Содержание
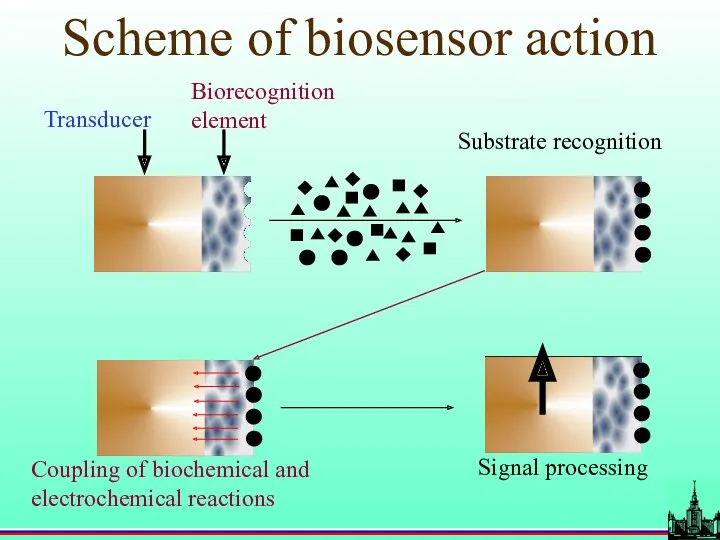
- 2. Scheme of biosensor action Transducer Biorecognition element Substrate recognition Coupling of biochemical and electrochemical reactions Signal
- 3. Requirements: detection directly in object without pretreatment; a possibility for continuous monitoring; a possibility for miniaturization;
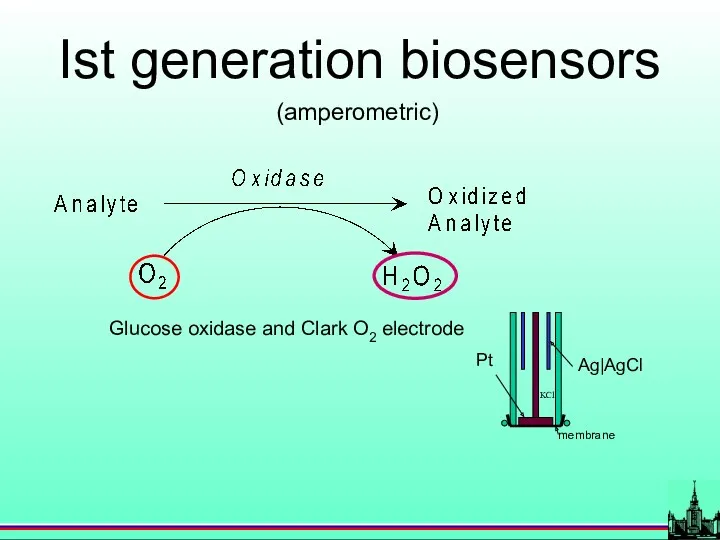
- 4. History Glucose oxidase and Clark O2 electrode L. C. Clark, and C. Lyons, Ann.NY Acad.Sci. 102,
- 5. Volume 102 Issue Automated and Semi-Automated Systems in Clinical Chemistry , Pages 3 - 180 (October
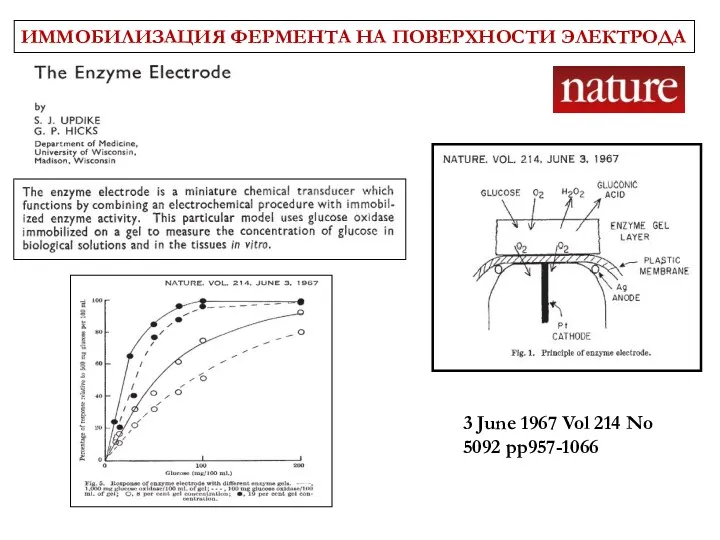
- 6. 3 June 1967 Vol 214 No 5092 pp957-1066 ИММОБИЛИЗАЦИЯ ФЕРМЕНТА НА ПОВЕРХНОСТИ ЭЛЕКТРОДА
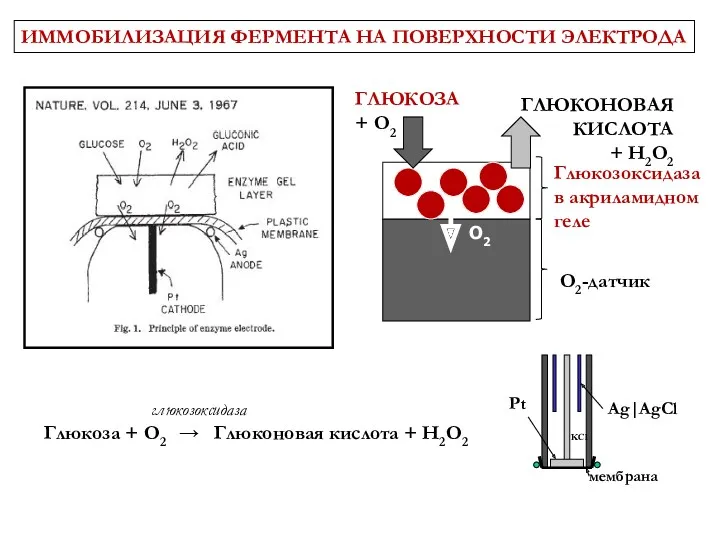
- 7. ИММОБИЛИЗАЦИЯ ФЕРМЕНТА НА ПОВЕРХНОСТИ ЭЛЕКТРОДА ГЛЮКОЗА + O2 Глюкозоксидаза в акриламидном геле ГЛЮКОНОВАЯ КИСЛОТА + H2O2
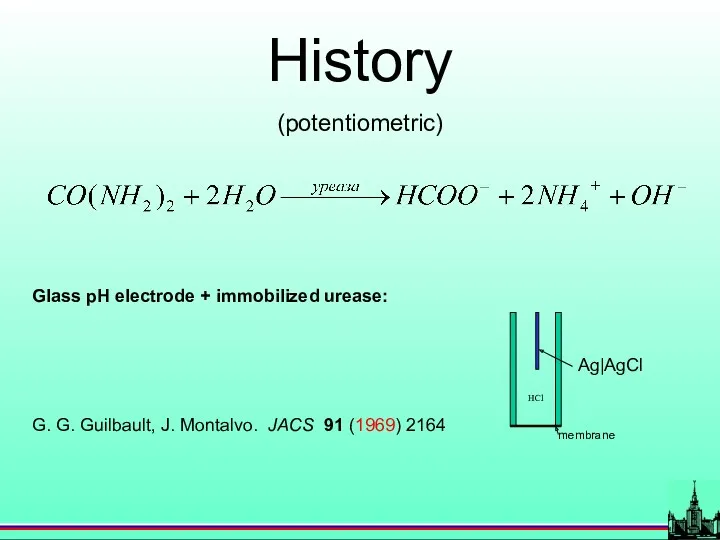
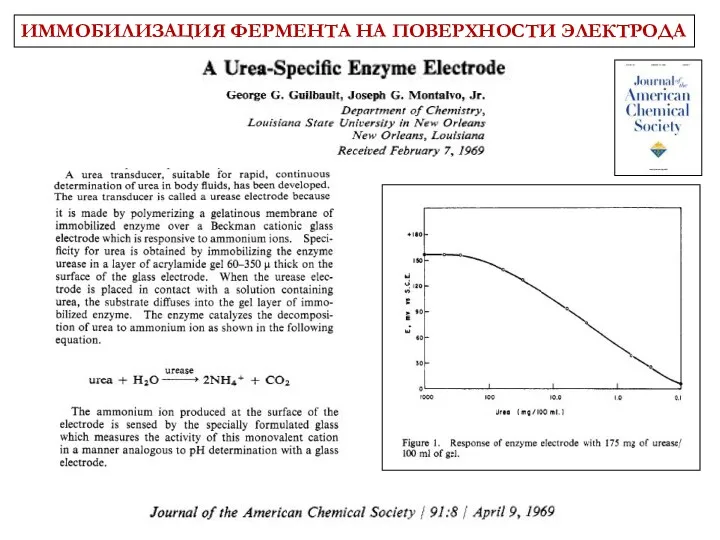
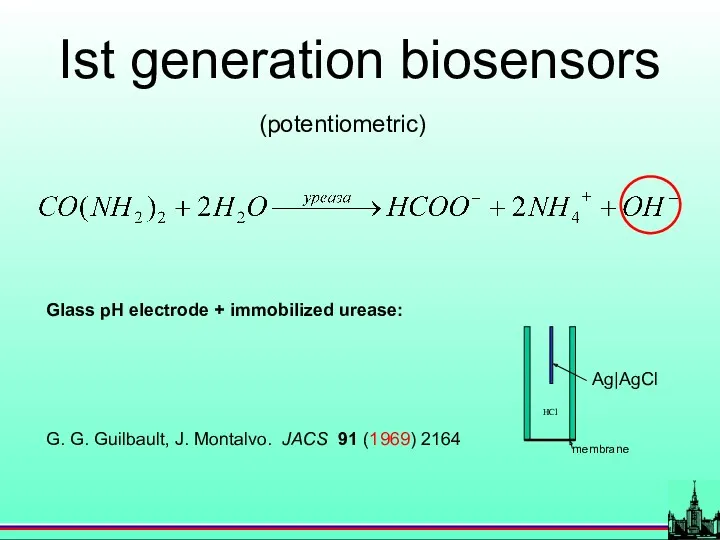
- 8. History (potentiometric) Glass pH electrode + immobilized urease: G. G. Guilbault, J. Montalvo. JACS 91 (1969)
- 9. ИММОБИЛИЗАЦИЯ ФЕРМЕНТА НА ПОВЕРХНОСТИ ЭЛЕКТРОДА
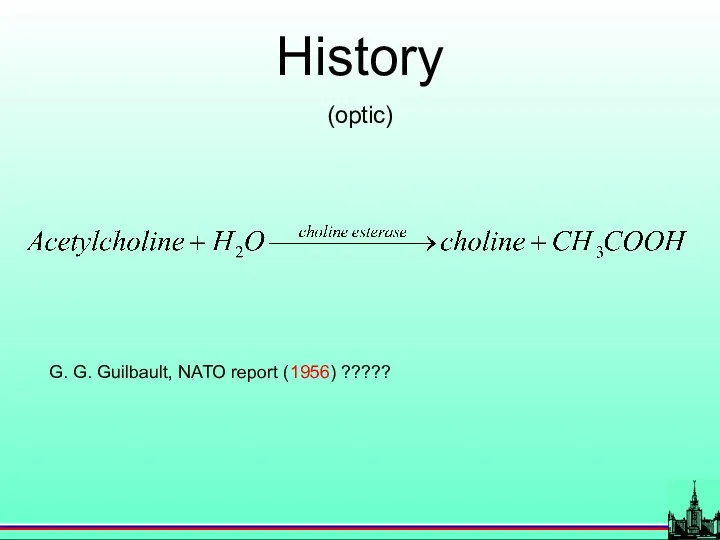
- 10. History (optic) G. G. Guilbault, NATO report (1956) ?????
