Содержание
- 2. History of Earth’s Climate Earth formed ~4.6 billion years ago Originally very hot Sun’s energy output
- 3. History of Earth’s Climate Life appeared ~3.8 billion years ago Photosynthesis began 3.5-2.5 billion years ago
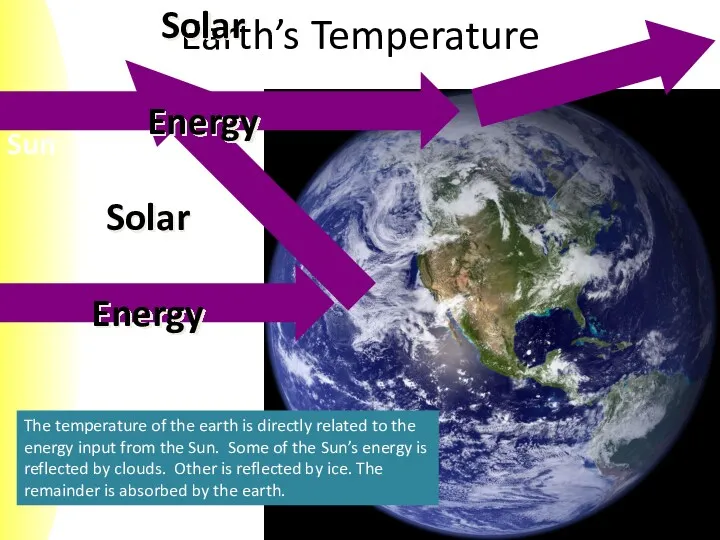
- 4. Earth’s Temperature The temperature of the earth is directly related to the energy input from the
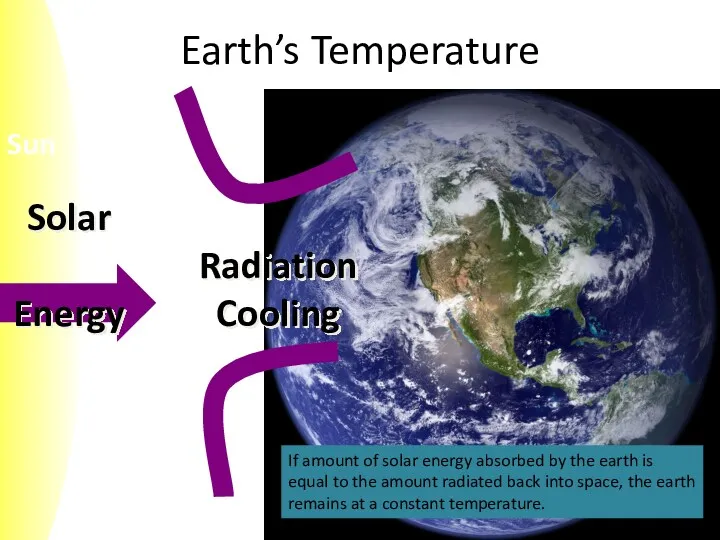
- 5. Earth’s Temperature If amount of solar energy absorbed by the earth is equal to the amount
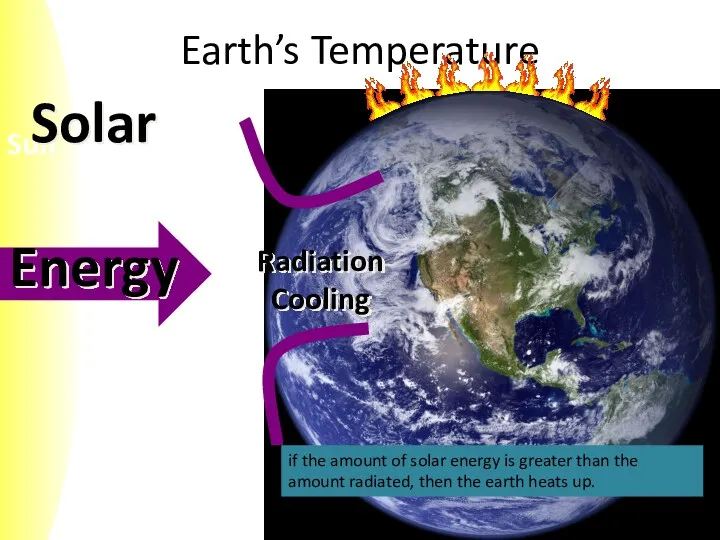
- 6. Earth’s Temperature if the amount of solar energy is greater than the amount radiated, then the
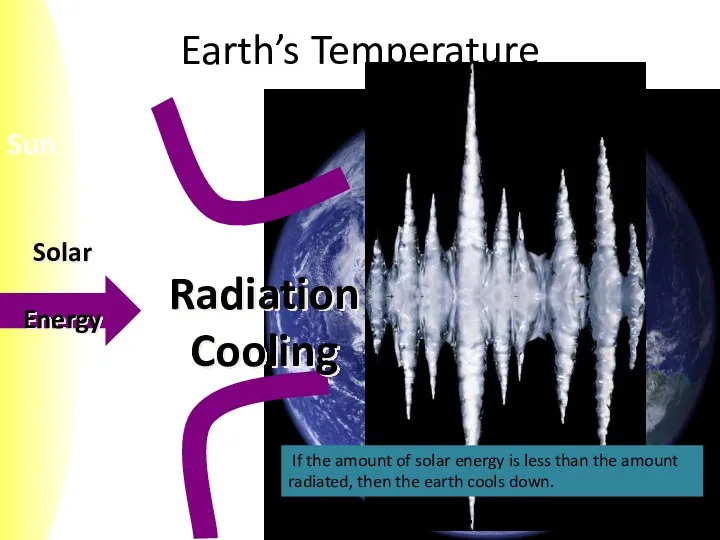
- 7. Earth’s Temperature If the amount of solar energy is less than the amount radiated, then the
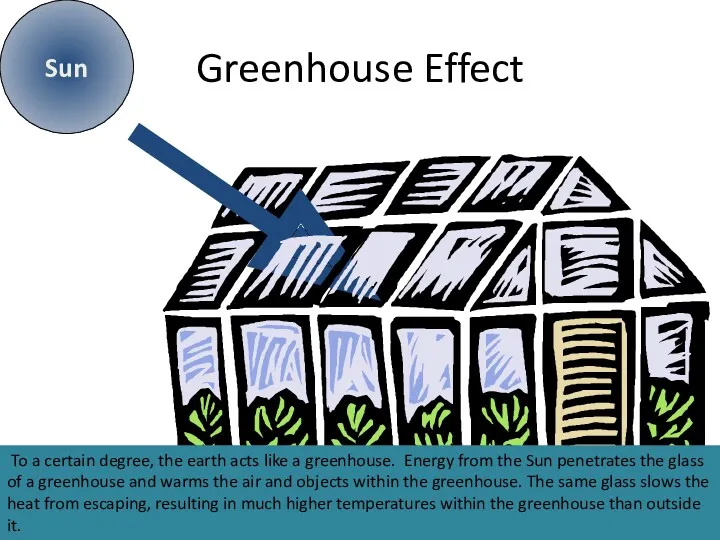
