Содержание
- 2. LECTURE No. 5 PROPERTIES OF NANOSTRUCTURED MATERIALS
- 3. INTRODUCTION Metal based nanomaterials. Importance of the size effect in properties of nanomaterials. Properties of metal
- 4. OBJECTIVES To analyze the influence of size factor on the chemical equilibrium. To discuss the adsorption
- 5. OUTLINE Thermodynamic features of nanoparticles. The adsorption properties of nanoparticles and nanomaterials.
- 6. Some thermodynamic features of nanoparticles Number of atoms 13 atoms (12 on the surface) 92% 55
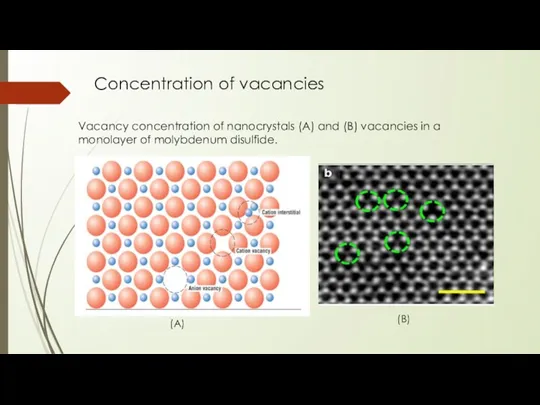
- 7. Concentration of vacancies Vacancy concentration of nanocrystals (A) and (B) vacancies in a monolayer of molybdenum
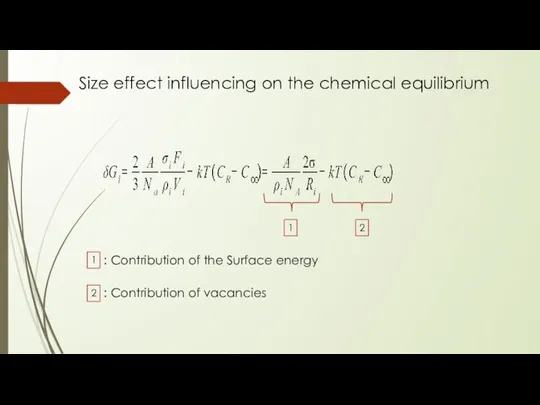
- 8. Size effect influencing on the chemical equilibrium
- 9. Size effect influencing on the chemical equilibrium Standard change in the Gibbs energy equation: Where: k
- 10. Size effect influencing on the chemical equilibrium Standard state ΔGO : (Normal T and P) Where:
- 11. Size effect influencing on the chemical equilibrium For reactions between substances in a state of change
- 12. Size effect influencing on the chemical equilibrium For reactions between substances in a state of change
- 13. Size effect influencing on the chemical equilibrium 1 2 1 : Contribution of the Surface energy
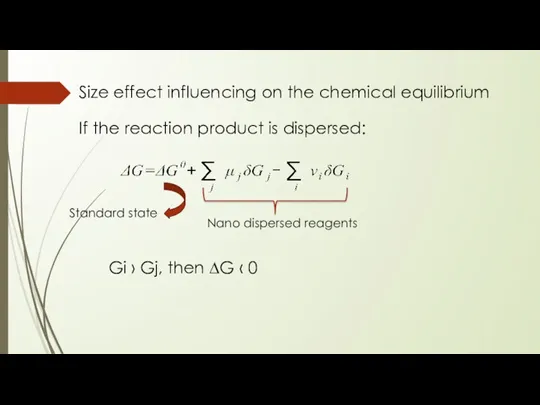
- 14. Size effect influencing on the chemical equilibrium If the reaction product is dispersed: Nano dispersed reagents
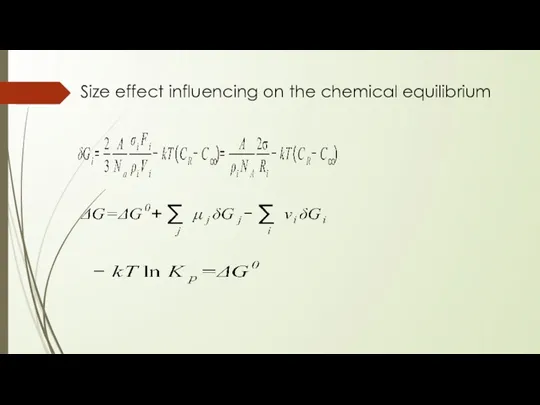
- 15. Size effect influencing on the chemical equilibrium
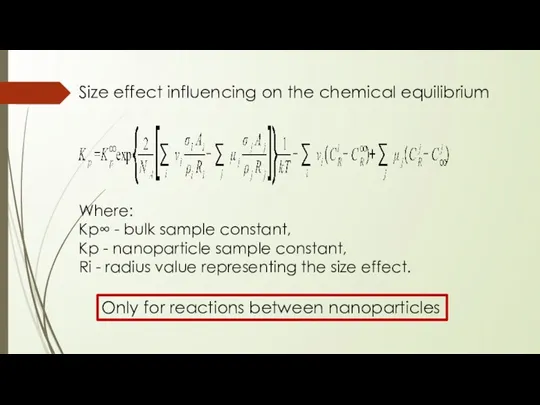
- 16. Size effect influencing on the chemical equilibrium Where: Kp∞ - bulk sample constant, Kp - nanoparticle