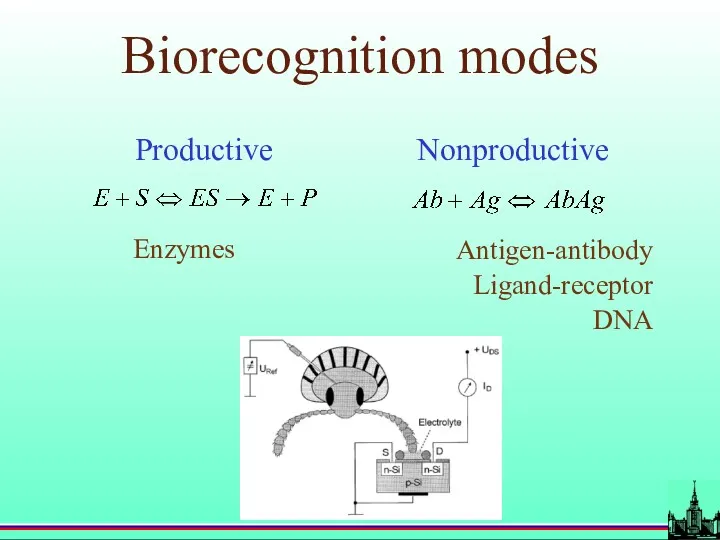
- 11. Biorecognition modes Productive Nonproductive Enzymes Antigen-antibody Ligand-receptor DNA
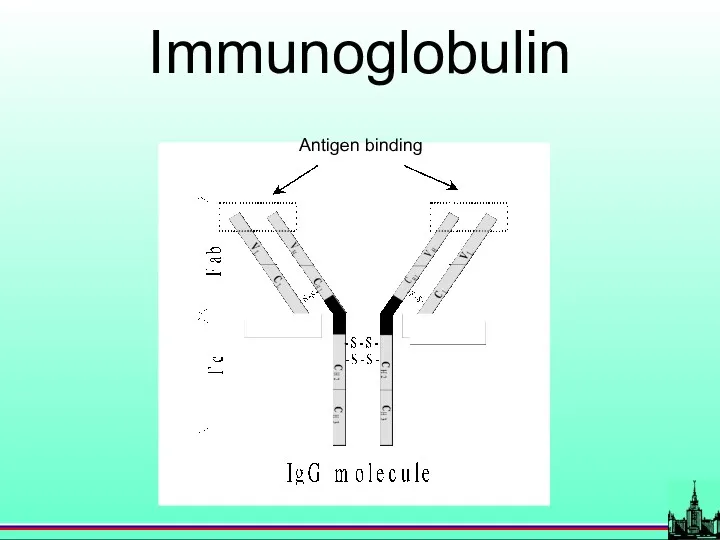
- 13. Antigen binding Immunoglobulin
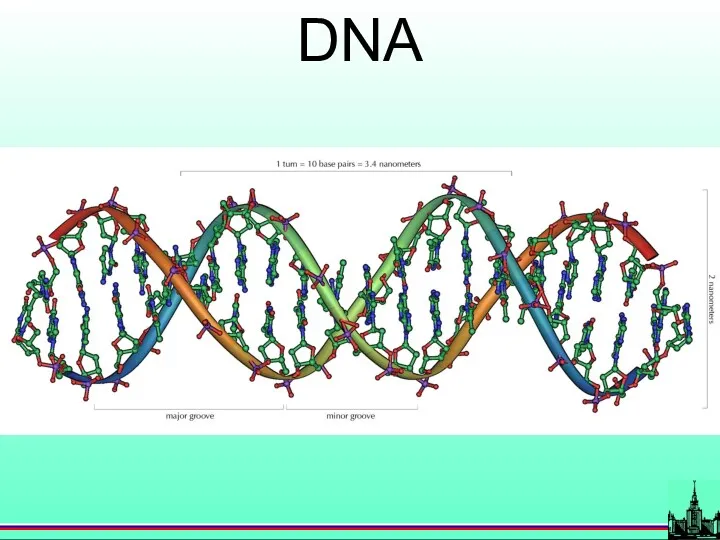
- 14. DNA
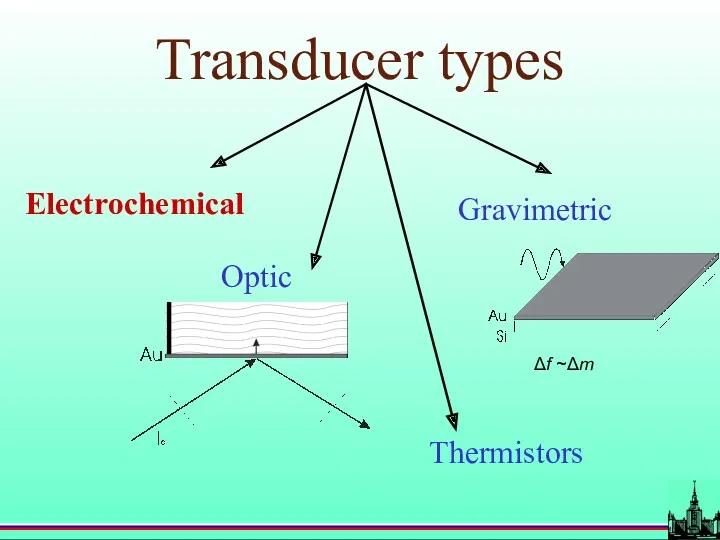
- 15. Transducer types Electrochemical Optic Gravimetric Thermistors Δf ~Δm
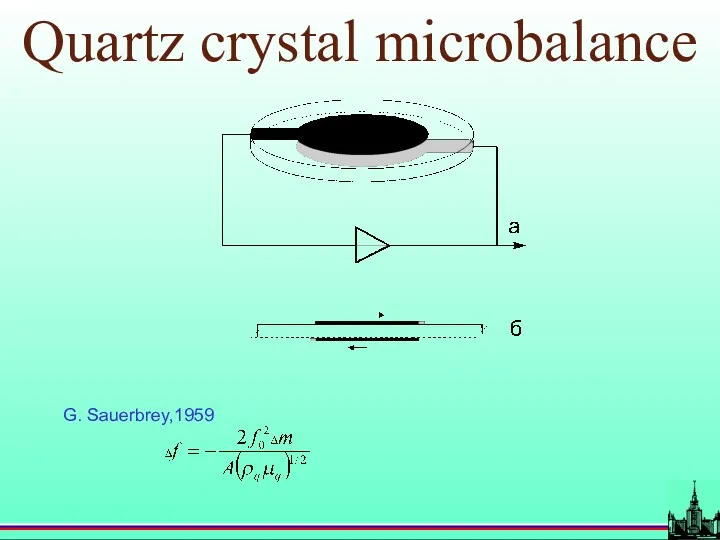
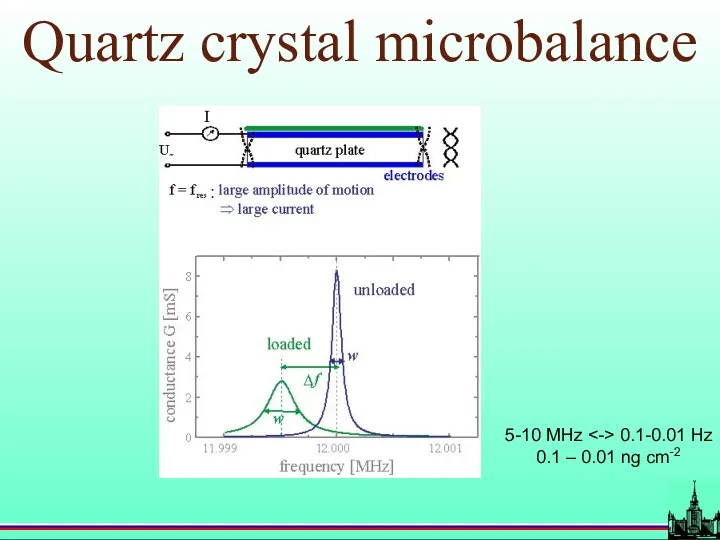
- 16. Quartz crystal microbalance G. Sauerbrey,1959
- 17. Quartz crystal microbalance 5-10 MHz 0.1-0.01 Hz 0.1 – 0.01 ng cm-2
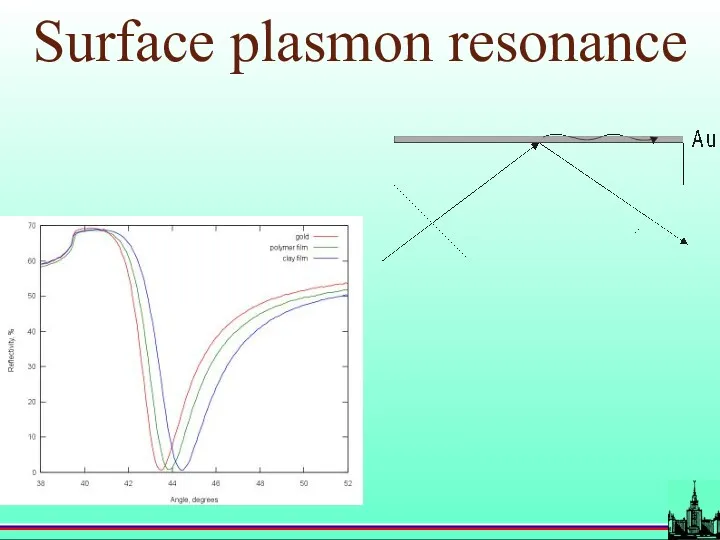
- 18. Surface plasmon resonance
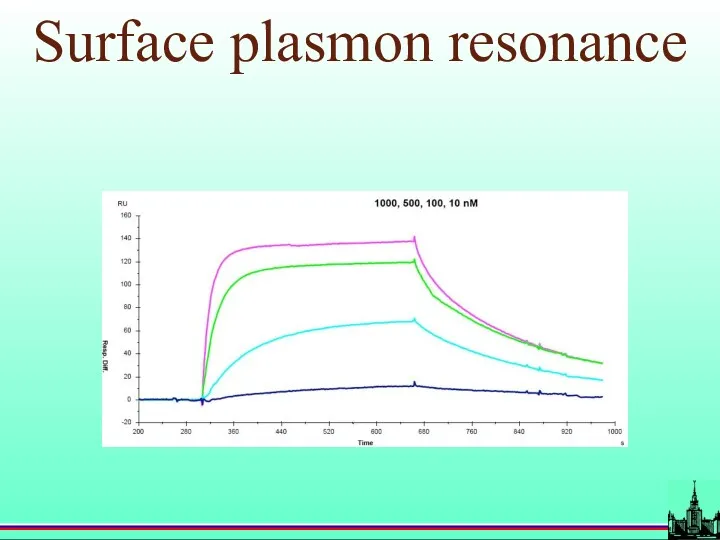
- 19. Surface plasmon resonance
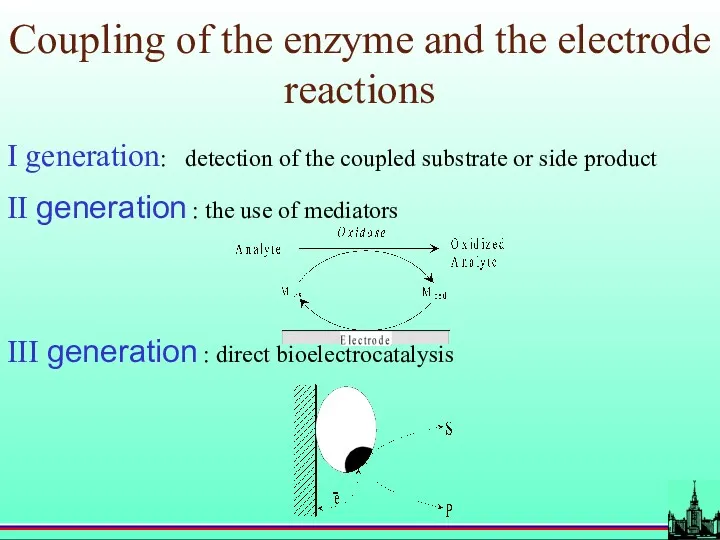
- 20. Coupling of the enzyme and the electrode reactions I generation: detection of the coupled substrate or
- 21. Ist generation biosensors (amperometric) Glucose oxidase and Clark O2 electrode
- 22. (potentiometric) Ist generation biosensors Glass pH electrode + immobilized urease: G. G. Guilbault, J. Montalvo. JACS
