Содержание
- 2. How many colours of CARBON do you know?
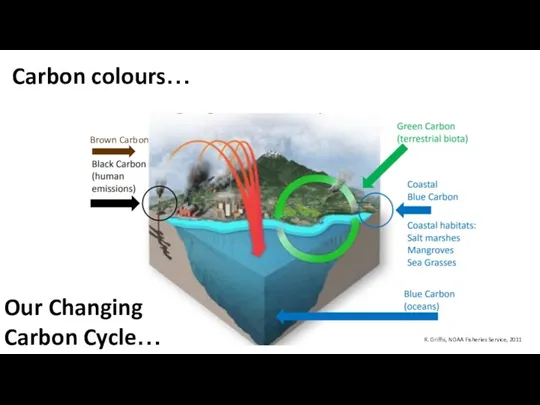
- 3. Carbon colours… R. Griffis, NOAA Fisheries Service, 2011 Our Changing Carbon Cycle… Brown Carbon
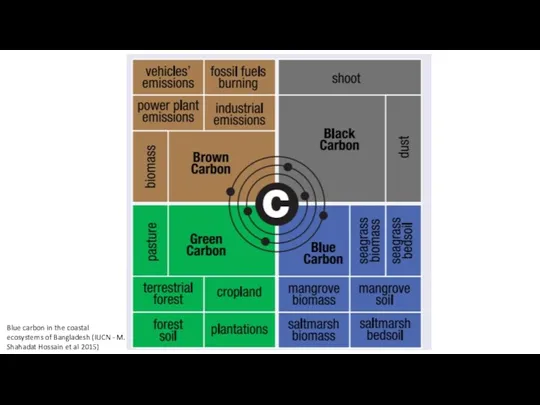
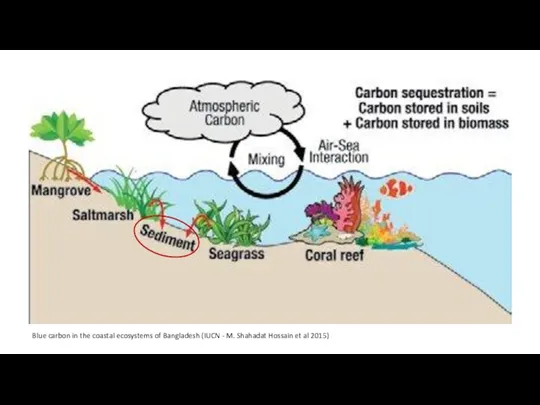
- 4. Blue carbon in the coastal ecosystems of Bangladesh (IUCN - M. Shahadat Hossain et al 2015)
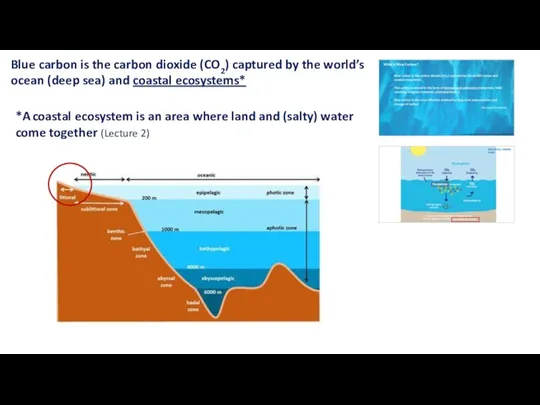
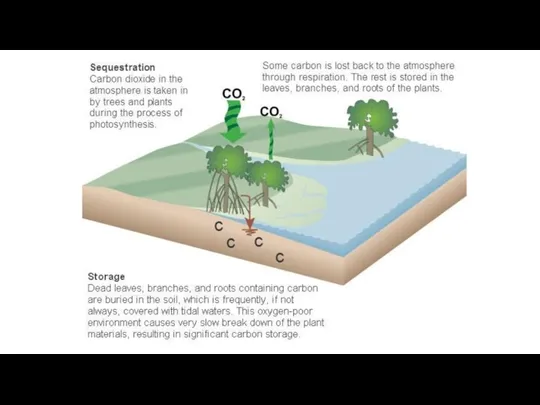
- 5. What is Blue Carbon? Blue carbon is the carbon dioxide (CO2) captured by the world’s ocean
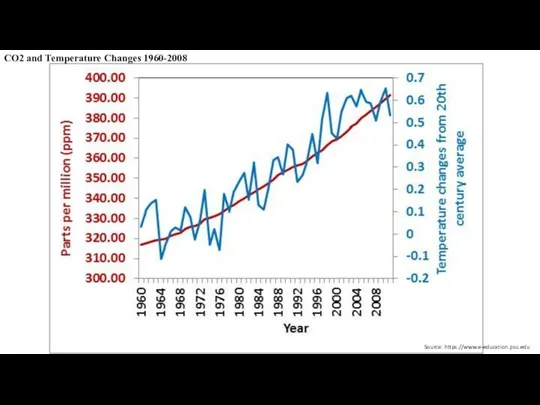
- 6. CO2 and Temperature Changes 1960-2008 Source: https://www.e-education.psu.edu
