Содержание
- 2. The Greek Word of “Atomos” means “Indivisible” Around 440 BC, Leucippus originated the atom concept. One
- 3. Dalton’s Atomic Theory (1803-1808) 1.Elements are composed of extremely small particles called atoms原子. 2. All atoms
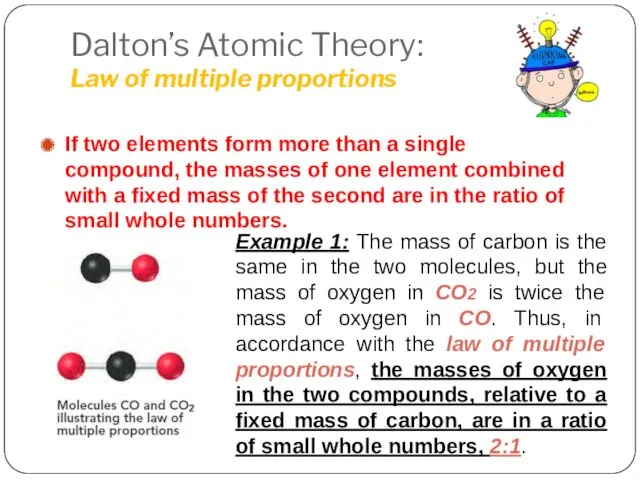
- 4. Dalton’s Atomic Theory: Law of multiple proportions If two elements form more than a single compound,
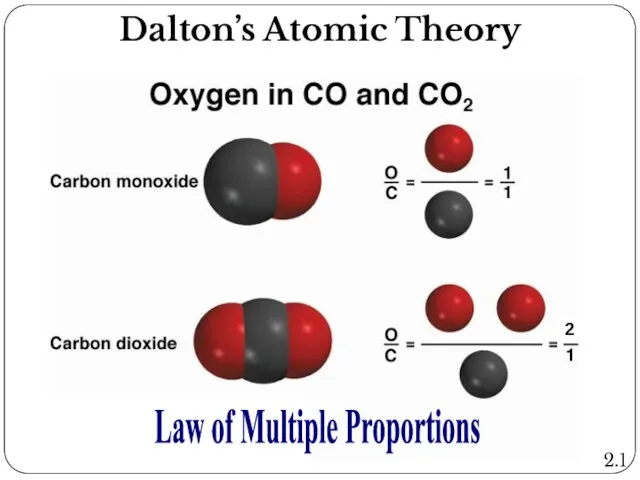
- 5. Law of Multiple Proportions 2.1 Dalton’s Atomic Theory
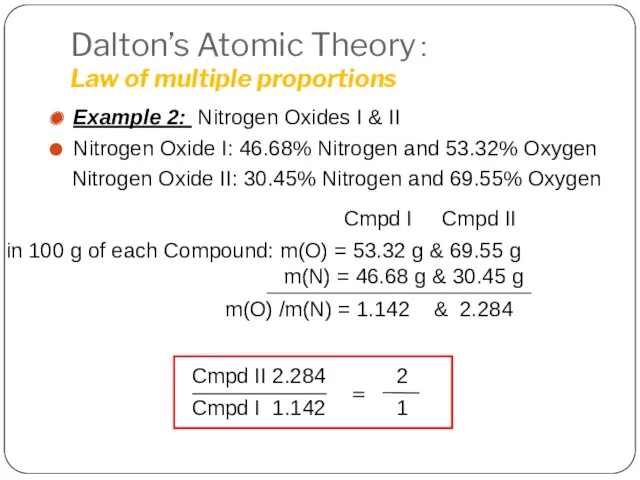
- 6. Dalton’s Atomic Theory: Law of multiple proportions Example 2: Nitrogen Oxides I & II Nitrogen Oxide
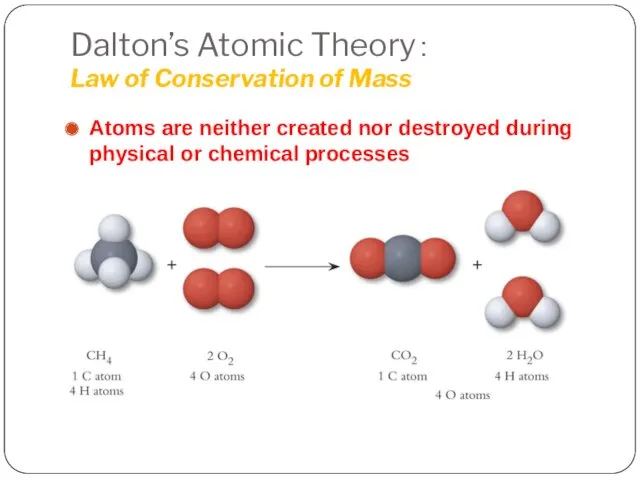
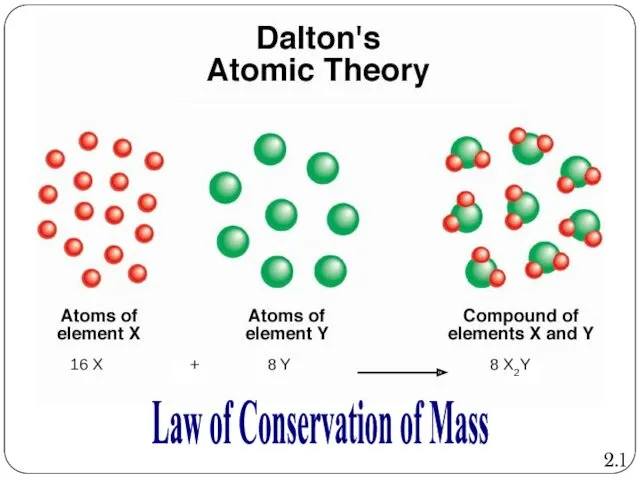
- 7. Dalton’s Atomic Theory: Law of Conservation of Mass Atoms are neither created nor destroyed during physical
- 8. 8 X2Y Law of Conservation of Mass 2.1
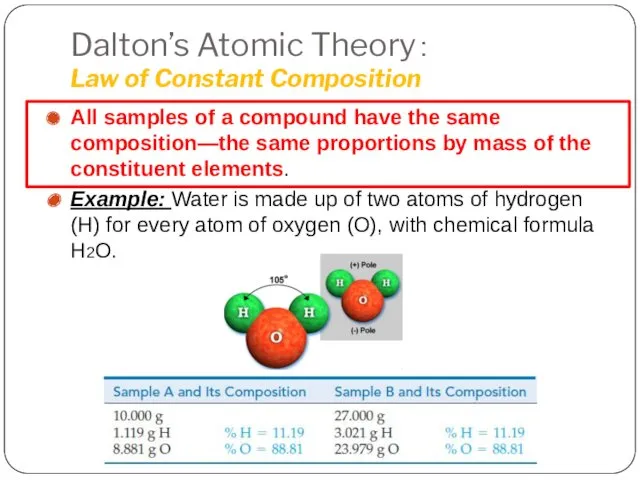
- 9. Dalton’s Atomic Theory: Law of Constant Composition All samples of a compound have the same composition—the