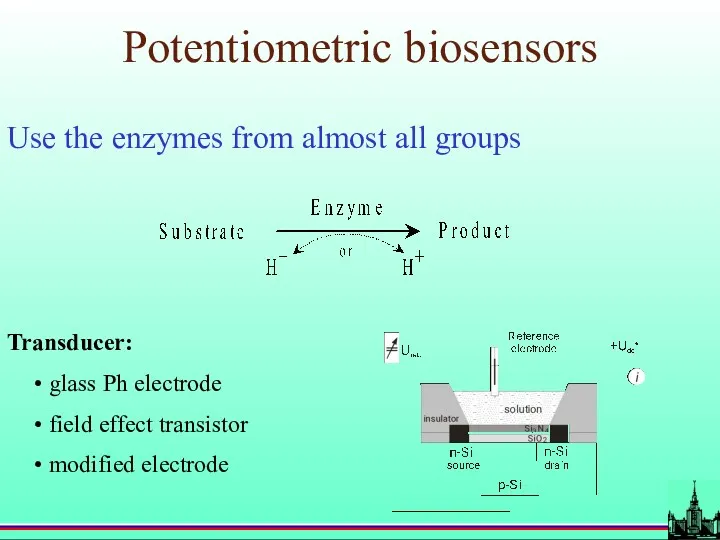
- 23. Potentiometric biosensors Use the enzymes from almost all groups Transducer: glass Ph electrode field effect transistor
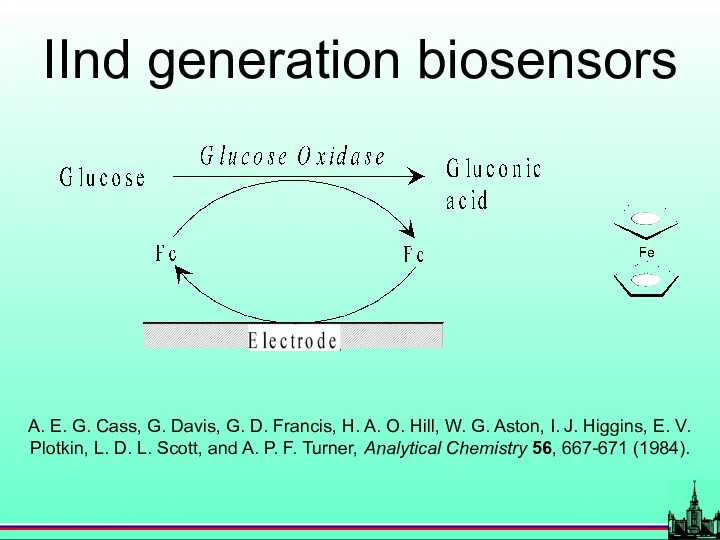
- 24. IInd generation biosensors A. E. G. Cass, G. Davis, G. D. Francis, H. A. O. Hill,
- 25. What Is Diabetes? Can cause: Blindness Heart attack Poor circulation Gangrene Kidney dysfunction Death No cure,
- 26. Glucose tests
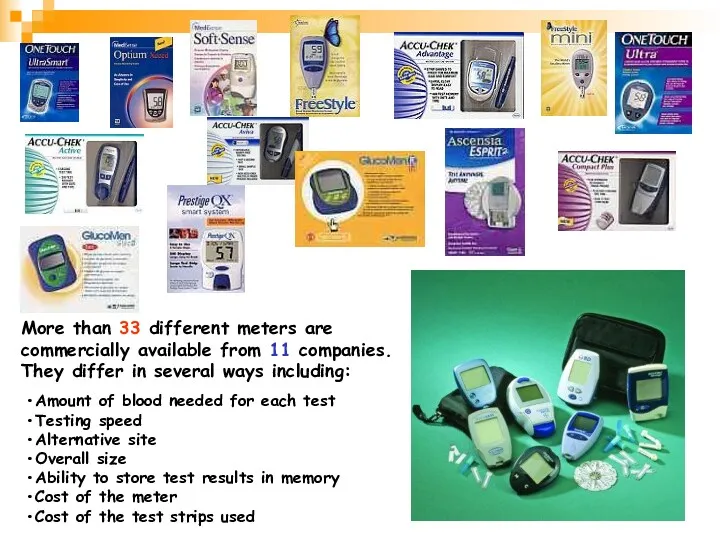
- 27. More than 33 different meters are commercially available from 11 companies. They differ in several ways
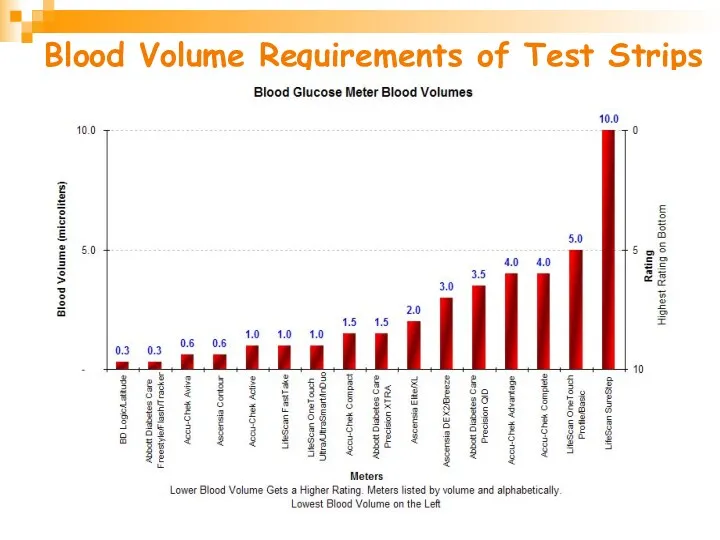
- 28. Blood Volume Requirements of Test Strips
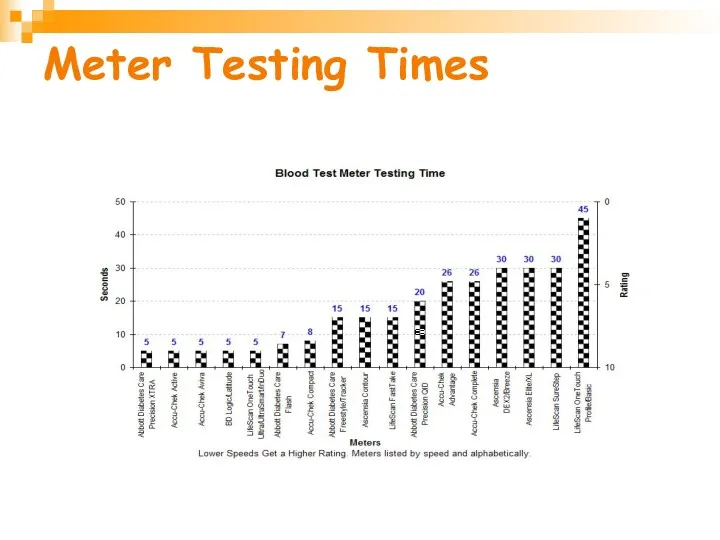
- 29. Meter Testing Times
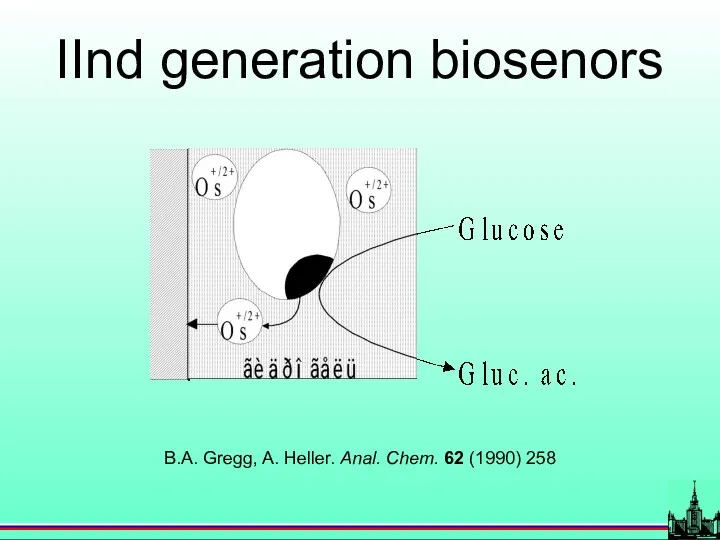
- 30. IInd generation biosenors B.A. Gregg, A. Heller. Anal. Chem. 62 (1990) 258
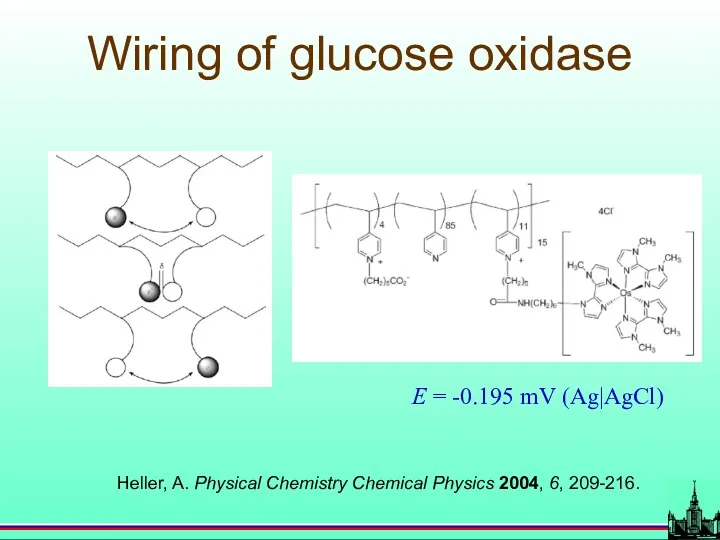
- 31. Wiring of glucose oxidase Heller, A. Physical Chemistry Chemical Physics 2004, 6, 209-216. E = -0.195
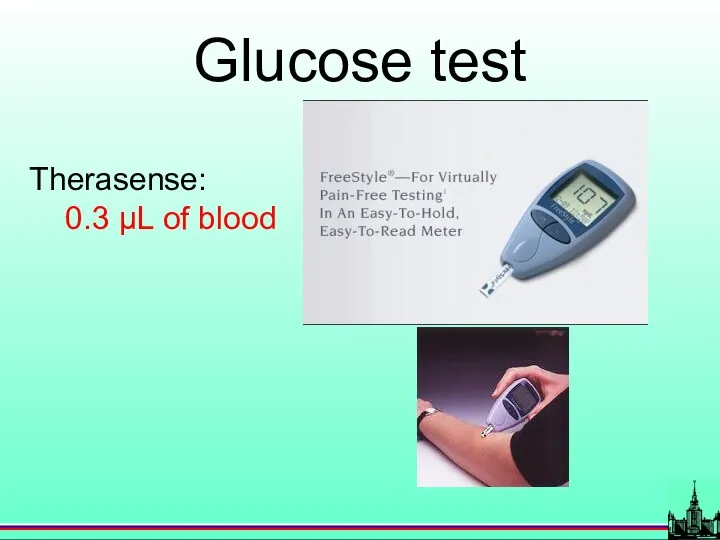