- 7. Oceans absorb greenhouse gases (GHG) Source: Conservation International. E.Pidgeon, S. Troëng, 2011 Blue Carbon Reducing our
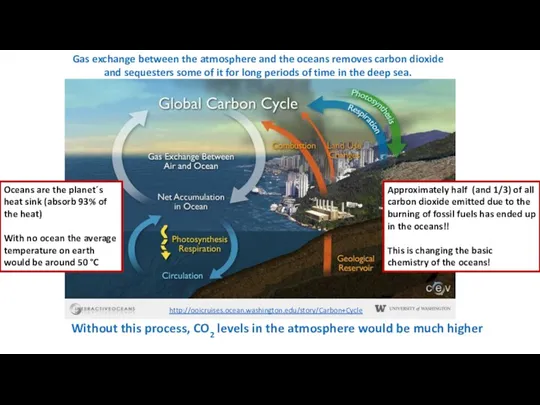
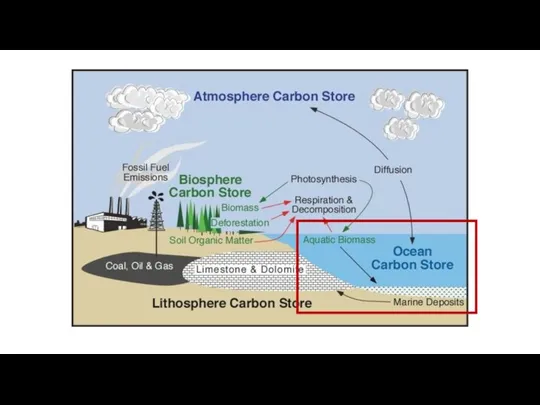
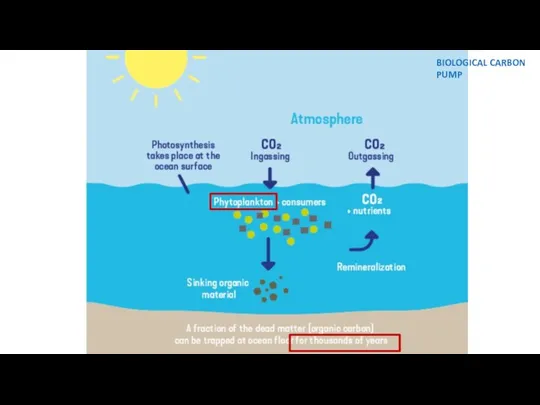
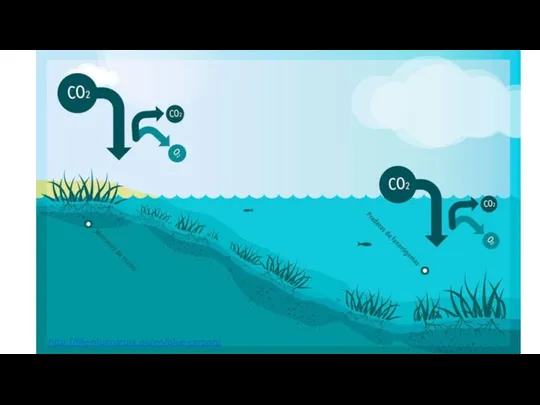
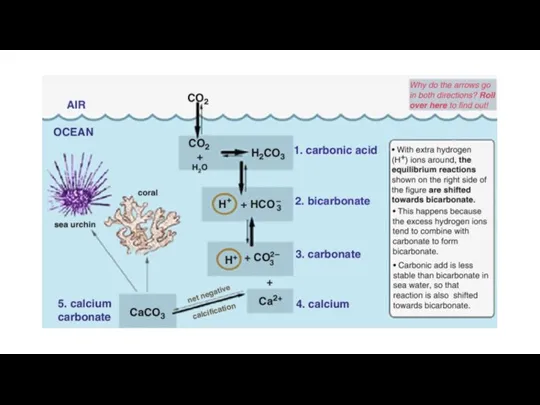
- 8. Gas exchange between the atmosphere and the oceans removes carbon dioxide and sequesters some of it
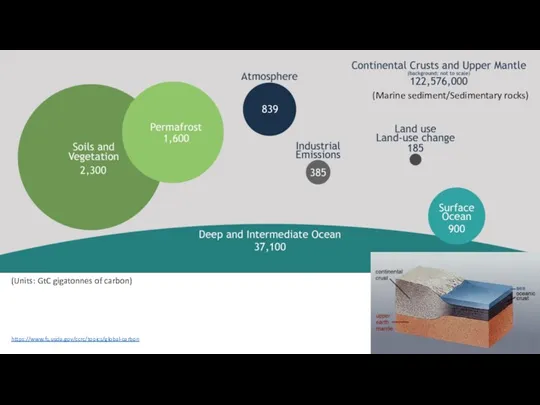
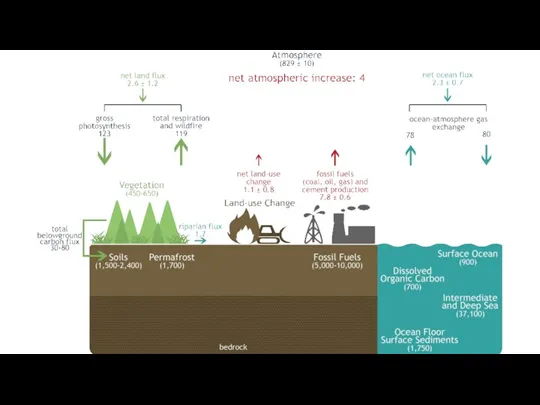
- 10. https://www.fs.usda.gov/ccrc/topics/global-carbon (Marine sediment/Sedimentary rocks) (Units: GtC gigatonnes of carbon)
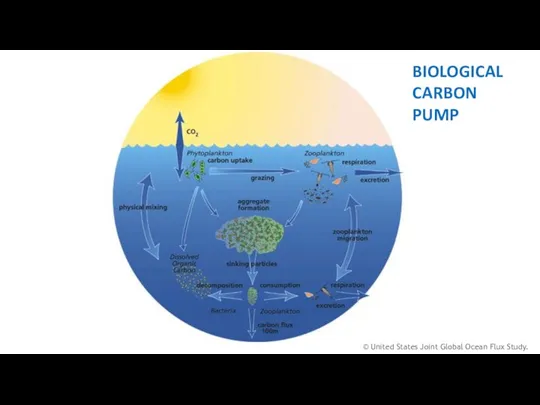