- 8. Greenhouse Effect Sun To a certain degree, the earth acts like a greenhouse. Energy from the
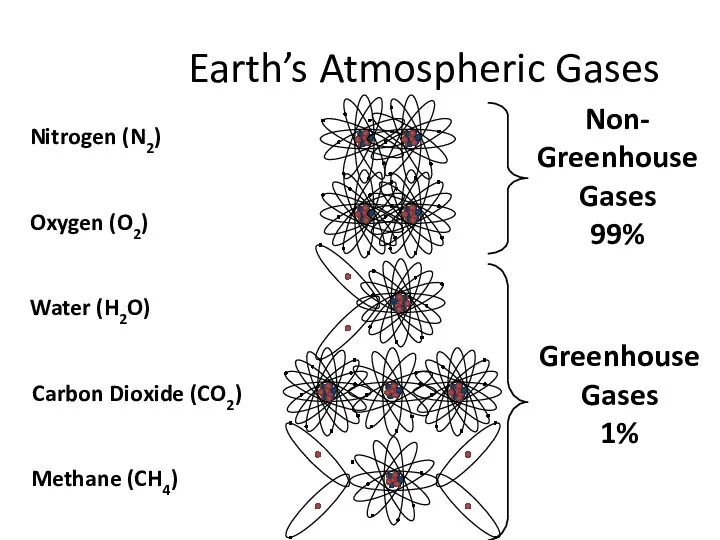
- 9. Earth’s Atmospheric Gases Non- Greenhouse Gases 99% Greenhouse Gases 1%
- 10. A trace gas is a gas that makes up an extremely small portion of a mixture
- 11. by Laurent Cousineau (Montreal) http://www.climate-change-guide.com/global-warming-potential-definition.html Global Warming Potential Definition The global warming potential (GWP) of a
- 12. Climate Feedback Definition A climate feedback is a process that will either amplify or reduce climate
- 13. Carbon Dioxide Definition Carbon dioxide (CO2) is the primary anthropogenic greenhouse gasresponsible for global warming. Although
- 14. Methane Definition Methane (CH4) is a hydrocarbon and an important greenhouse gas. According to the IPCC’s