- 10. BUT!!! Atoms are still DIVISIBLE!!! Atom is made up of smaller parts, which can only be
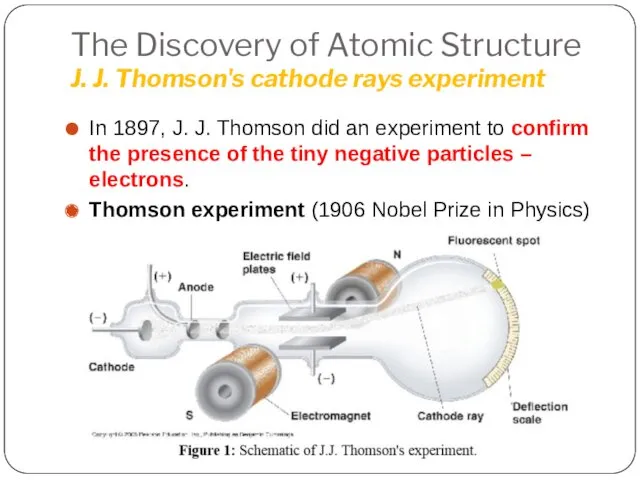
- 11. The Discovery of Atomic Structure J. J. Thomson's cathode rays experiment In 1897, J. J. Thomson
- 12. The Discovery of Atomic Structure J. J. Thomson's cathode rays experiment CRT, the abbreviation for cathode-ray
- 13. The Discovery of Atomic Structure J. J. Thomson's cathode rays experiment When gases are subjected to
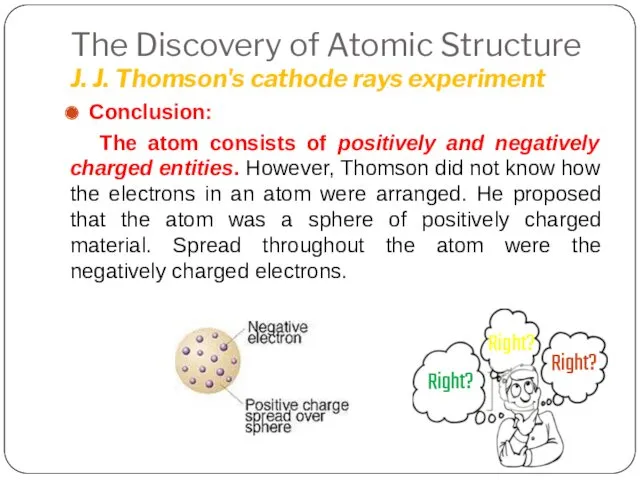
- 14. The Discovery of Atomic Structure J. J. Thomson's cathode rays experiment Conclusion: The atom consists of
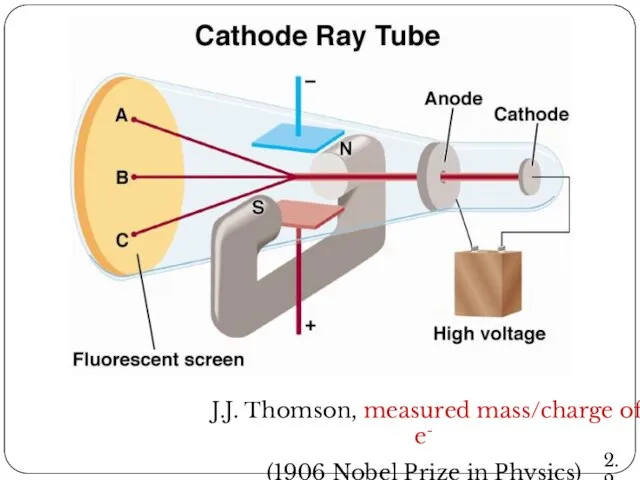
- 15. J.J. Thomson, measured mass/charge of e- (1906 Nobel Prize in Physics) 2.2
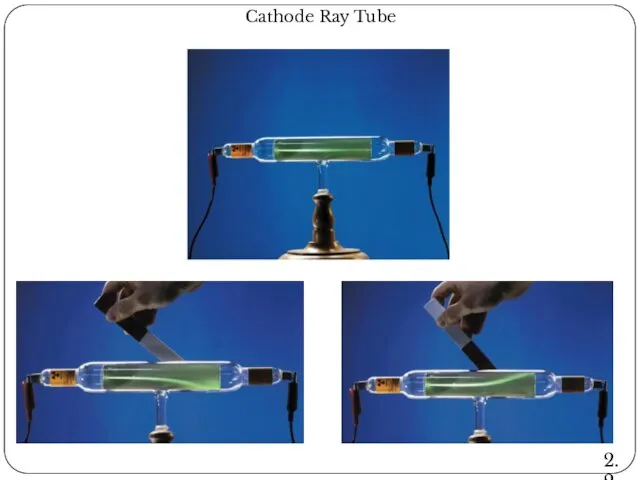
- 16. Cathode Ray Tube 2.2
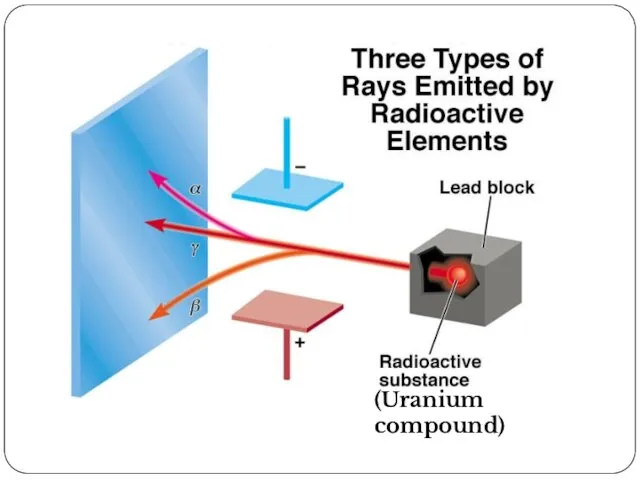
- 17. (Uranium compound)
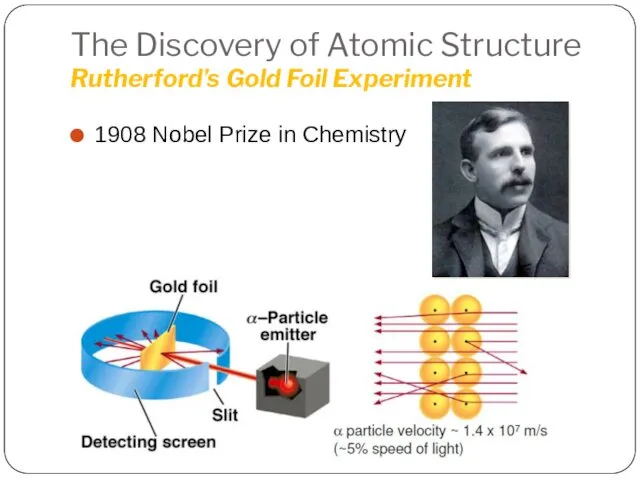