- 32. Glucose test Therasense: 0.3 µL of blood
- 33. Enzyme bioelectrocatalysis
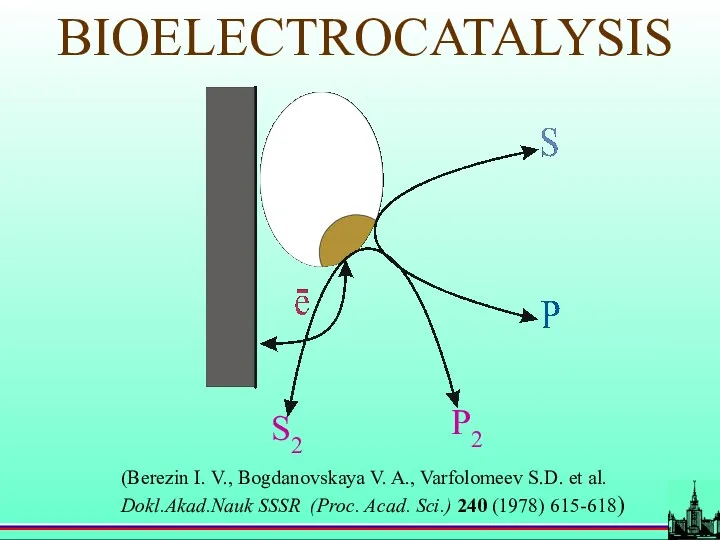
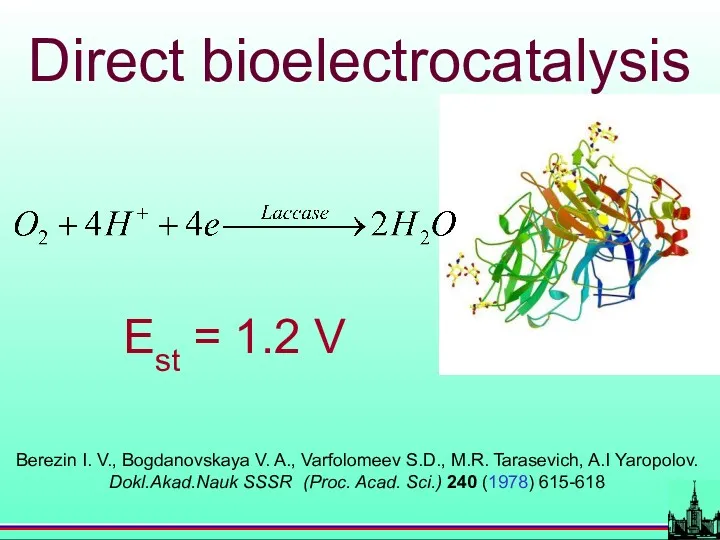
- 34. BIOELECTROCATALYSIS (Berezin I. V., Bogdanovskaya V. A., Varfolomeev S.D. et al. Dokl.Akad.Nauk SSSR (Proc. Acad. Sci.)
- 35. Direct enzyme bioelectrocatalysis
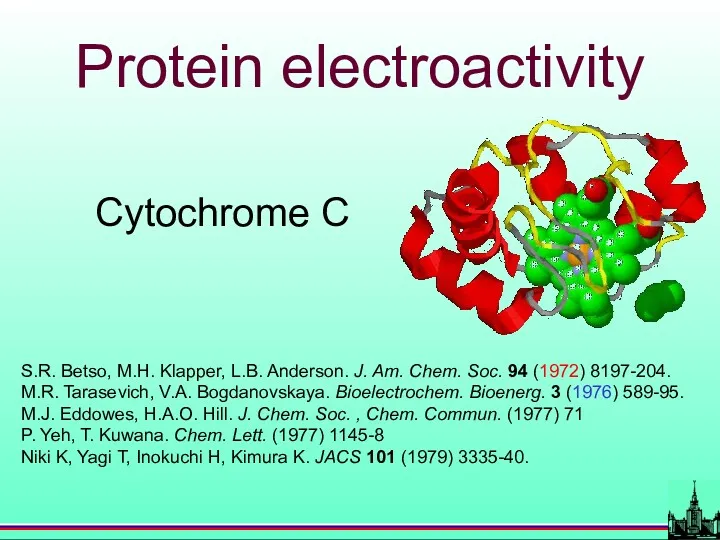
- 36. Protein electroactivity Cytochrome C S.R. Betso, M.H. Klapper, L.B. Anderson. J. Am. Chem. Soc. 94 (1972)
- 37. ВОССТАНОВЛЕНИЕ ЦИТОХРОМА С НА ПОВЕРХНОСТИ ЭЛЕКТРОДА Fe3+ + e → Fe2+
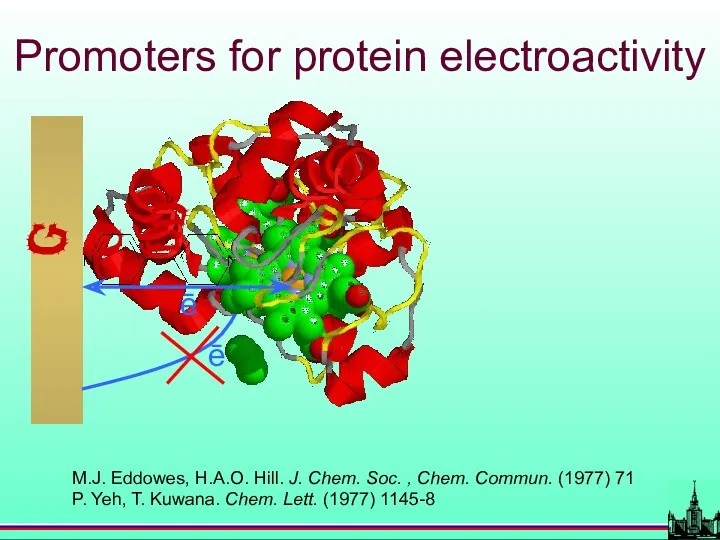
- 38. Promoters for protein electroactivity M.J. Eddowes, H.A.O. Hill. J. Chem. Soc. , Chem. Commun. (1977) 71
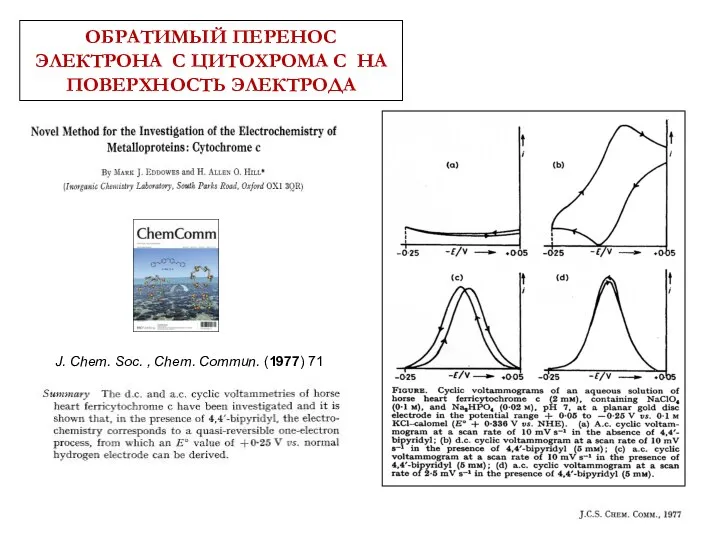
- 39. ОБРАТИМЫЙ ПЕРЕНОС ЭЛЕКТРОНА С ЦИТОХРОМА С НА ПОВЕРХНОСТЬ ЭЛЕКТРОДА
- 40. J. Chem. Soc. , Chem. Commun. (1977) 71 ОБРАТИМЫЙ ПЕРЕНОС ЭЛЕКТРОНА С ЦИТОХРОМА С НА ПОВЕРХНОСТЬ
- 41. Berezin I. V., Bogdanovskaya V. A., Varfolomeev S.D., M.R. Tarasevich, A.I Yaropolov. Dokl.Akad.Nauk SSSR (Proc. Acad.