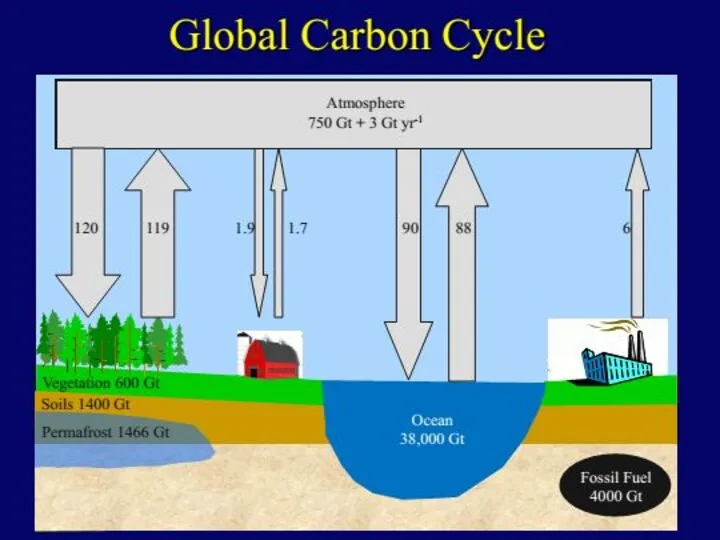
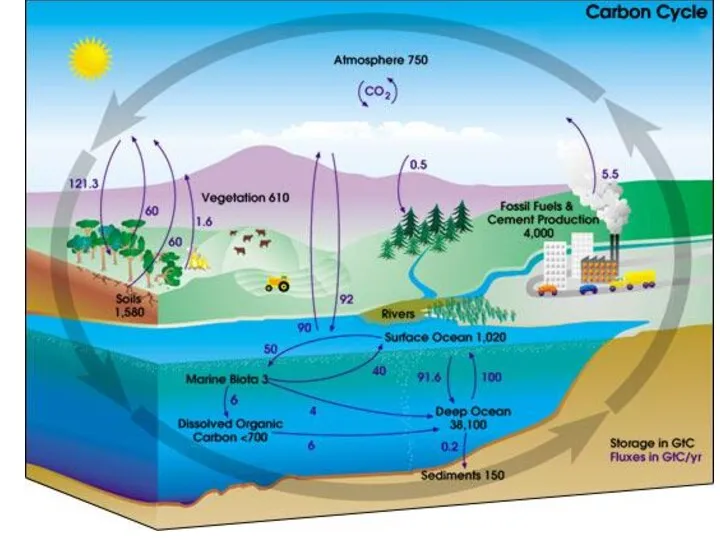
- 17. Natural sources of atmospheric carbon dioxide include volcanic outgassing, the combustion of organic matter, wildfires and
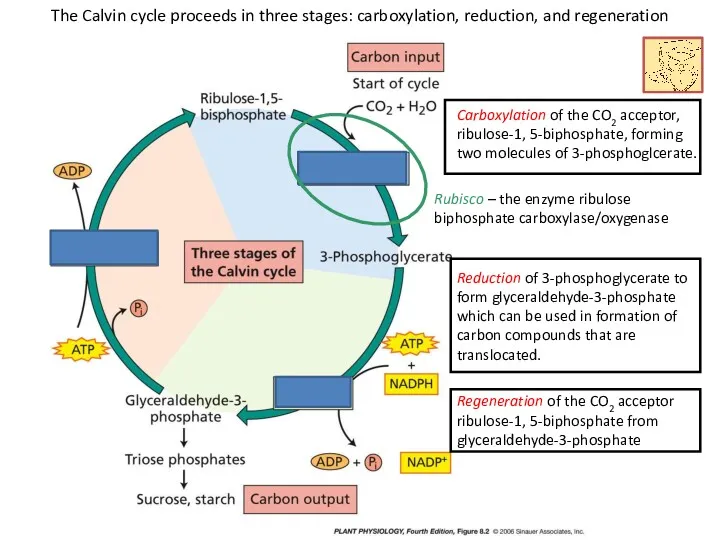
- 18. The Calvin cycle proceeds in three stages: carboxylation, reduction, and regeneration Carboxylation of the CO2 acceptor,
- 22. • There is a clear correlation between the amount of anthropogenic CO2 released to the atmosphere
- 23. На эти две реакции с ОН приходится около 90% удаления метана из атмосферы. Кроме реакции с
- 24. Pervasiveness of Life Snow algae on glacier Sierra Nevada, CA Earth life extraordinarily successful Natural selection
- 25. Five Things You Need to Have Life Stable Environment be able to adapt to changes Liquid
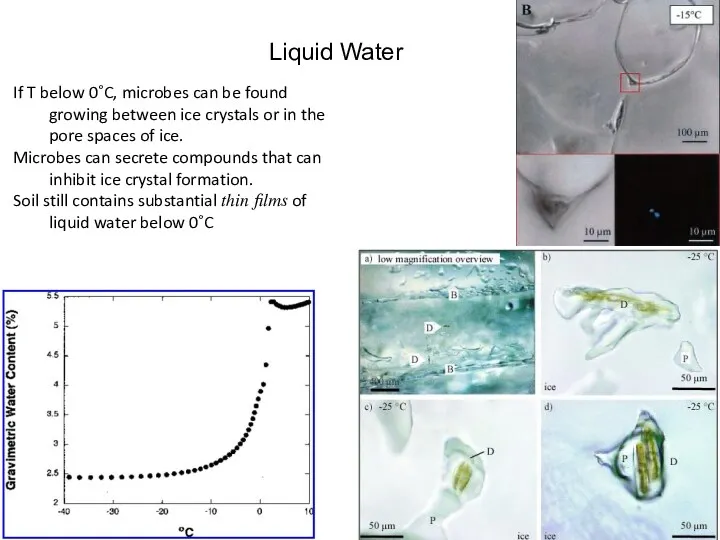