- 18. The Discovery of Atomic Structure Rutherford’s Gold Foil Experiment 1908 Nobel Prize in Chemistry
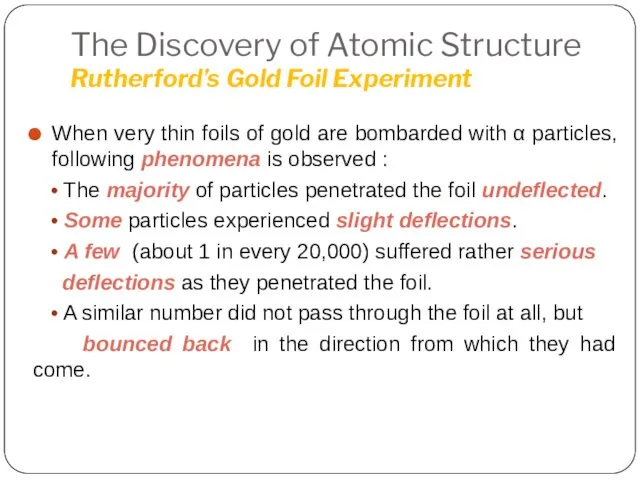
- 19. The Discovery of Atomic Structure Rutherford’s Gold Foil Experiment When very thin foils of gold are
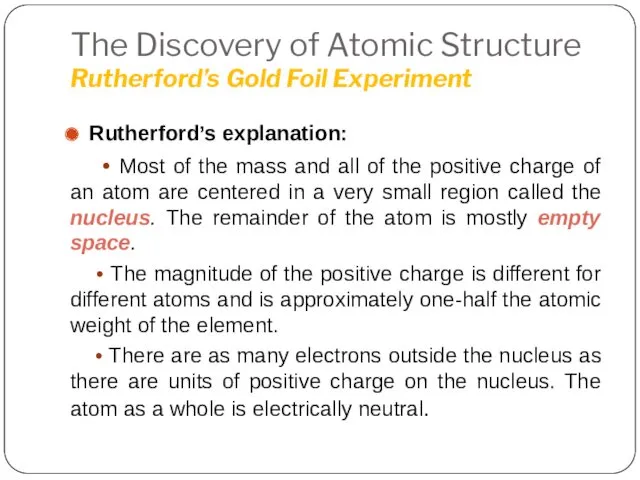
- 20. The Discovery of Atomic Structure Rutherford’s Gold Foil Experiment Rutherford’s explanation: • Most of the mass
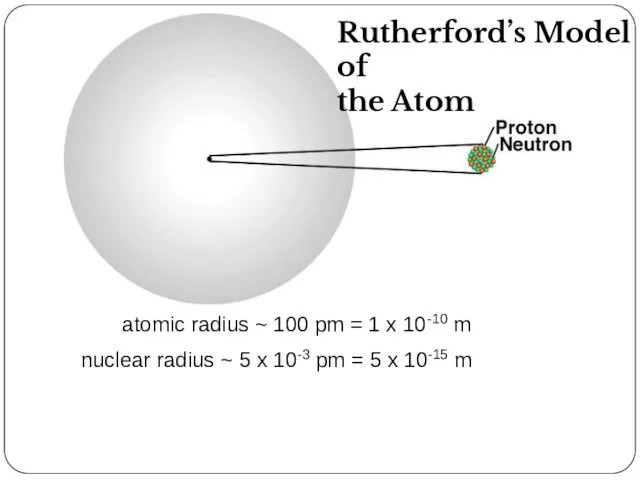
- 21. The Discovery of Atomic Structure Rutherford’s Gold Foil Experiment 1. undeflected straight-line paths exhibited by most
- 22. atomic radius ~ 100 pm = 1 x 10-10 m nuclear radius ~ 5 x 10-3
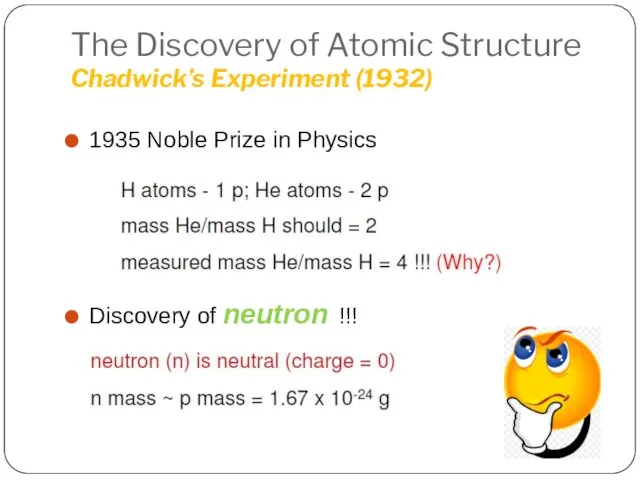
- 23. The Discovery of Atomic Structure Chadwick’s Experiment (1932) 1935 Noble Prize in Physics Discovery of neutron
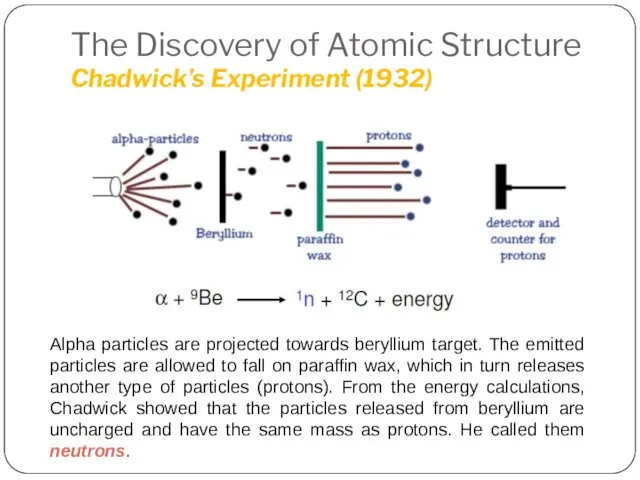
- 24. The Discovery of Atomic Structure Chadwick’s Experiment (1932) Alpha particles are projected towards beryllium target. The