- 12. BIOLOGICAL CARBON PUMP
- 13. © United States Joint Global Ocean Flux Study. BIOLOGICAL CARBON PUMP
- 14. Blue carbon is the carbon dioxide (CO2) captured by the world’s ocean (deep sea) and coastal
- 15. Three key ecosystems… Seagrass Mangroves Salt Marshes Coastal ecosystems transfer carbon from the atmosphere and ocean
- 16. BLUE FOREST https://www.unenvironment.org/news-and-stories/story/blue-forests-finding-coastal-and-marine-solutions-meet-paris-agreement ”TWO MINUTES ON OCEANS” (video youtube) (focused on mangroves but extrapolated to other
- 18. Blue carbon in the coastal ecosystems of Bangladesh (IUCN - M. Shahadat Hossain et al 2015)
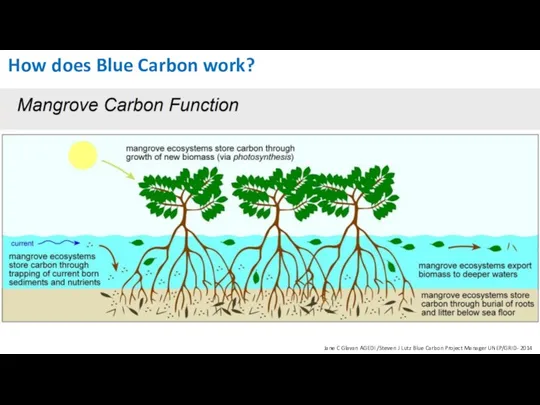
- 19. How does Blue Carbon work? Jane C Glavan AGEDI /Steven J Lutz Blue Carbon Project Manager
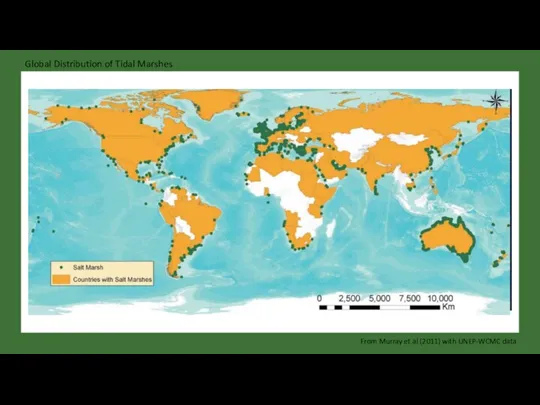
- 21. Global Distribution of Tidal Marshes From Murray et al (2011) with UNEP-WCMC data
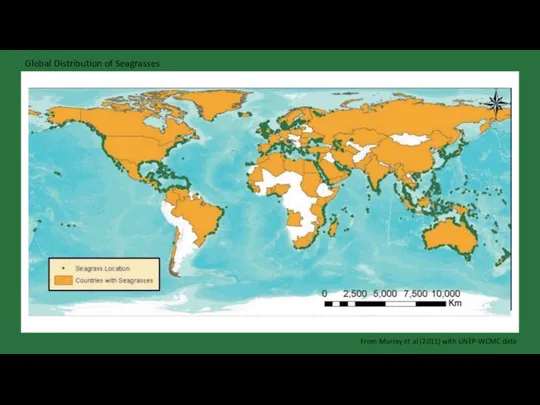
- 22. Global Distribution of Seagrasses From Murray et al (2011) with UNEP-WCMC data
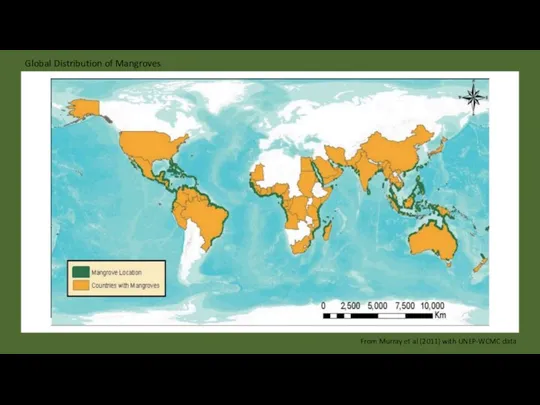
- 23. Global Distribution of Mangroves From Murray et al (2011) with UNEP-WCMC data
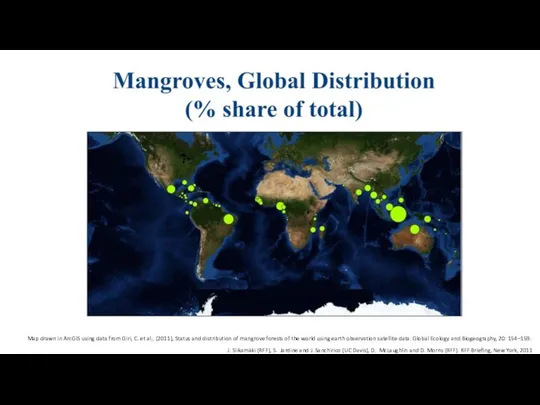
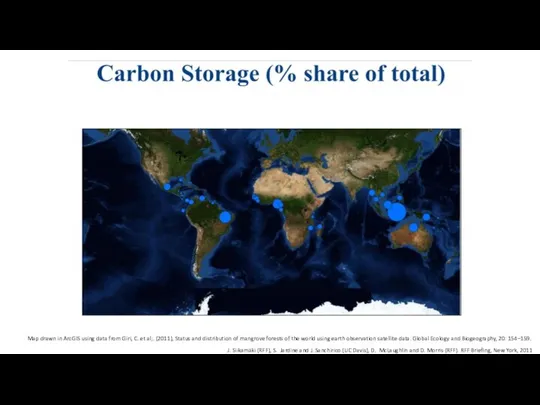
- 24. J. Siikamäki (RFF), S. Jardine and J. Sanchirico (UC Davis), D. McLaughlin and D. Morris (RFF).