- 26. Liquid Water If T below 0˚C, microbes can be found growing between ice crystals or in
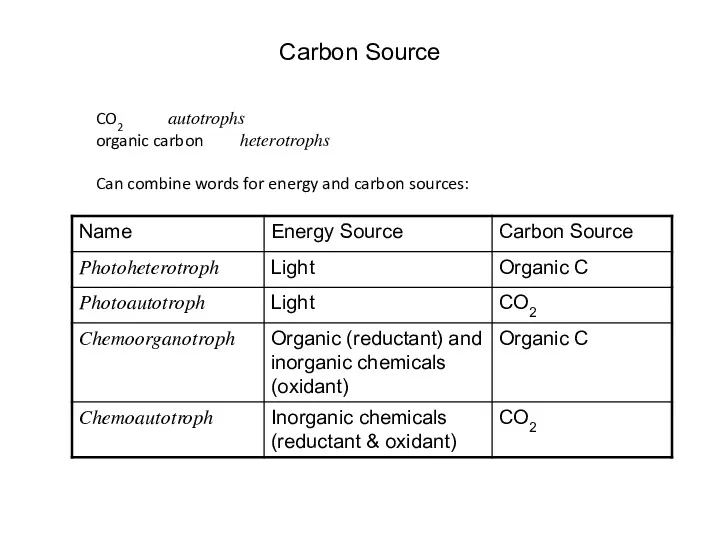
- 27. Carbon Source CO2 autotrophs organic carbon heterotrophs Can combine words for energy and carbon sources:
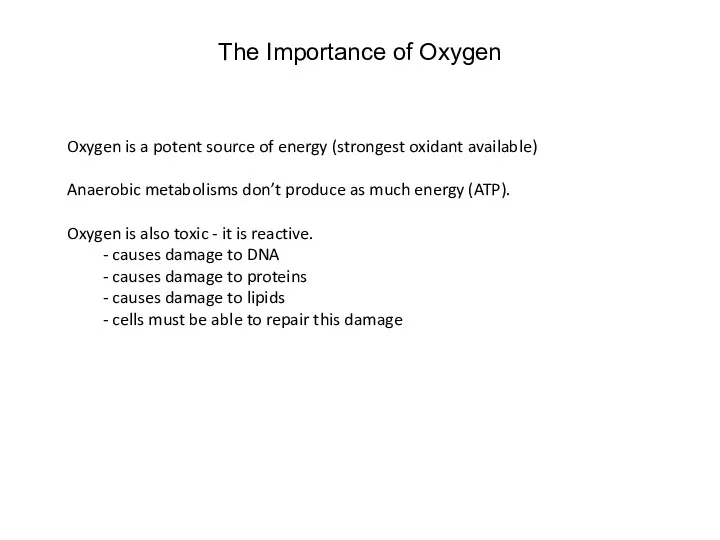
- 28. The Importance of Oxygen Oxygen is a potent source of energy (strongest oxidant available) Anaerobic metabolisms
- 29. Aerobic Metabolisms (Aerobes) Animals “CH2O” + O2 -? CO2 + H2O organotrophy Manganese Mn2+ + O2
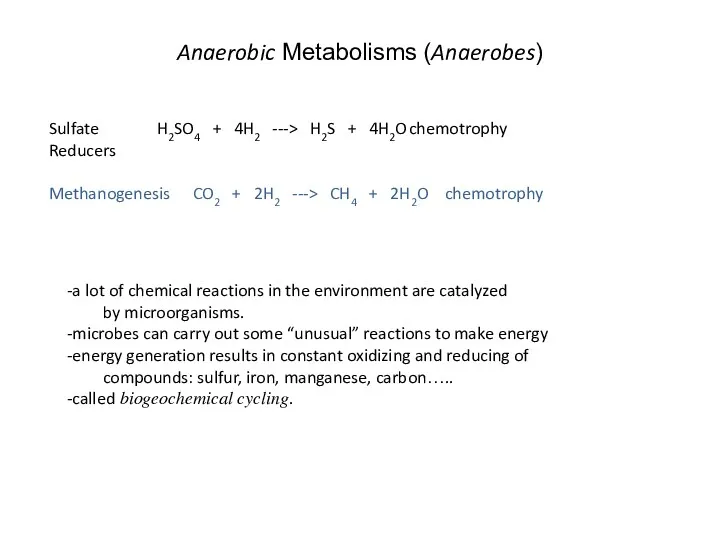
- 30. Anaerobic Metabolisms (Anaerobes) Sulfate H2SO4 + 4H2 ---> H2S + 4H2O chemotrophy Reducers Methanogenesis CO2 +
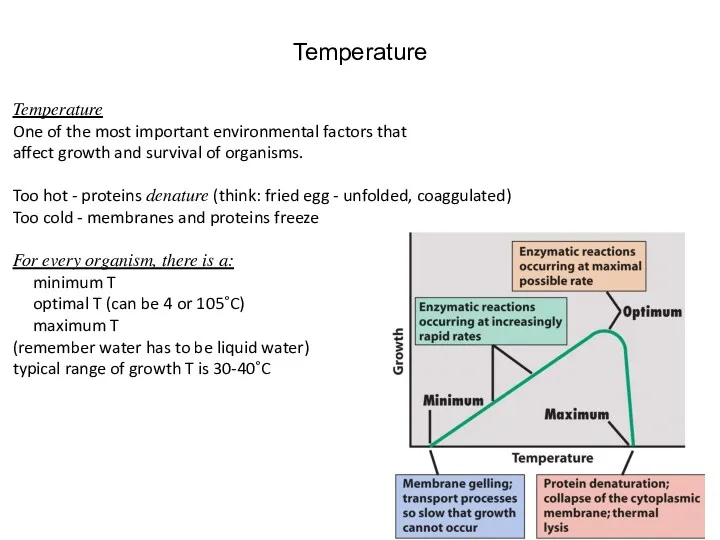
- 31. Temperature Temperature One of the most important environmental factors that affect growth and survival of organisms.
