Слайд 2

The reactions accompanied by a change of atom oxidation number of
elements are called
oxidation-reduction reactions.
Слайд 3

The particles which accept electrons are called oxidizers.
The particles which
donate electrons are called reducers.
Слайд 4

Fе2+ + Се4+ ↔ Fе3+ + Се3+
Oxidized and reduced forms of
one substance involved in the half-reaction compose redox couple:
Fе2+ – ē ↔ Fе3+
Се4+ + ē ↔ Се3+
Слайд 5

Electrode or redox potential (E) is the quantitative measure of redox
power of different redox reactions.
Слайд 6
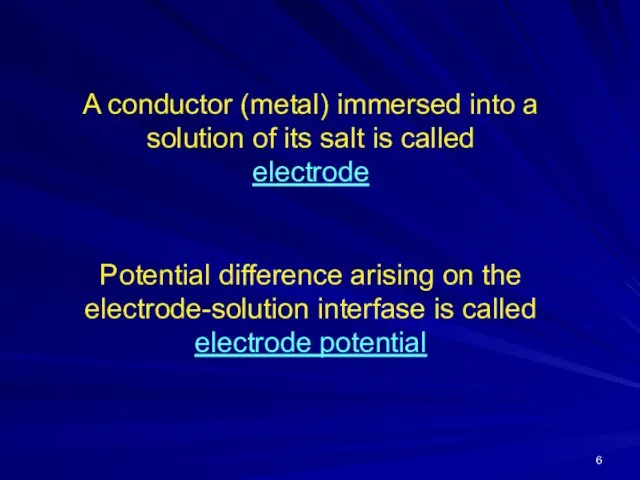
A conductor (metal) immersed into a solution of its salt is
called
electrode
Potential difference arising on the electrode-solution interfase is called electrode potential
Слайд 7

Potential difference between electrodes is known as electromotive force (EMF)
EMF =
Ecathode - Eanode
Слайд 8
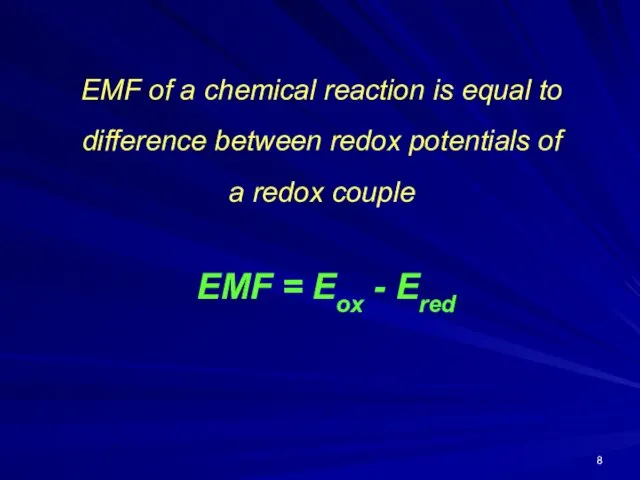
EMF of a chemical reaction is equal to difference between redox
potentials of
a redox couple
EMF = Eox - Ered
Слайд 9

The potentials difference that occurs in the tissues of living organisms
is called
bioelectric potential.








 Законы взаимодействия и движения тел Механические колебания и волны. Звук Электромагнитное поле
Законы взаимодействия и движения тел Механические колебания и волны. Звук Электромагнитное поле Физические методы исследования материалов
Физические методы исследования материалов Презентация Солнечная система
Презентация Солнечная система Техническое обслуживание и ремонт автомобильного транспорта
Техническое обслуживание и ремонт автомобильного транспорта Урок решения задач на расчёт количества теплоты
Урок решения задач на расчёт количества теплоты Электромагнитные колебания
Электромагнитные колебания Греет ли снег?
Греет ли снег? Ультразвук. Источники и применение
Ультразвук. Источники и применение Динамика твердого тела. Уравнения движения твердого тела
Динамика твердого тела. Уравнения движения твердого тела Магнитное поле
Магнитное поле Michael Faraday
Michael Faraday Молекулалы сәулелік эпитаксия
Молекулалы сәулелік эпитаксия Колебания, их виды и характеристики. 11 класс
Колебания, их виды и характеристики. 11 класс Электрические явления в природе
Электрические явления в природе Кванттық физиканың негізін қалаушы
Кванттық физиканың негізін қалаушы Модернизация рулевого управления автомобиля ГАЗ-3308
Модернизация рулевого управления автомобиля ГАЗ-3308 Применение законов сохранения импульса и энергии
Применение законов сохранения импульса и энергии Поляризация света
Поляризация света Великие ученые XIX-XX веков
Великие ученые XIX-XX веков Система электрооборудования Лада 2190 (Гранта)
Система электрооборудования Лада 2190 (Гранта) Всё для фронта, всё для победы!
Всё для фронта, всё для победы! Зубчатые передачи
Зубчатые передачи Electrical Charges
Electrical Charges Теплообмен излучением
Теплообмен излучением Линзы. Оптическая сила линзы
Линзы. Оптическая сила линзы Совершенствование технического облуживания сельскохозяйственных машин в условиях ООО КОЛОС
Совершенствование технического облуживания сельскохозяйственных машин в условиях ООО КОЛОС Методы наблюдения и регистрации элементарных частиц
Методы наблюдения и регистрации элементарных частиц Замедление нейтронов. Уравнение переноса
Замедление нейтронов. Уравнение переноса