- 25. J. Siikamäki (RFF), S. Jardine and J. Sanchirico (UC Davis), D. McLaughlin and D. Morris (RFF).
- 26. http://life-bluenatura.eu/en/blue-carbon/
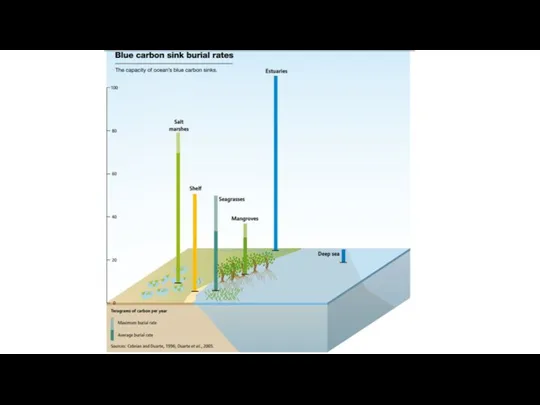
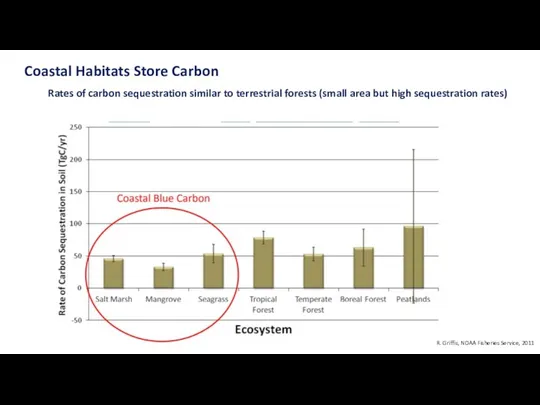
- 27. Coastal ecosystems are smaller, but the rate of sequestration are larger Traditionally, terrestrial ecosystems have been
- 28. Coastal Habitats Store Carbon Rates of carbon sequestration similar to terrestrial forests (small area but high
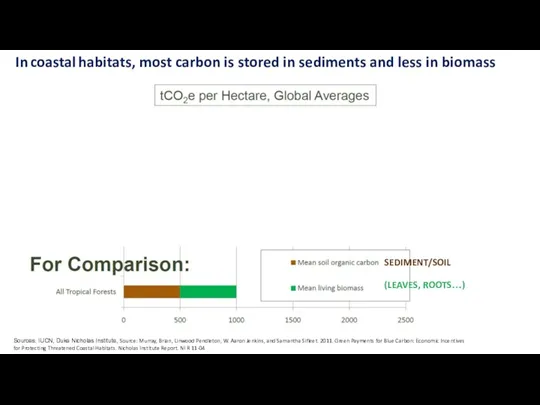
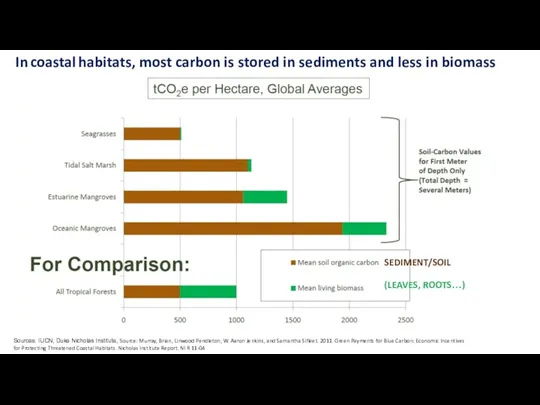
- 29. In coastal habitats, most carbon is stored in sediments and less in biomass Sources: IUCN, Duke
- 30. In coastal habitats, most carbon is stored in sediments and less in biomass Sources: IUCN, Duke
- 31. These coastal systems are being rapidly lost and degraded Source: Conservation International. E.Pidgeon, S. Troëng, 2011
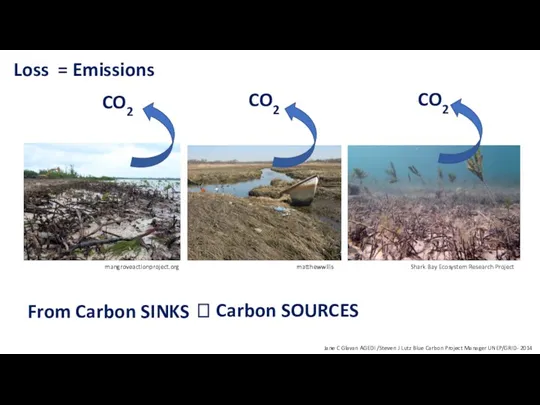
- 32. Loss From Carbon SINKS Jane C Glavan AGEDI /Steven J Lutz Blue Carbon Project Manager UNEP/GRID-
- 33. Carbon sequestration (“Blue Carbon”) Seas absorb a third of CO2 emitted annually!!