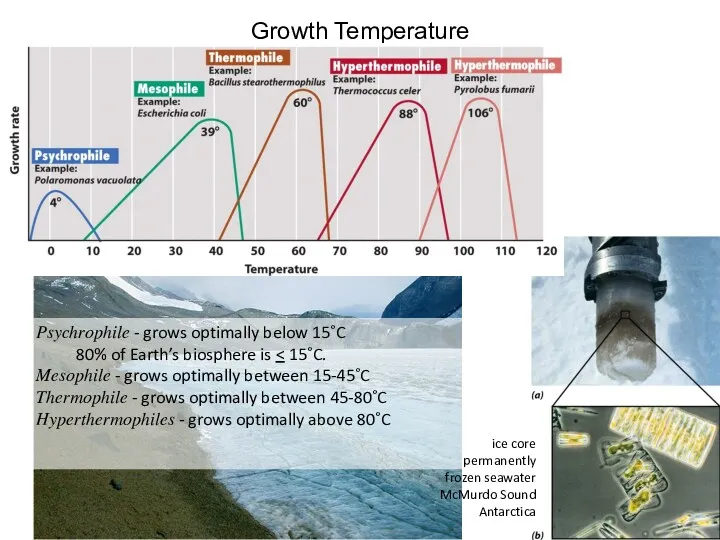
- 32. Growth Temperature Psychrophile - grows optimally below 15˚C 80% of Earth’s biosphere is Mesophile - grows
- 33. Extremophiles What is extreme for one organism is necessary for another. Organisms are all highly adapted
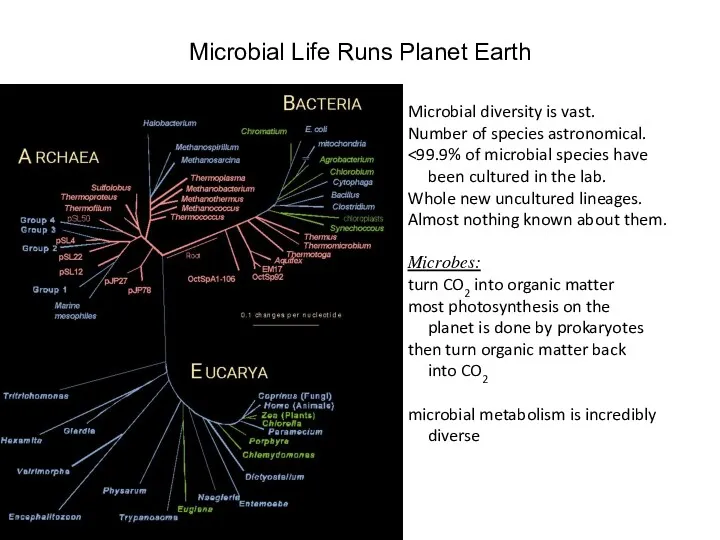
- 34. Microbial Life Runs Planet Earth Microbial diversity is vast. Number of species astronomical. been cultured in
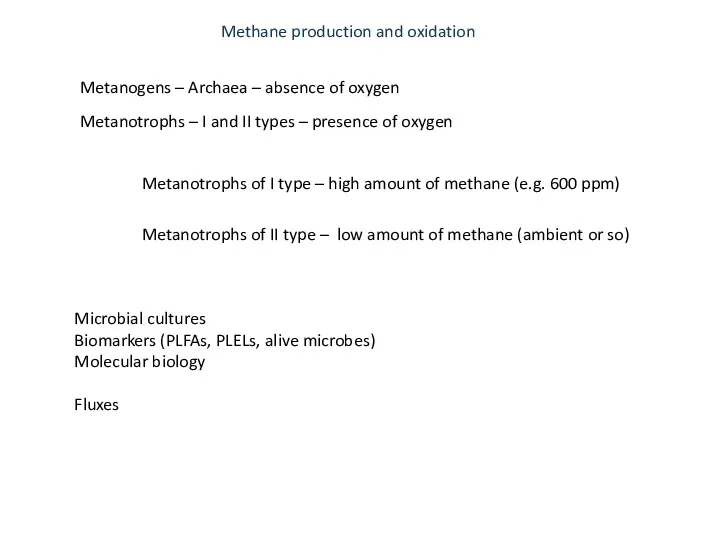
- 35. Metanogens – Archaea – absence of oxygen Metanotrophs – I and II types – presence of
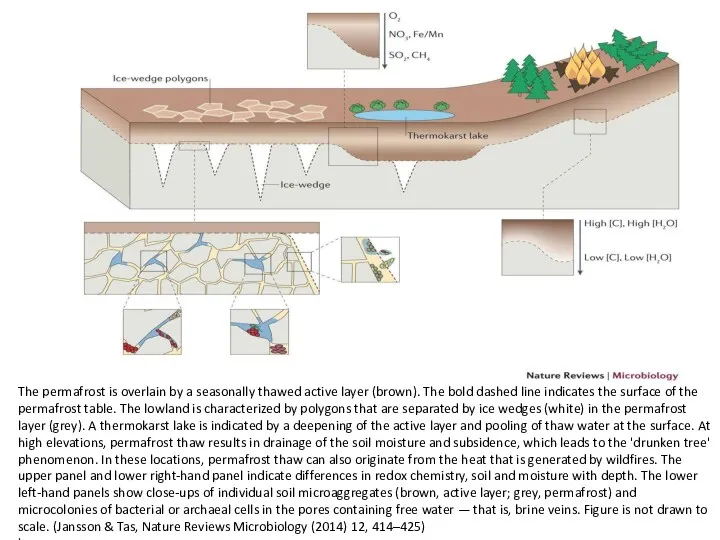
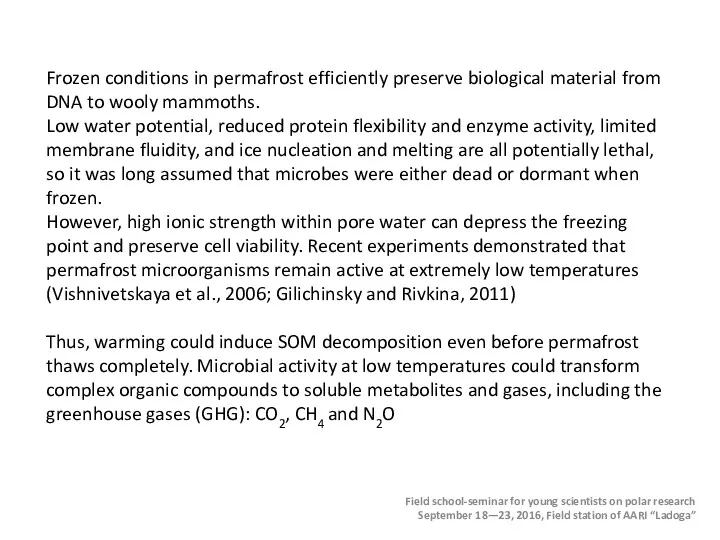
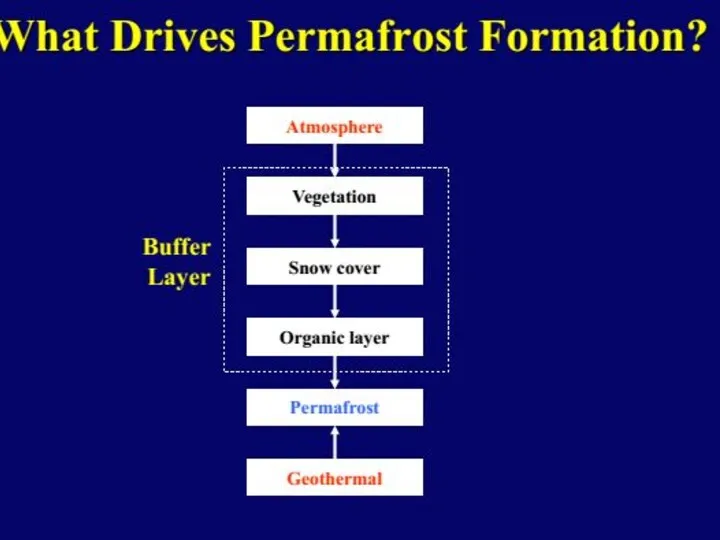
- 36. The permafrost is overlain by a seasonally thawed active layer (brown). The bold dashed line indicates
- 37. Field school-seminar for young scientists on polar research September 18—23, 2016, Field station of AARI “Ladoga”
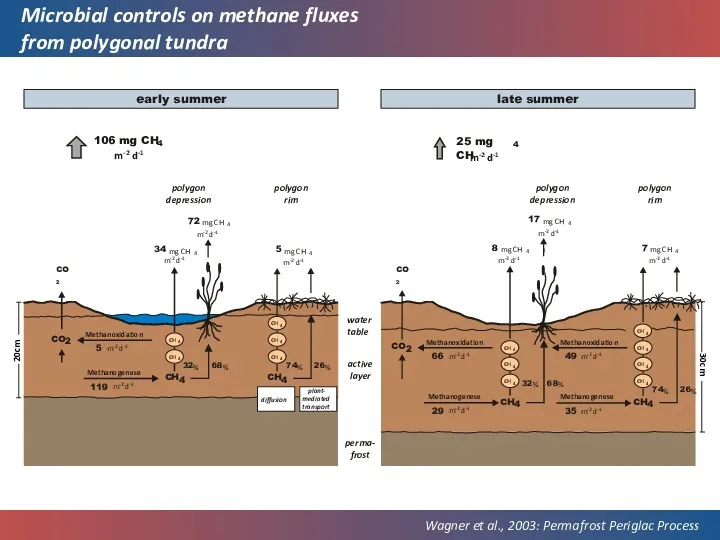
- 38. Microbial controls on methane fluxes from polygonal tundra Wagner et al., 2003: Permafrost Periglac Process
- 39. Field school-seminar for young scientists on polar research September 18—23, 2016, Field station of AARI “Ladoga”
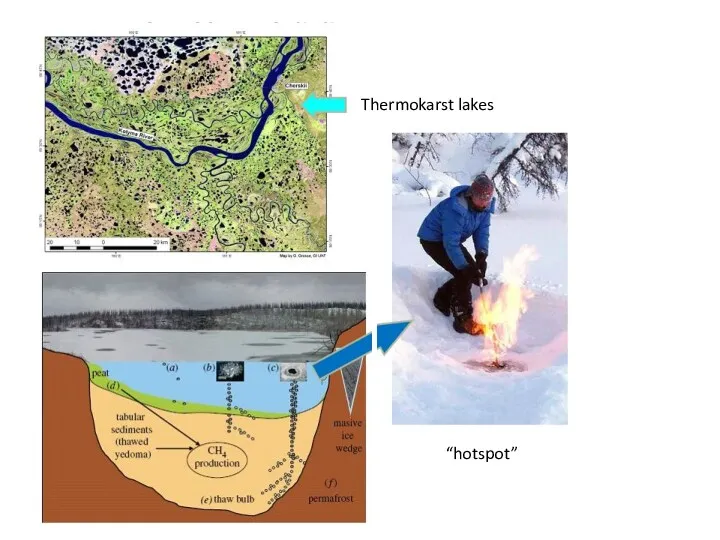
- 41. Thermokarst lakes “hotspot”