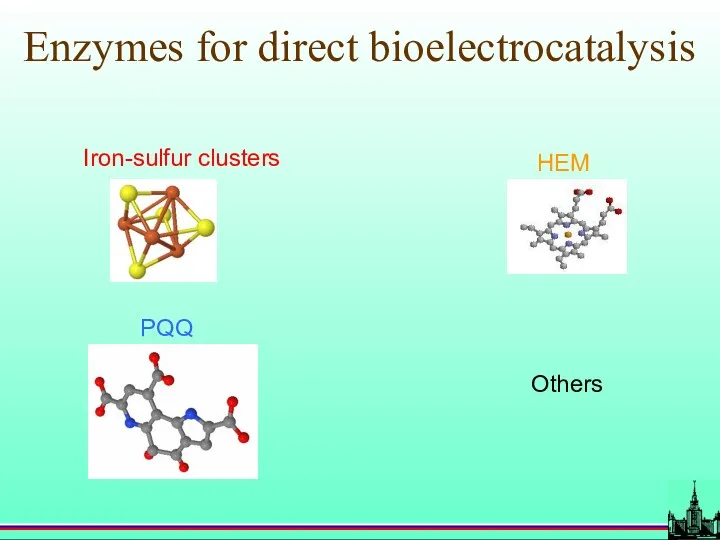
- 42. Enzymes for direct bioelectrocatalysis Others
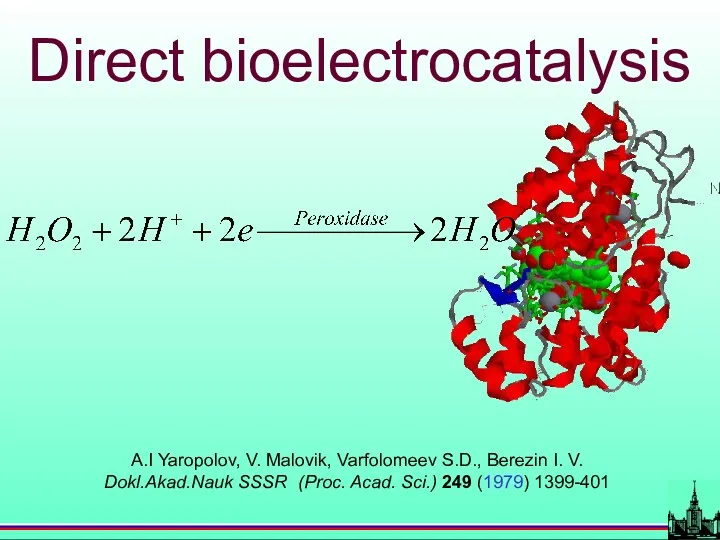
- 43. A.I Yaropolov, V. Malovik, Varfolomeev S.D., Berezin I. V. Dokl.Akad.Nauk SSSR (Proc. Acad. Sci.) 249 (1979)
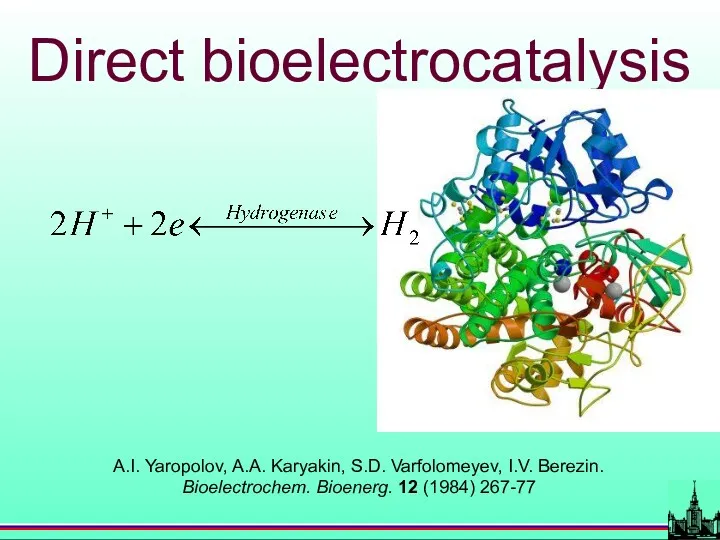
- 44. A.I. Yaropolov, A.A. Karyakin, S.D. Varfolomeyev, I.V. Berezin. Bioelectrochem. Bioenerg. 12 (1984) 267-77 Direct bioelectrocatalysis
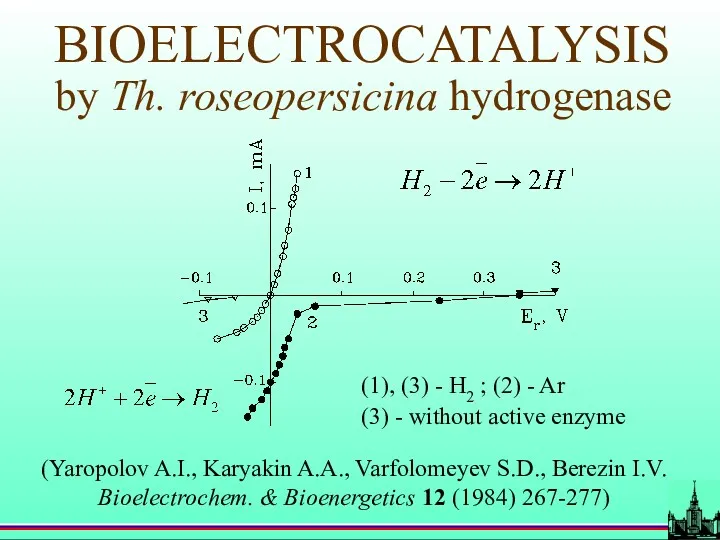
- 45. BIOELECTROCATALYSIS by Th. roseopersicina hydrogenase (1), (3) - H2 ; (2) - Ar (3) - without
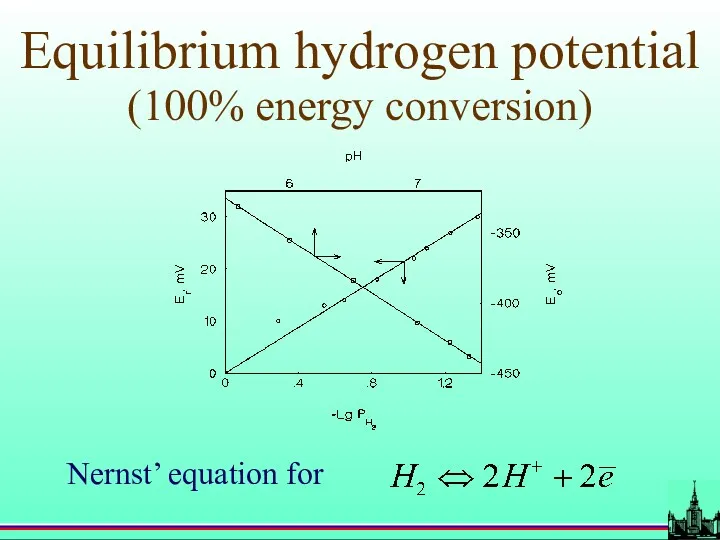
- 46. Equilibrium hydrogen potential (100% energy conversion)
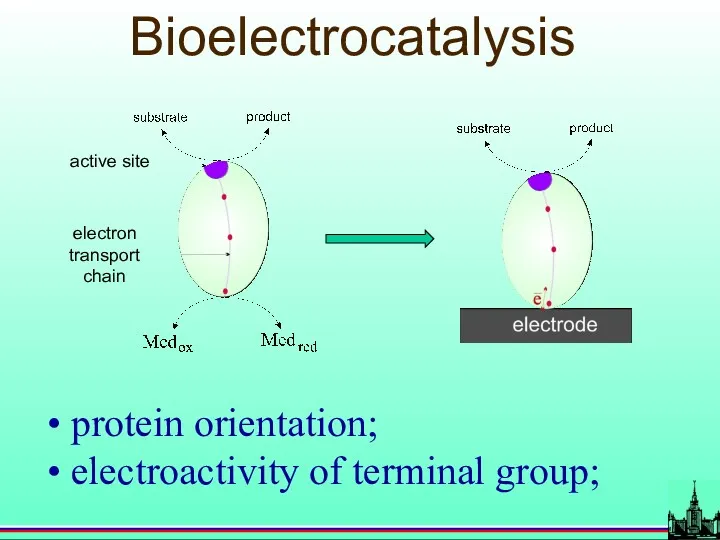
- 47. Bioelectrocatalysis active site electron transport chain protein orientation; electroactivity of terminal group;
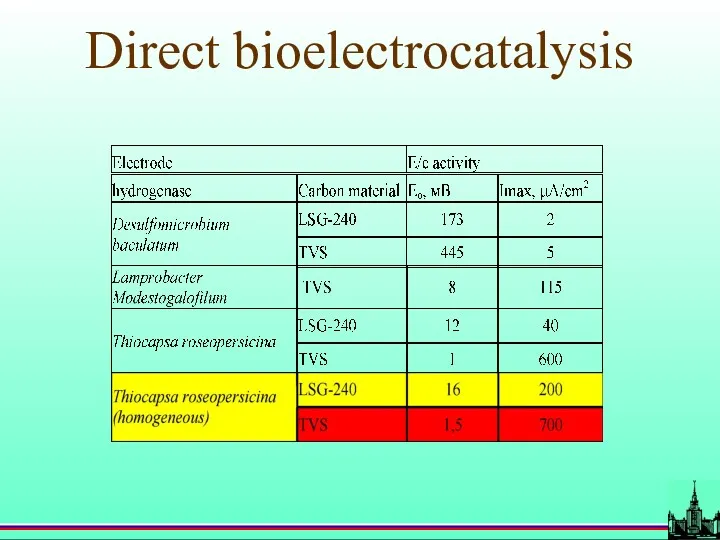
- 48. Direct bioelectrocatalysis
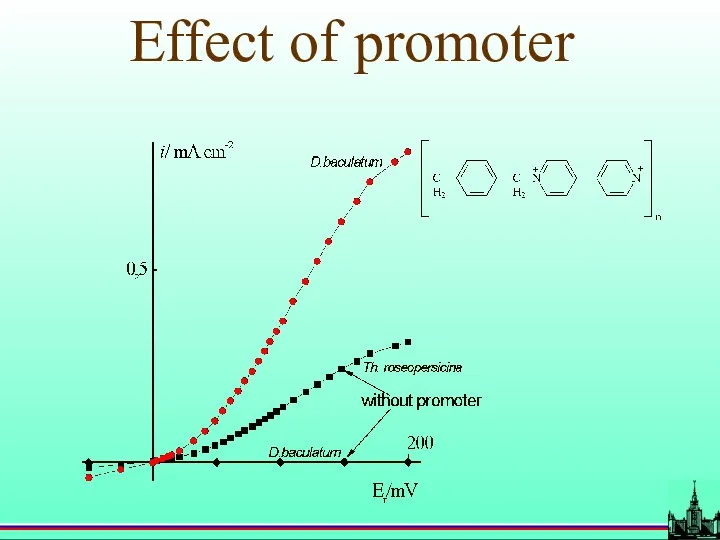
- 49. Effect of promoter
