биотехнология и биоэкономика: состояние и перспективы
Биотехнология и биоэкономика: состояние и перспективы Виды движений в биомеханике. Лекция 5
Виды движений в биомеханике. Лекция 5 Анатомия лицевого черепа
Анатомия лицевого черепа Фрукти і овочі: характеристика, вимоги до якості, умови зберігання
Фрукти і овочі: характеристика, вимоги до якості, умови зберігання Обмен углеводов: значение, переваривание. Гликолиз. Пентозофосфатный путь окисления глюкозы
Обмен углеводов: значение, переваривание. Гликолиз. Пентозофосфатный путь окисления глюкозы Психофизиология воли
Психофизиология воли Особенности строения безногих ящериц
Особенности строения безногих ящериц Медоносные растения Донбасса
Медоносные растения Донбасса Анатомия и физиология человека. Предмет и его значение. Методы изучения. История
Анатомия и физиология человека. Предмет и его значение. Методы изучения. История Класс птицы
Класс птицы Растительноядные, хищные, паразиты и сверх паразиты среди представителей насекомых
Растительноядные, хищные, паразиты и сверх паразиты среди представителей насекомых Дигибридное скрещивание. Третий закон Г. Менделя
Дигибридное скрещивание. Третий закон Г. Менделя Мочевыделительная система человека. Анатомия органов мочевыделительной системы
Мочевыделительная система человека. Анатомия органов мочевыделительной системы Медико-біологічні основи паразитизму. Медична протозоологія. Найпростіші паразити людини
Медико-біологічні основи паразитизму. Медична протозоологія. Найпростіші паразити людини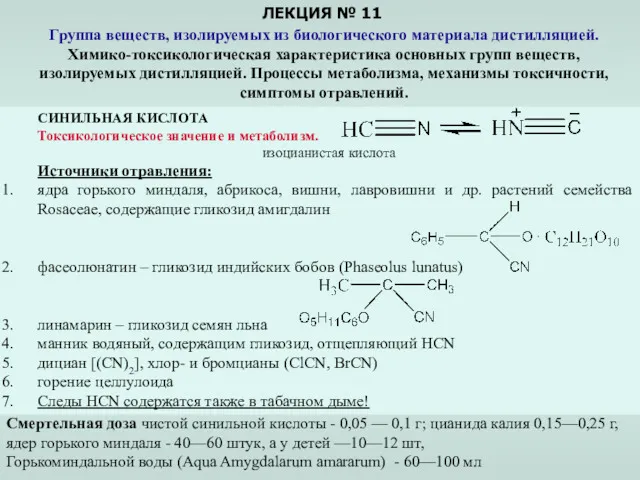 Группа веществ, изолируемых из биологического материала дистилляцией. (Лекция 11)
Группа веществ, изолируемых из биологического материала дистилляцией. (Лекция 11) ДНК-геномы
ДНК-геномы Мендель Грегор Иоганн
Мендель Грегор Иоганн Вид, популяция
Вид, популяция Селекция. Задачи селекции
Селекция. Задачи селекции Строение и функции репродуктивной системы
Строение и функции репродуктивной системы Царство животные. Тип хордовые. Класс птицы
Царство животные. Тип хордовые. Класс птицы