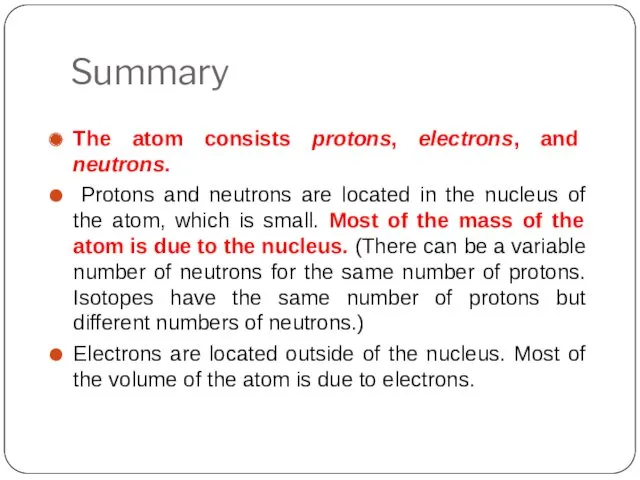
- 25. Summary The atom consists protons, electrons, and neutrons. Protons and neutrons are located in the nucleus
- 27. Скачать презентацию



 Нагревание проводников электрическим током. Закон Джоуля-Ленца
Нагревание проводников электрическим током. Закон Джоуля-Ленца Типы волоконной оптики, способы изготовления и применения
Типы волоконной оптики, способы изготовления и применения Презентация по теме Разделы механики для 10 класса
Презентация по теме Разделы механики для 10 класса презентация Сообщающиеся сосуды
презентация Сообщающиеся сосуды Тема №5. Энергетические системы самолета. Занятие №2. Гидравлическая система самолета МИГ-29. Общая гидросистема
Тема №5. Энергетические системы самолета. Занятие №2. Гидравлическая система самолета МИГ-29. Общая гидросистема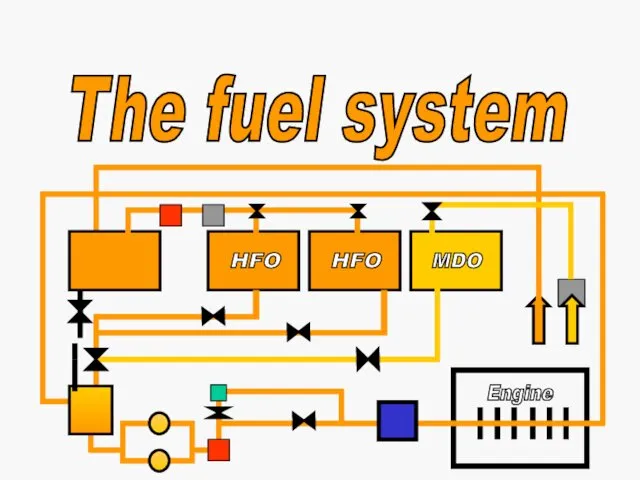 The fuel system
The fuel system Презентация к уроку Что изучает физика. Физические явления (7 класс)