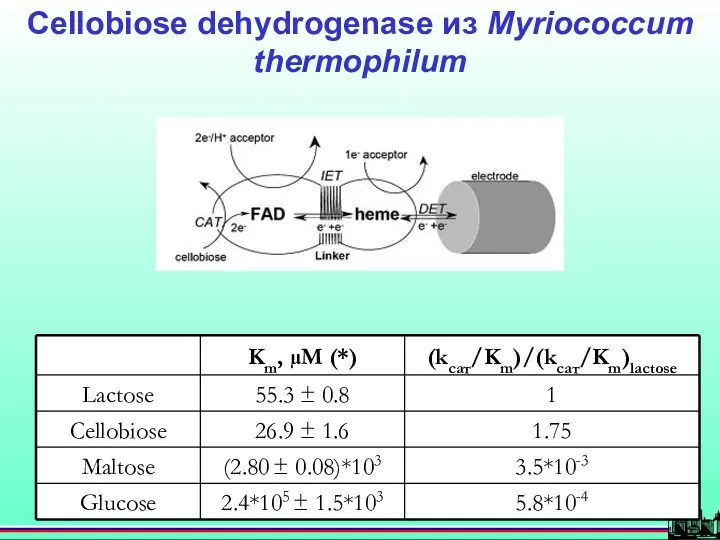
- 50. Cellobiose dehydrogenase из Myriococcum thermophilum
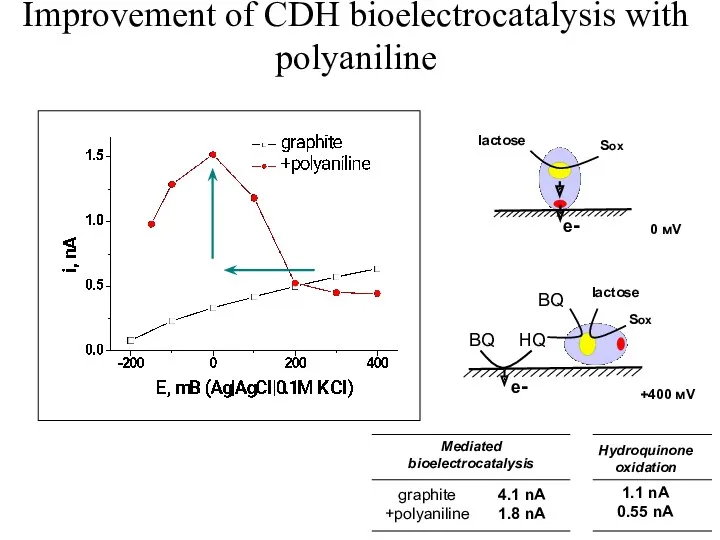
- 51. Improvement of CDH bioelectrocatalysis with polyaniline
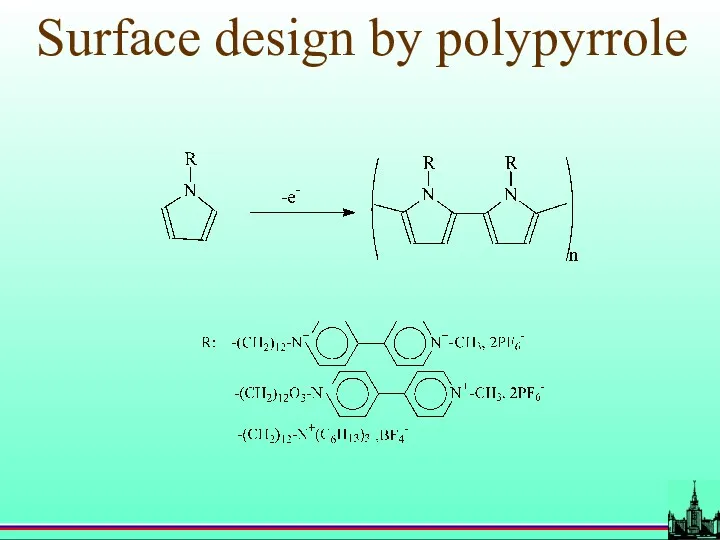
- 52. Surface design by polypyrrole
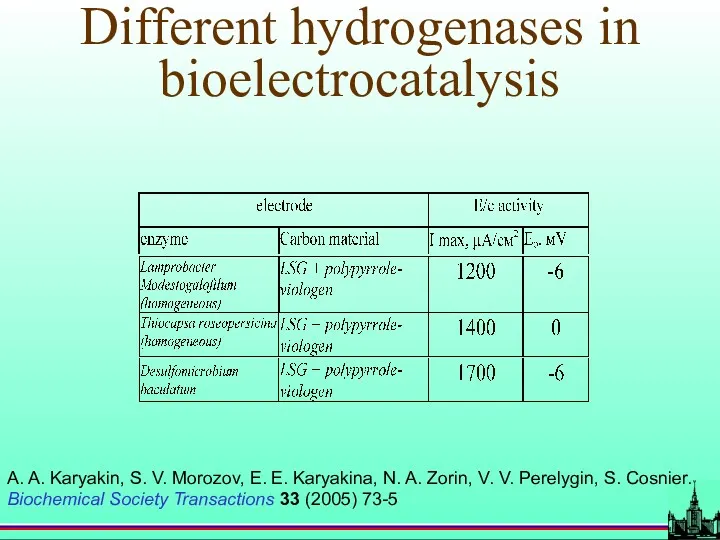
- 53. Different hydrogenases in bioelectrocatalysis A. A. Karyakin, S. V. Morozov, E. E. Karyakina, N. A. Zorin,
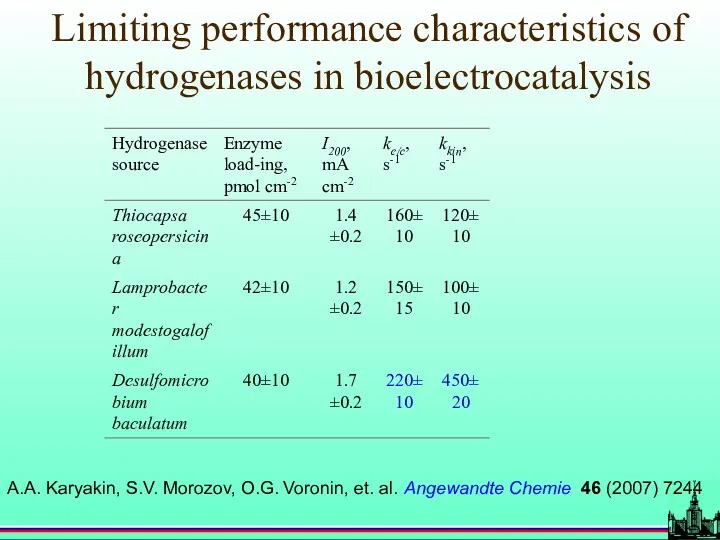
- 54. A.A. Karyakin, S.V. Morozov, O.G. Voronin, et. al. Angewandte Chemie 46 (2007) 7244 Limiting performance characteristics
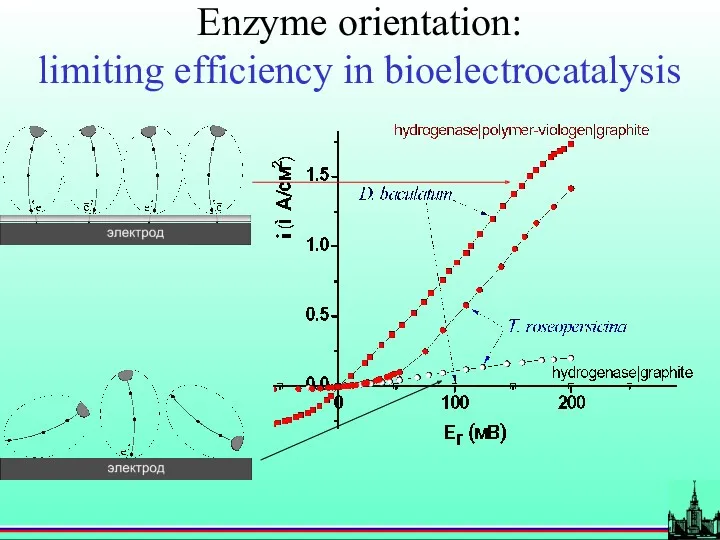
- 55. Enzyme orientation: limiting efficiency in bioelectrocatalysis
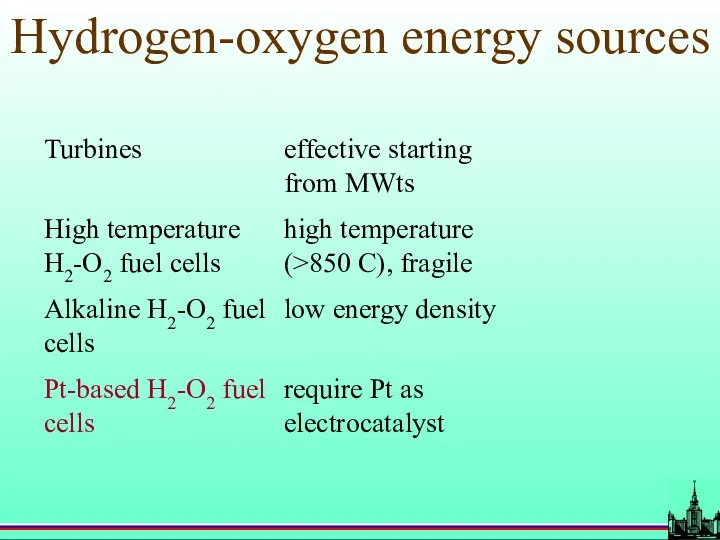
- 56. Hydrogen-oxygen energy sources
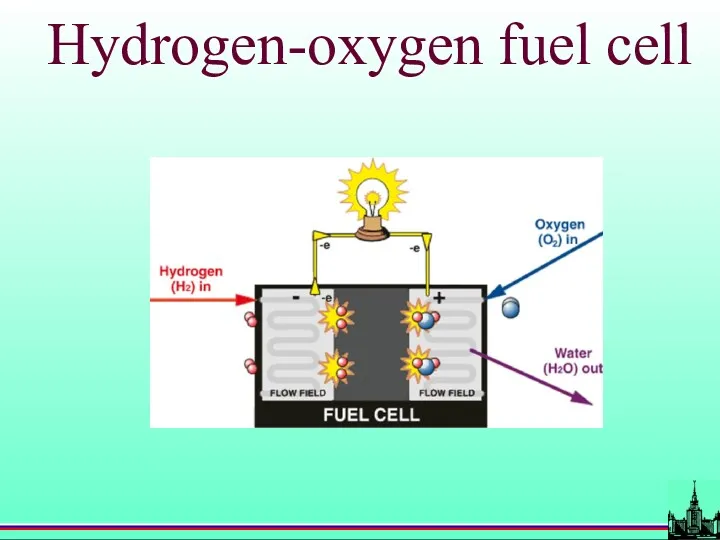
- 57. Hydrogen-oxygen fuel cell
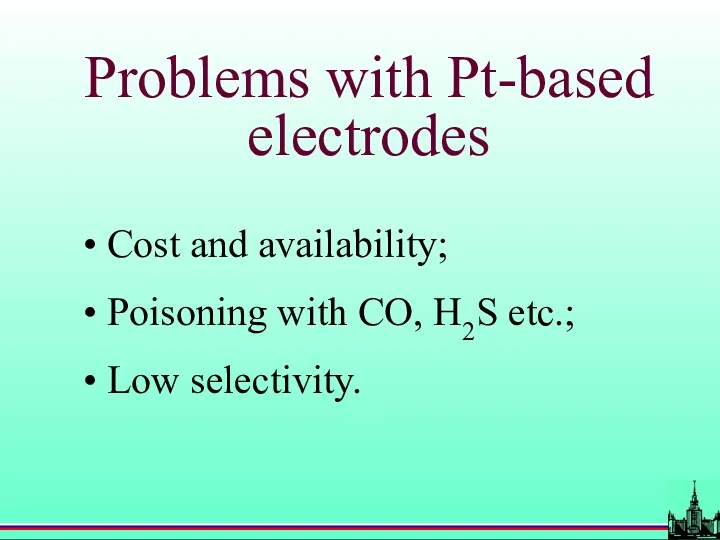
- 58. Problems with Pt-based electrodes Cost and availability; Poisoning with CO, H2S etc.; Low selectivity.