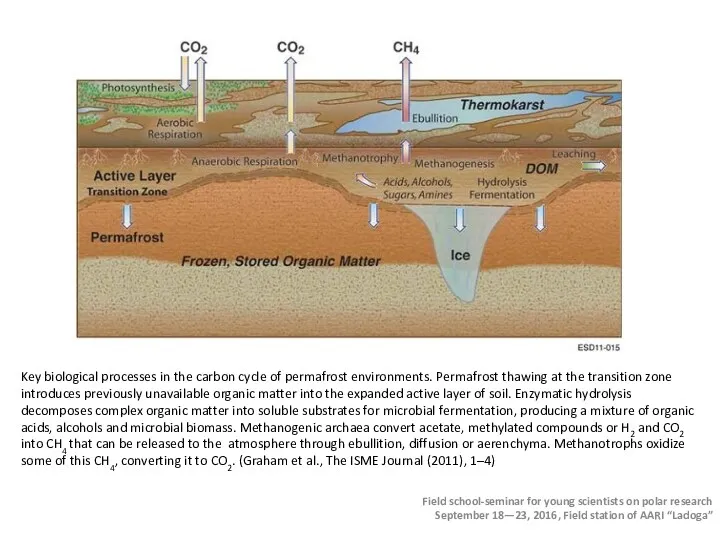
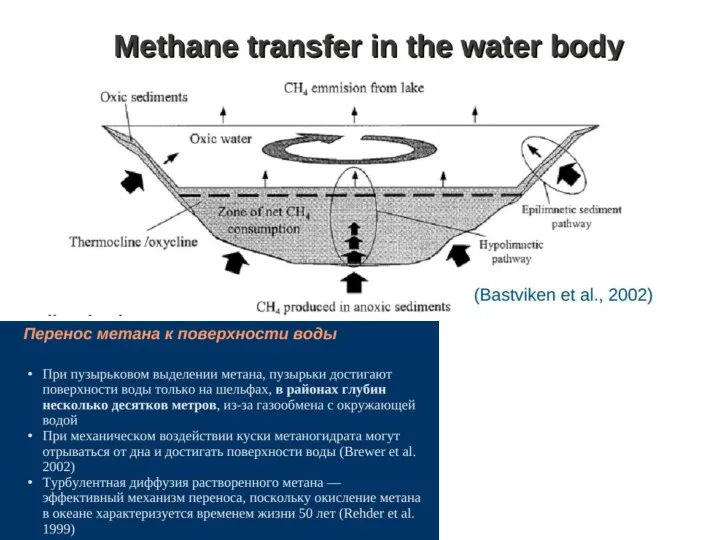
- 42. Methane emission: bogs and lakes Mechanisms of methane production: On bogs the substrate for methane production
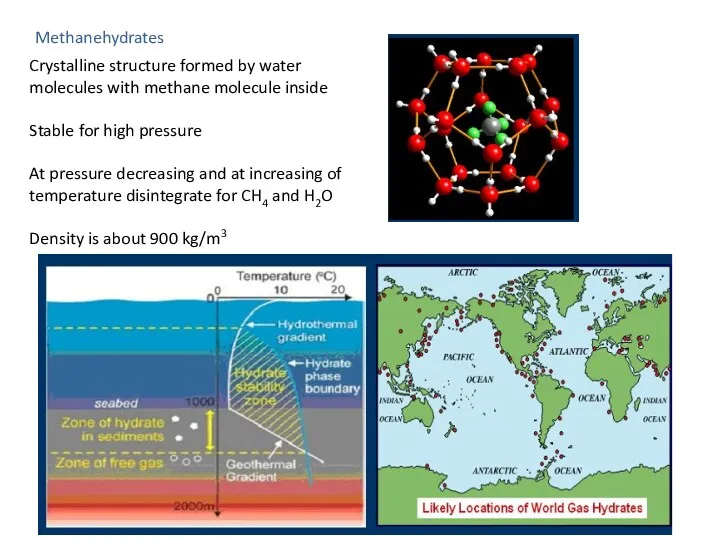
- 43. Methanehydrates Crystalline structure formed by water molecules with methane molecule inside Stable for high pressure At
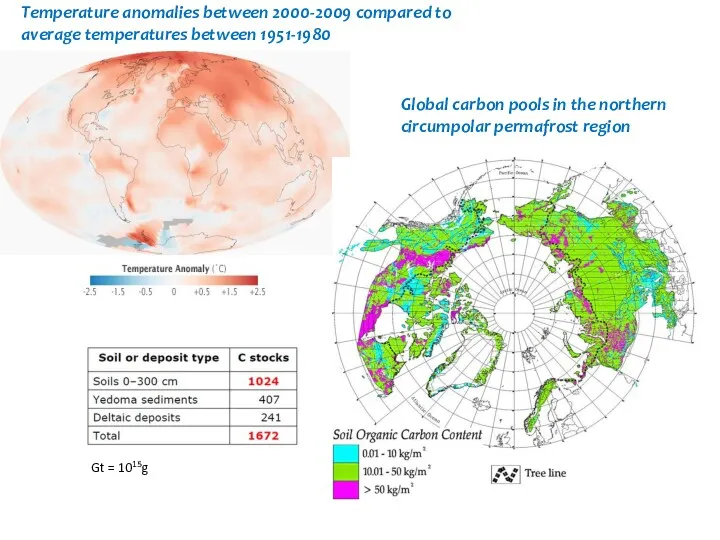
- 44. Temperature anomalies between 2000-2009 compared to average temperatures between 1951-1980 Global carbon pools in the northern
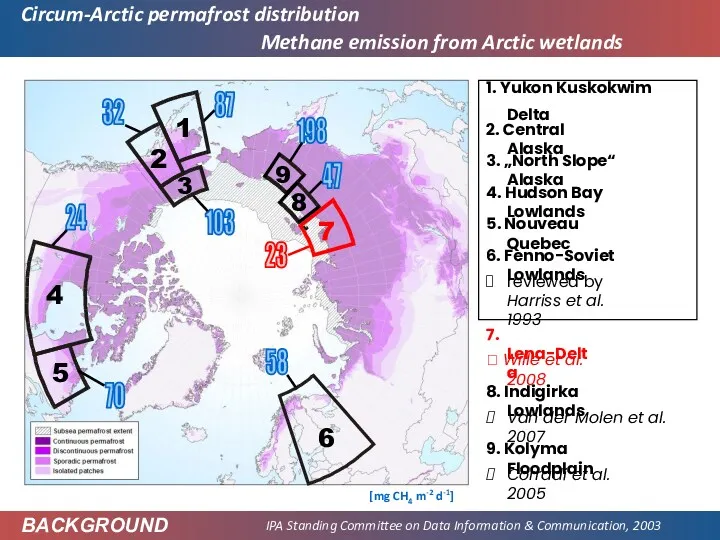
- 45. Circum-Arctic permafrost distribution IPA Standing Committee on Data Information & Communication, 2003 BACKGROUND
- 46. Field school-seminar for young scientists on polar research September 18—23, 2016, Field station of AARI “Ladoga”
- 47. Field school-seminar for young scientists on polar research September 18—23, 2016, Field station of AARI “Ladoga”
- 49. Field school-seminar for young scientists on polar research September 18—23, 2016, Field station of AARI “Ladoga”
- 50. В определении терминов Четвертого оценочного доклада IPCC , "время жизни" имеет несколько значений. Наиболее подходящим является:
- 52. Скачать презентацию













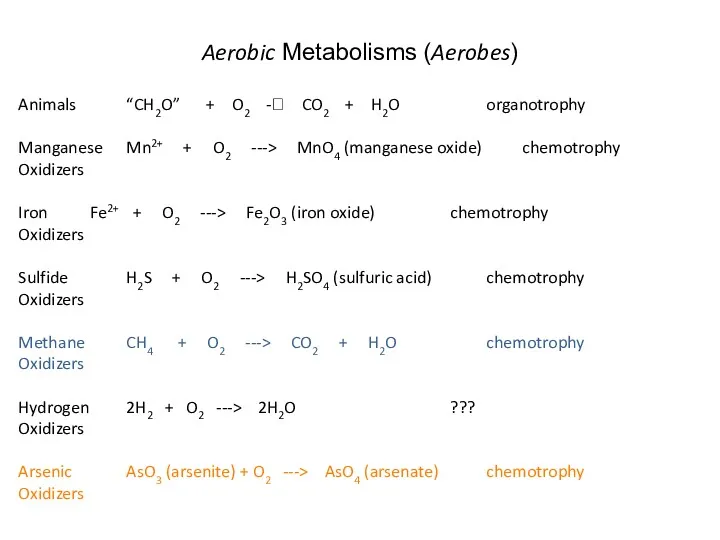