Презентация к уроку Что изучает физика. Физические явления (7 класс) Физические величины в электроэнергетике, их размерности
Физические величины в электроэнергетике, их размерности Сила тока в различных участках параллельной цепи
Сила тока в различных участках параллельной цепи Явление электромагнитной индукции
Явление электромагнитной индукции Свойства магнита. Исследовательская работа дошкольников
Свойства магнита. Исследовательская работа дошкольников 20231011_elektrizatsiya1
20231011_elektrizatsiya1 Магнит өрісінің тогы бар өткізгішке әрекеті. Электрқозғалтқыштар. Электр өлшеуіш аспаптар
Магнит өрісінің тогы бар өткізгішке әрекеті. Электрқозғалтқыштар. Электр өлшеуіш аспаптар Оценка снижения экологического воздействия энергетики при реализации ЗЯТЦ в проекте БРЕСТ
Оценка снижения экологического воздействия энергетики при реализации ЗЯТЦ в проекте БРЕСТ Есептеу әдістемесі мен механикалық құралжабдықтарды таңдау
Есептеу әдістемесі мен механикалық құралжабдықтарды таңдау Эволюция физических картин мира. (Лекция 3)
Эволюция физических картин мира. (Лекция 3) Аэродинамика автомобиля
Аэродинамика автомобиля Оќыту процессінде композициялыќ жобалау єдісін ќолдану
Оќыту процессінде композициялыќ жобалау єдісін ќолдану Количество теплоты
Количество теплоты Тепловые процессы. Теплообменники. Нагрев острым паром
Тепловые процессы. Теплообменники. Нагрев острым паром Балочные системы. (Тема 1.4)
Балочные системы. (Тема 1.4) Магнитное поле
Магнитное поле Закон всемирного тяготения
Закон всемирного тяготения Теплообмен в металлургических агрегатах
Теплообмен в металлургических агрегатах Презентация к уроку по физике Скорость 7 класс
Презентация к уроку по физике Скорость 7 класс Тренажер по теме МКТ к уроку Газовые законы
Тренажер по теме МКТ к уроку Газовые законы Термодинамика диэлектриков. Типы диэлектриков, свойства и применение. Лекция №5
Термодинамика диэлектриков. Типы диэлектриков, свойства и применение. Лекция №5 Устройство скутера
Устройство скутера