- 17. Size effect influencing on the chemical equilibrium могут возникать реакции между наночастицами, термодинамически запрещенными для массивных
- 18. The adsorption properties of nanoparticles and nanomaterials. The adsorption properties of nanoparticles and nanomaterials. Application: Chromatography
- 19. The adsorption properties of nanoparticles and nanomaterials. Normal alkanes (C6-C8) in 3 Å, 4 Å and
- 20. The adsorption properties of nanoparticles and nanomaterials. Cabon nanotubes: The nanotubes obtained by high-temperature annealing and
- 21. The adsorption properties of nanoparticles and nanomaterials. Metal nanoparticles deposited on oxide supports Gold and platinum
- 22. The adsorption properties of nanoparticles and nanomaterials. Metal nanoparticles deposited on oxide supports Gold and platinum
- 23. Control questions Explain two properties of metal based nanoparticles. Analyze some factors, that explain the change
- 25. Скачать презентацию

















 Ядерная физика (Лекция 9)
Ядерная физика (Лекция 9)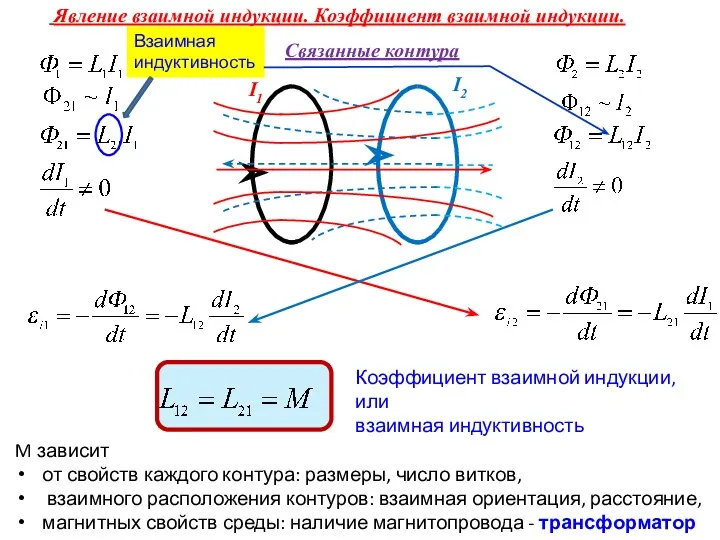 Явление взаимной индукции. Коэффициент взаимной индукции. Взаимная индуктивность
Явление взаимной индукции. Коэффициент взаимной индукции. Взаимная индуктивность Проводниковые материалы
Проводниковые материалы Швартовное устройство на судне
Швартовное устройство на судне Радиоактивность, как свидетельство сложного строения атома
Радиоактивность, как свидетельство сложного строения атома Презентация к игре Звездный час для учащихся 7-8 классов
Презентация к игре Звездный час для учащихся 7-8 классов Проницаемость
Проницаемость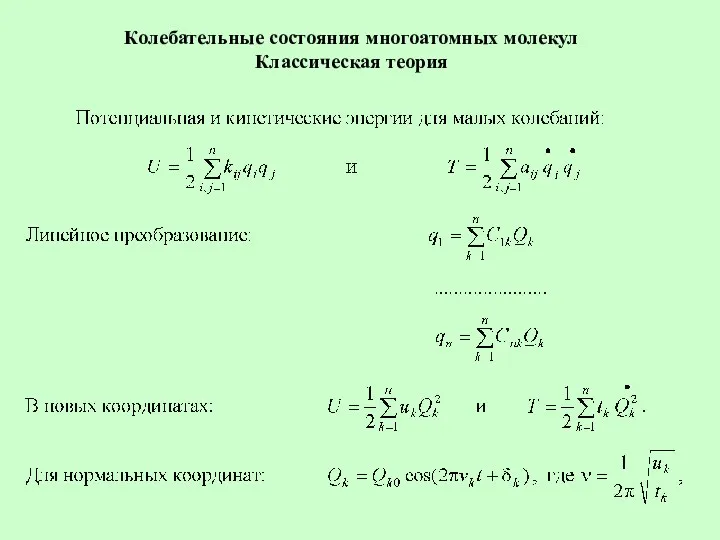 Колебательные состояния многоатомных молекул. Классическая теория