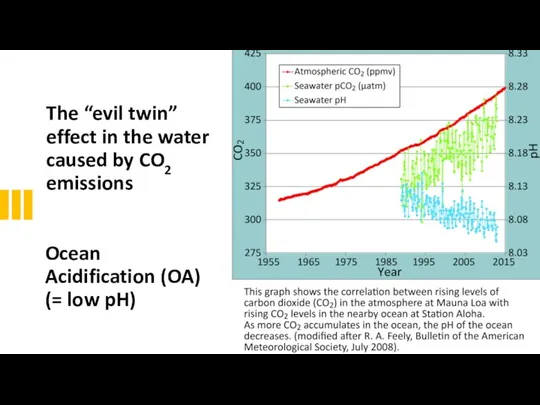
- 34. The “evil twin” effect in the water caused by CO2 emissions Ocean Acidification (OA) (= low
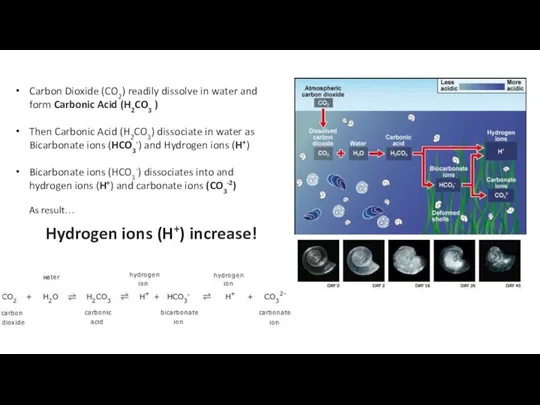
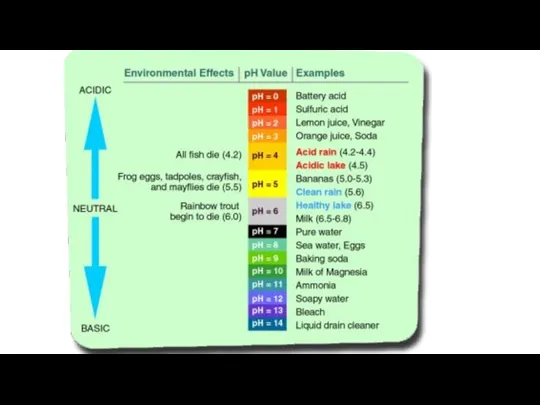
- 35. Ocean Acidification - Osteoporosis of the sea ECGS-601 Carbon Dioxide (CO2) readily dissolve in water and
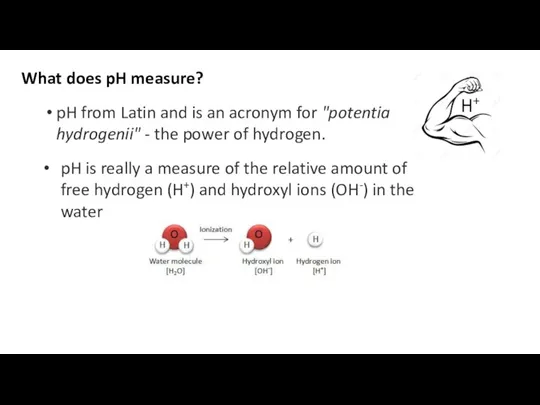
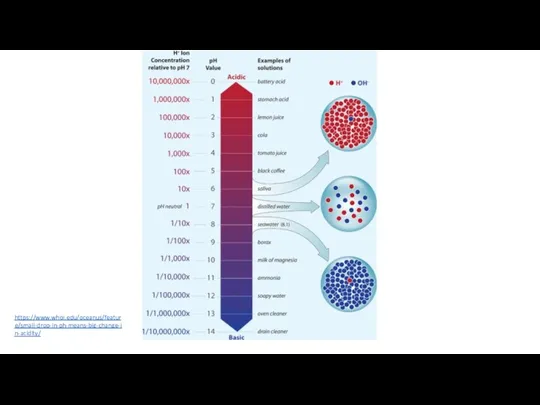
- 36. What does pH measure? pH from Latin and is an acronym for "potentia hydrogenii" - the
- 37. pH is reported in "logarithmic units“ Each number represents a 10-fold change in the acidity/basicness of
- 38. https://www.whoi.edu/oceanus/feature/small-drop-in-ph-means-big-change-in-acidity/
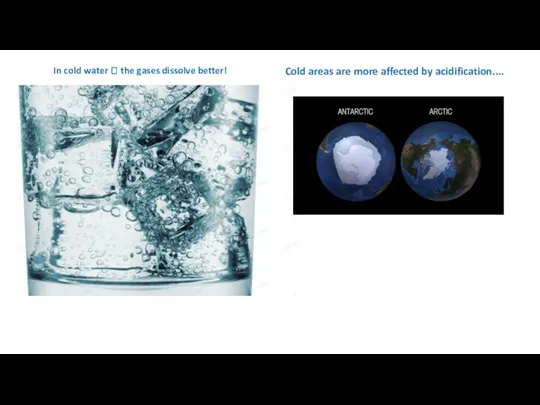
- 40. In cold water ? the gases dissolve better! Cold areas are more affected by acidification....