 Лекция 9. Основы популяционной экологии
Лекция 9. Основы популяционной экологии Эколого-экономическая оценка эффективности природоохранных мероприятий
Эколого-экономическая оценка эффективности природоохранных мероприятий Естественные сообщества живых организмов, компоненты биогеоценозов. 11 класс
Естественные сообщества живых организмов, компоненты биогеоценозов. 11 класс Красная книга России
Красная книга России Пресноводные экосистемы
Пресноводные экосистемы Экологические проблемы в сельском хозяйстве
Экологические проблемы в сельском хозяйстве Влияние жизнедеятельности бобров на экологию реки Малая Каменка
Влияние жизнедеятельности бобров на экологию реки Малая Каменка Захаровское озеро. Облагораживание
Захаровское озеро. Облагораживание Черное море и его экологические проблемы
Черное море и его экологические проблемы Техническое перевооружение очистных сооружений фабрики по переработке табачного сырья ООО Крес Нева
Техническое перевооружение очистных сооружений фабрики по переработке табачного сырья ООО Крес Нева Забруднення повітря об’єктами енергетики
Забруднення повітря об’єктами енергетики Особенности взаимодействия общества и природы
Особенности взаимодействия общества и природы Презентация к уроку экологического краеведения Озеро в опасности.
Презентация к уроку экологического краеведения Озеро в опасности. Презентация по темеОсобо охраняемые природные территории Брянской области в системе экологического каркаса
Презентация по темеОсобо охраняемые природные территории Брянской области в системе экологического каркаса Урбанизация и экология городской среды
Урбанизация и экология городской среды Khomutovskaya_step
Khomutovskaya_step Ecological Problems
Ecological Problems Les principales dispositions legislatives et reglementaires regissant le HSE
Les principales dispositions legislatives et reglementaires regissant le HSE Экоурок Свобода от отходов
Экоурок Свобода от отходов Әлемдік мұхиттардың ластануы
Әлемдік мұхиттардың ластануы Bio-monitoring and bio-indicators in the aspect of climate change
Bio-monitoring and bio-indicators in the aspect of climate change ПРО отходы!
ПРО отходы! Пути сохранения биоразнообразия и генофонда биосферы
Пути сохранения биоразнообразия и генофонда биосферы Влияние промышленного транспорта на окружающую среду
Влияние промышленного транспорта на окружающую среду Природоохороні технології
Природоохороні технології Экологические проблемы Казахстана
Экологические проблемы Казахстана Изменение климата
Изменение климата Crafts from waste
Crafts from waste