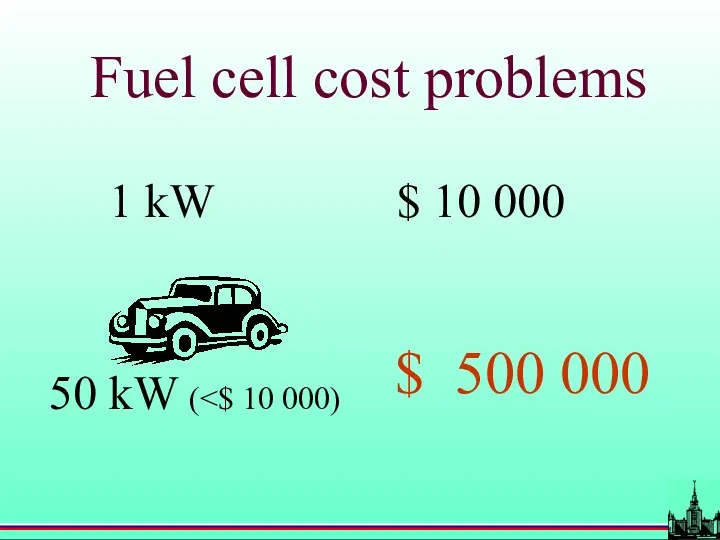
- 59. Fuel cell cost problems 1 kW $ 10 000 $ 500 000
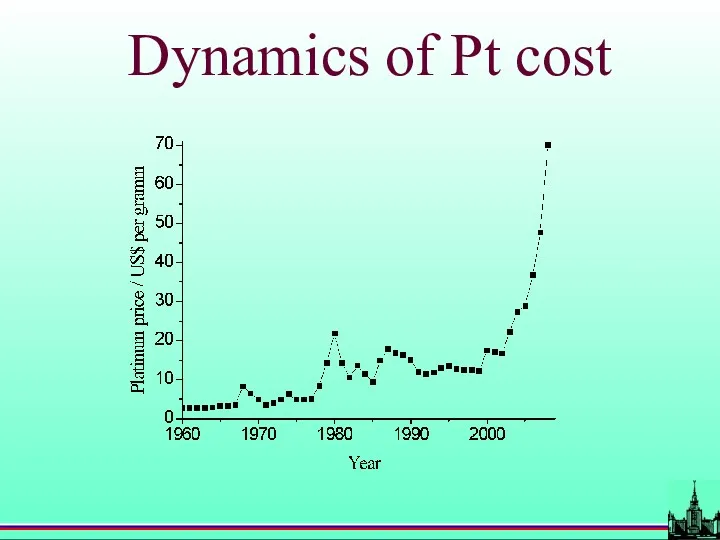
- 60. Dynamics of Pt cost
- 61. Available amount of Pt Annual production: 130 tonnes Assured resources: 100 000 tonnes every year: >60
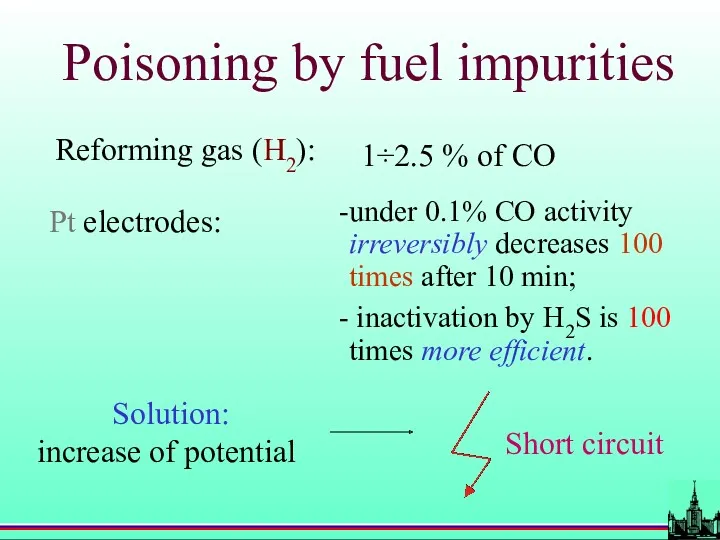
- 62. Poisoning by fuel impurities Reforming gas (H2): 1÷2.5 % of CO Pt electrodes: under 0.1% CO
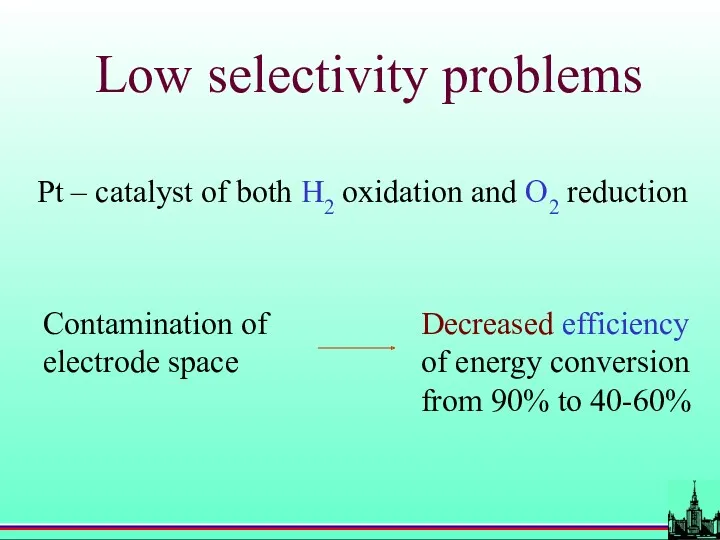
- 63. Low selectivity problems Contamination of electrode space Pt – catalyst of both H2 oxidation and O2
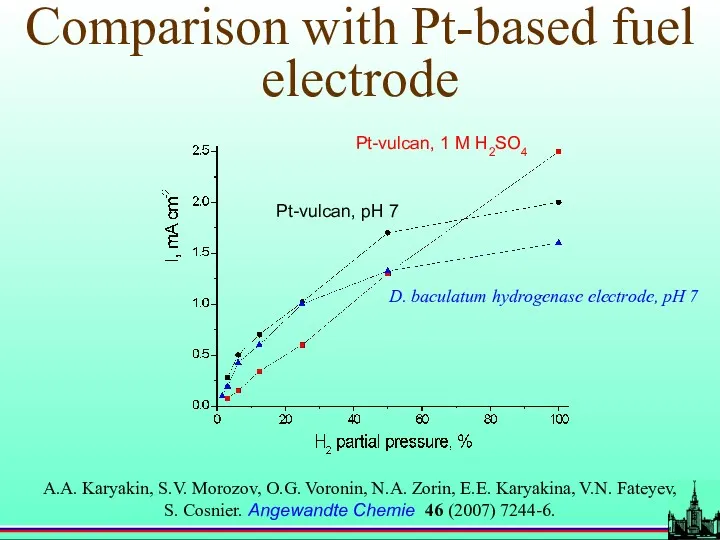
- 64. Comparison with Pt-based fuel electrode D. baculatum hydrogenase electrode, pH 7 Pt-vulcan, 1 M H2SO4 Pt-vulcan,
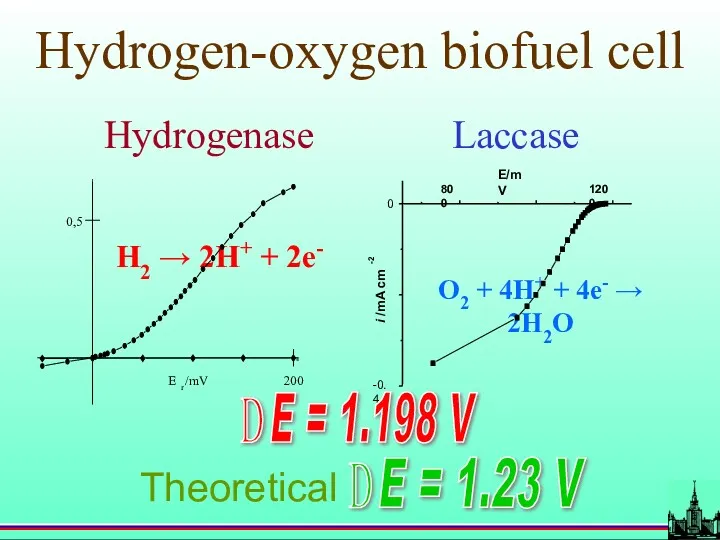
- 65. Hydrogen-oxygen biofuel cell
- 66. Direct bioelectrocatalysis by intact cells
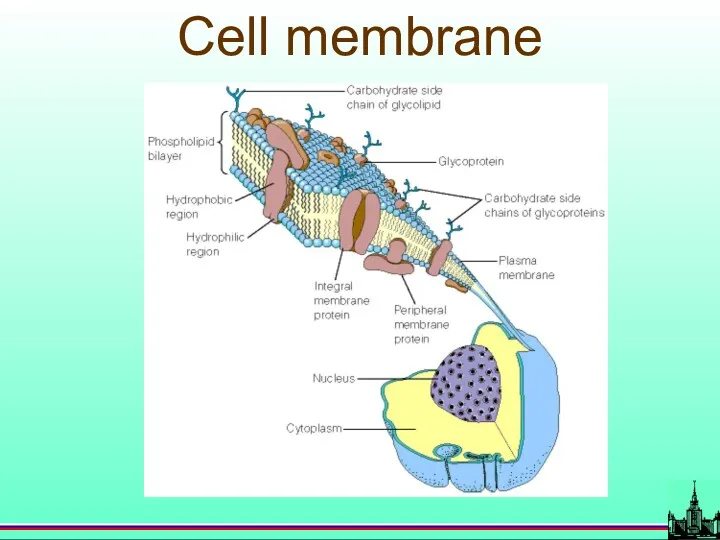
- 67. Cell membrane
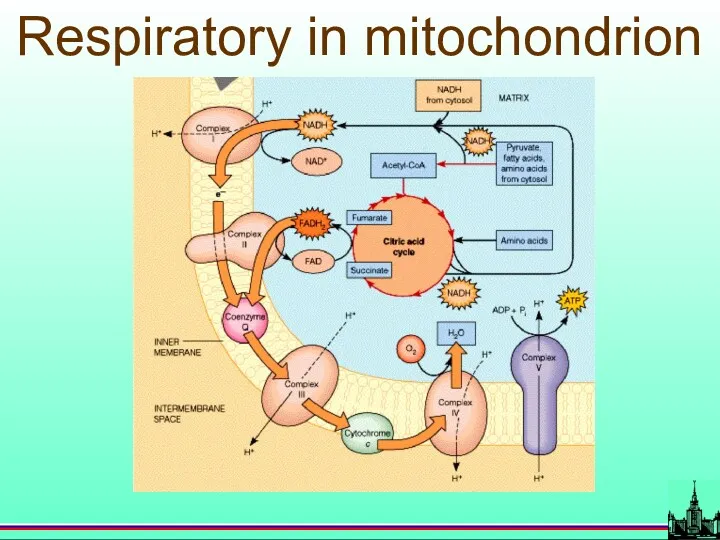
- 68. Respiratory in mitochondrion
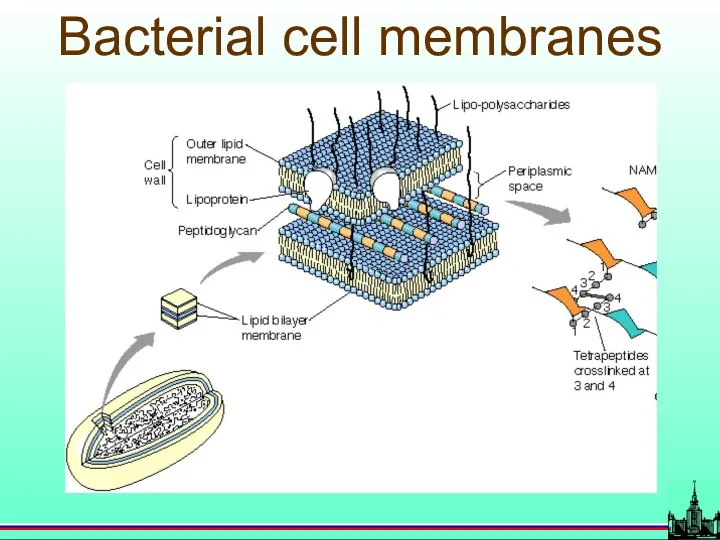
- 69. Bacterial cell membranes
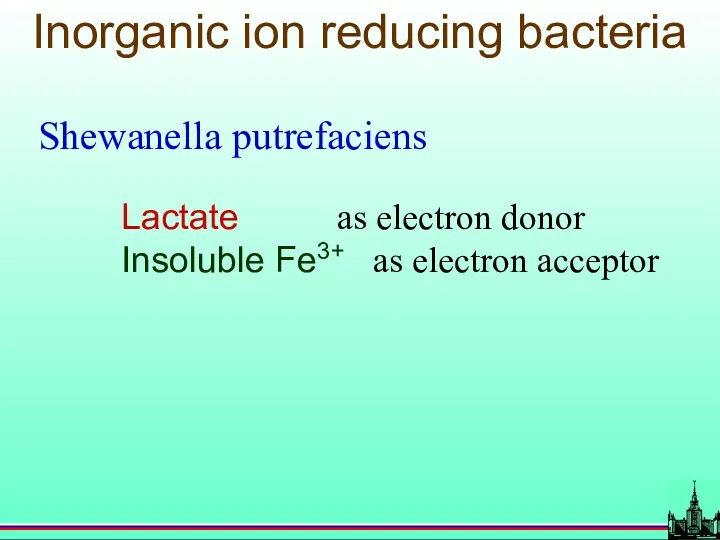
- 70. Inorganic ion reducing bacteria Shewanella putrefaciens Lactate as electron donor Insoluble Fe3+ as electron acceptor
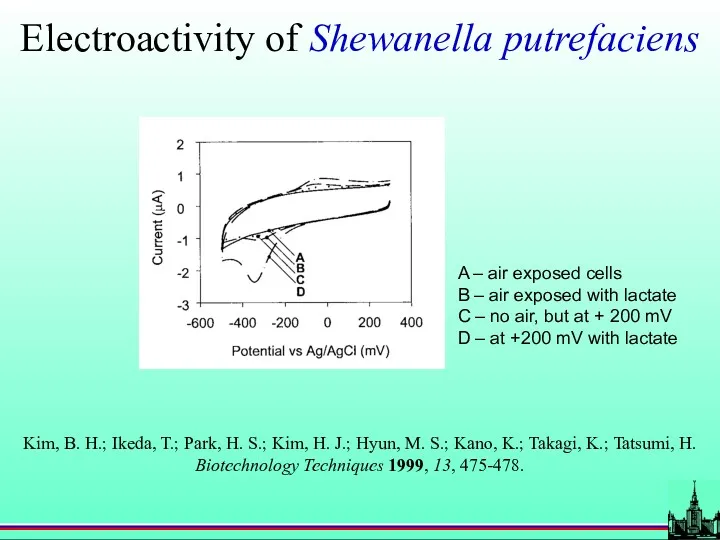
- 71. Electroactivity of Shewanella putrefaciens A – air exposed cells B – air exposed with lactate C
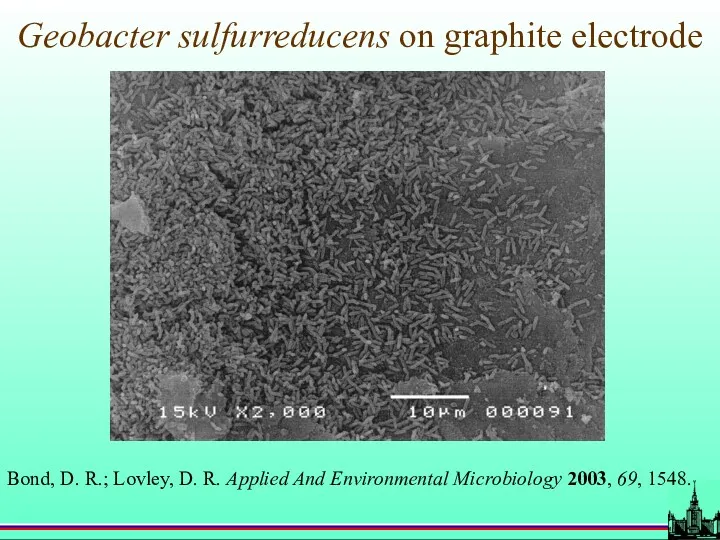
- 72. Geobacter sulfurreducens on graphite electrode Bond, D. R.; Lovley, D. R. Applied And Environmental Microbiology 2003,
- 73. Acetate enriched consortium on graphite electrode Lee, J. Y.; Phung, N. T.; Chang, I. S.; Kim,
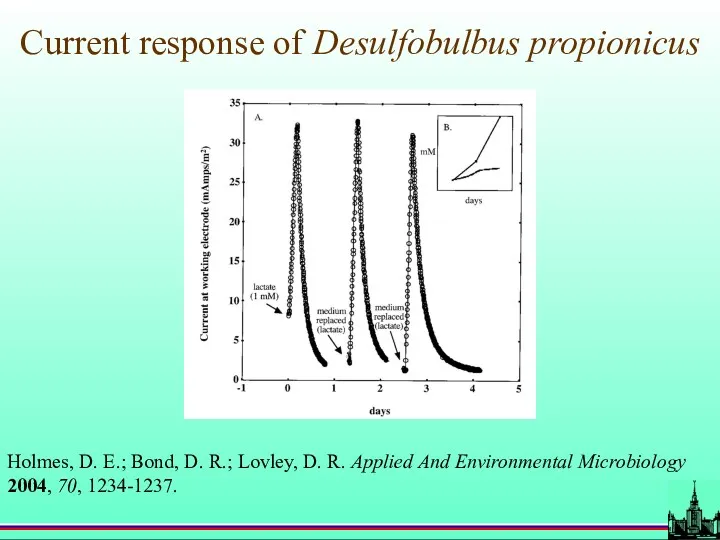
- 74. Current response of Desulfobulbus propionicus Holmes, D. E.; Bond, D. R.; Lovley, D. R. Applied And
- 76. Скачать презентацию









 Инструментальные методы исследования органических веществ. Спектроскопические методы – ЯМР (часть 1)
Инструментальные методы исследования органических веществ. Спектроскопические методы – ЯМР (часть 1) Закон Ома. Сопротивление. Что такое электрический ток?
Закон Ома. Сопротивление. Что такое электрический ток? Выяснение условия равновесия рычага
Выяснение условия равновесия рычага Diesel engines
Diesel engines Температуралық тепе-теңдік күйіндегі денелердің сәуле шығаруы. Қара дененің сәуле шығару заңдары. (Лекция 14)
Температуралық тепе-теңдік күйіндегі денелердің сәуле шығаруы. Қара дененің сәуле шығару заңдары. (Лекция 14) Атом ядросының физикасы
Атом ядросының физикасы Лазерные импульсные дальномеры. Принцип действия импульсных дальномеров