- 41. Water that has more free hydrogen ions (H+) is acidic, whereas water that has more free
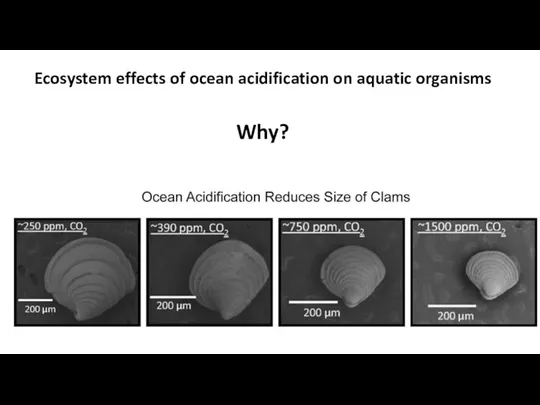
- 42. Ecosystem effects of ocean acidification on aquatic organisms
- 43. Ecosystem effects of ocean acidification on aquatic organisms Why?
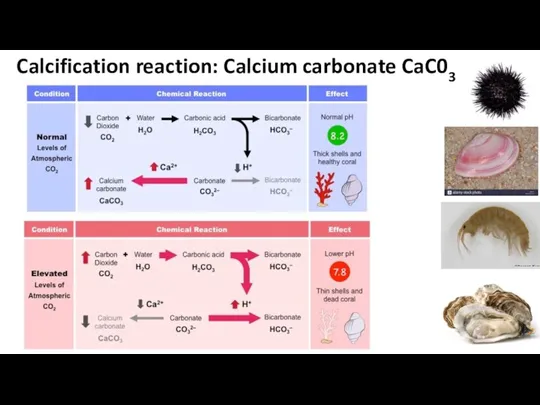
- 44. Calcification reaction: Calcium carbonate CaC03
- 45. Calcification = Building a brick house….
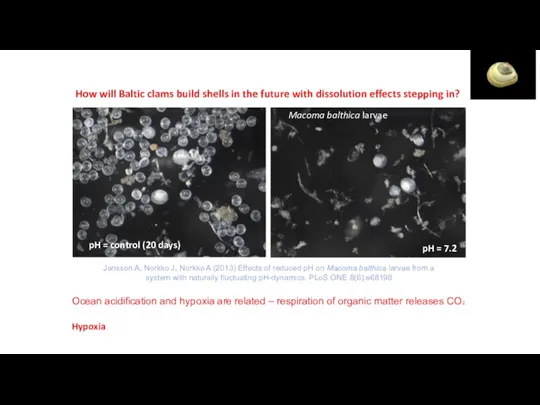
- 47. How will Baltic clams build shells in the future with dissolution effects stepping in? Ocean acidification
- 49. Castello Aragonese (Italy) Underwater It is a 14th century castle off the coast of Italy
- 50. There are volcanic vents naturally release bubbles of carbon dioxide gas, creating different levels of acidity
- 51. In Moodle (Literature) Video
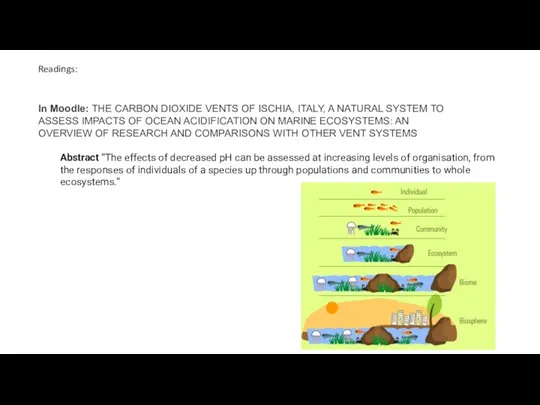
- 52. Readings: Abstract “The effects of decreased pH can be assessed at increasing levels of organisation, from
- 54. Algal bloom ? increase or decrease the pH? Why?