Колебательные состояния многоатомных молекул. Классическая теория Рулевое управление тракторов
Рулевое управление тракторов История фрезерного станка
История фрезерного станка Неподвижные элементы и неисправности газораспределительных механизмов
Неподвижные элементы и неисправности газораспределительных механизмов История паровых двигателей
История паровых двигателей Реактивное движение. Ракеты
Реактивное движение. Ракеты Презентация по теме Спектры
Презентация по теме Спектры Типовые соединения, применяемые в электроустановках. Методы и средства контроля размеров и качества сборки
Типовые соединения, применяемые в электроустановках. Методы и средства контроля размеров и качества сборки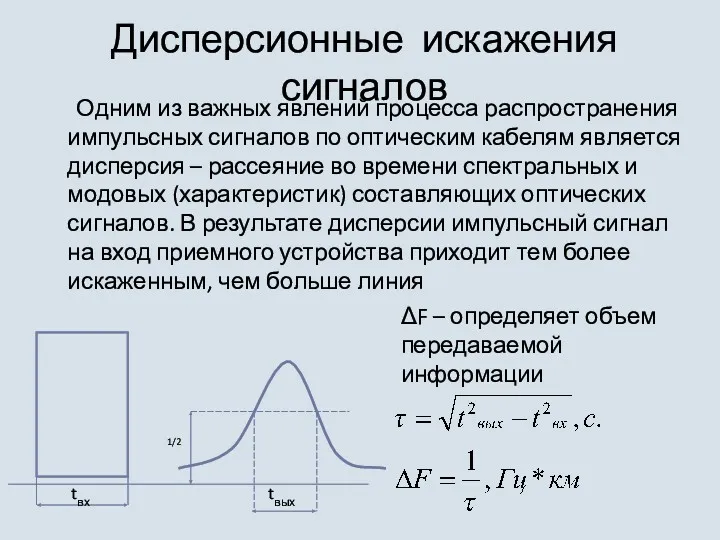 Дисперсионные искажения сигналов
Дисперсионные искажения сигналов Бытовая швейная машина
Бытовая швейная машина Интерференция света
Интерференция света Конспект урока на тему: Плотность вещества 7 класс
Конспект урока на тему: Плотность вещества 7 класс Презентация к уроку физики 8 класс
Презентация к уроку физики 8 класс Анимации на уроках физики.
Анимации на уроках физики. Система распределения тормозных усилий. Урок № 189
Система распределения тормозных усилий. Урок № 189 Ядерные реакции, атомная энергия
Ядерные реакции, атомная энергия Своя игра по физике - 7
Своя игра по физике - 7 Презентация к уроку физики Закон всемирного тяготения
Презентация к уроку физики Закон всемирного тяготения Основные понятия термодинамики
Основные понятия термодинамики Two types of transformers
Two types of transformers Алгоритм решения задач по теме Законы сохранения
Алгоритм решения задач по теме Законы сохранения