Лазерные импульсные дальномеры. Принцип действия импульсных дальномеров Технология проведения технического обслуживания и ремонта смазочной системы
Технология проведения технического обслуживания и ремонта смазочной системы Превращение энергии. (Окружающий мир, 3 класс)
Превращение энергии. (Окружающий мир, 3 класс) Определение частоты вращения и крутящих моментов на всех валах привода и подбор электродвигателя
Определение частоты вращения и крутящих моментов на всех валах привода и подбор электродвигателя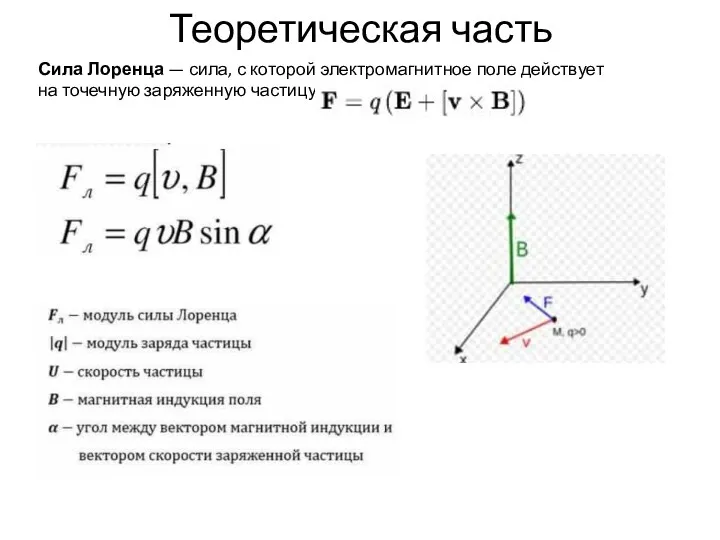 Сила Лоренца. Второй закон Ньютона. Напряженность электрического поля
Сила Лоренца. Второй закон Ньютона. Напряженность электрического поля Транспортирующие машины. (Лекция № 5)
Транспортирующие машины. (Лекция № 5) Правило буравчика. Правило правой и левой руки. (Урок 41)
Правило буравчика. Правило правой и левой руки. (Урок 41) Закон всемирного тяготения. Решение задач
Закон всемирного тяготения. Решение задач Лазерные и радио-лазерные системы для прецизионной передачи шкал времени и частотно-временных измерений в ГЛОНАСС
Лазерные и радио-лазерные системы для прецизионной передачи шкал времени и частотно-временных измерений в ГЛОНАСС Движение под действием силы тяжести. Решение задач
Движение под действием силы тяжести. Решение задач Техническая эксплуатация подъёмно-транспортных, строительных, дорожных машин и оборудования (по отраслям)
Техническая эксплуатация подъёмно-транспортных, строительных, дорожных машин и оборудования (по отраслям) Механика деформируемых тел. Основные допущения и принципы сопротивления материалов
Механика деформируемых тел. Основные допущения и принципы сопротивления материалов Формирование познавательного интереса на уроках физики ,с помощью современных образовательных технологий
Формирование познавательного интереса на уроках физики ,с помощью современных образовательных технологий Изотопы. Радиоактивные превращения атомных ядер
Изотопы. Радиоактивные превращения атомных ядер Аэрогазодинамика. Тела вращения в сверхзвуковом потоке (лекции 22, 23)
Аэрогазодинамика. Тела вращения в сверхзвуковом потоке (лекции 22, 23) Биоэлектромагнетизм. Основы электрокардиографии и реографии
Биоэлектромагнетизм. Основы электрокардиографии и реографии Игра Поле чудес по теме Нобелевские лауреаты по физике
Игра Поле чудес по теме Нобелевские лауреаты по физике Внутренняя энергия тела
Внутренняя энергия тела Давление в жидкости и газе. Решение задач
Давление в жидкости и газе. Решение задач Дифракция механических волн
Дифракция механических волн Аморфные тела
Аморфные тела Основные положения сопротивления материалов
Основные положения сопротивления материалов