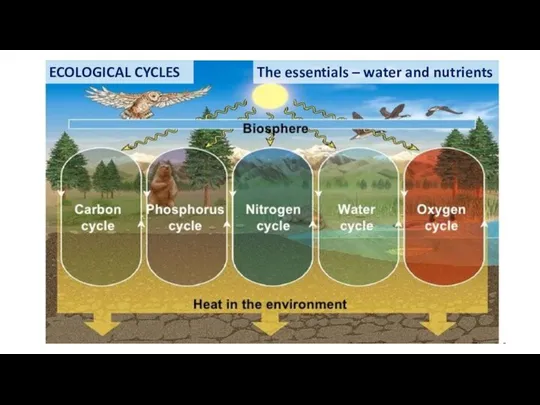
- 55. ECOLOGICAL CYCLES The essentials – water and nutrients
- 58. Скачать презентацию

















 презентация био 0804
презентация био 0804 Отчет о деятельности СПбРО РЭА и МОО Природоохранный союз
Отчет о деятельности СПбРО РЭА и МОО Природоохранный союз Викиди хімічної промисловості
Викиди хімічної промисловості Влияние полиэтилена на экологическую ситуацию
Влияние полиэтилена на экологическую ситуацию Рациональное природопользование. Предотвращение загрязнения морской среды
Рациональное природопользование. Предотвращение загрязнения морской среды Красная книга России. 2 класс
Красная книга России. 2 класс Suv–hayot manbayi
Suv–hayot manbayi Построение и реализация уровневой подготовки по техносферной безопасности в транспортном вузе
Построение и реализация уровневой подготовки по техносферной безопасности в транспортном вузе Организация и проведение производственного экологического контроля для предприятий и организаций
Организация и проведение производственного экологического контроля для предприятий и организаций Предмет и задачи экологии
Предмет и задачи экологии Экологические проблемы животных
Экологические проблемы животных Экология, как наука. Задания на лето
Экология, как наука. Задания на лето Опале листя: користь чи шкода
Опале листя: користь чи шкода презентация наш воздухкасающийся проблемы
презентация наш воздухкасающийся проблемы Методическая разработка урока географии по теме Глобальные проблемы человечества
Методическая разработка урока географии по теме Глобальные проблемы человечества Экологические проблемы Казахстана
Экологические проблемы Казахстана Проект эко-кварталов Полис на Комендантском
Проект эко-кварталов Полис на Комендантском Экологические аспекты рекреационного лесопользования. Воздействие рекреационного лесопользования на лесные экосистемы
Экологические аспекты рекреационного лесопользования. Воздействие рекреационного лесопользования на лесные экосистемы Экологическая викторина
Экологическая викторина Экологический квест
Экологический квест Экологическая проблема г. Кургана и Курганской области: анализ, воздействие на окружающую среду и стратегии решения
Экологическая проблема г. Кургана и Курганской области: анализ, воздействие на окружающую среду и стратегии решения Культура обращения с отходами
Культура обращения с отходами Проблемы охраны растений и животных в Казахстане
Проблемы охраны растений и животных в Казахстане Чистота воды в Панинских водоёмах
Чистота воды в Панинских водоёмах Предмет экологии. История развития
Предмет экологии. История развития Экологияның мәселелері арада әлемде және Қазақстанда
Экологияның мәселелері арада әлемде және Қазақстанда Анализ изменения состояния воздушного бассейна в результате реализации проекта строительства Томинского ГОКа
Анализ изменения состояния воздушного бассейна в результате реализации проекта строительства Томинского ГОКа Структура экосистемы
Структура экосистемы