Содержание
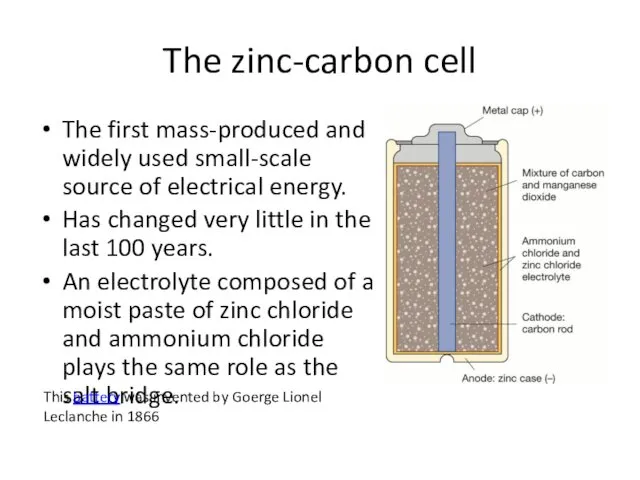
- 2. The zinc-carbon cell The first mass-produced and widely used small-scale source of electrical energy. Has changed
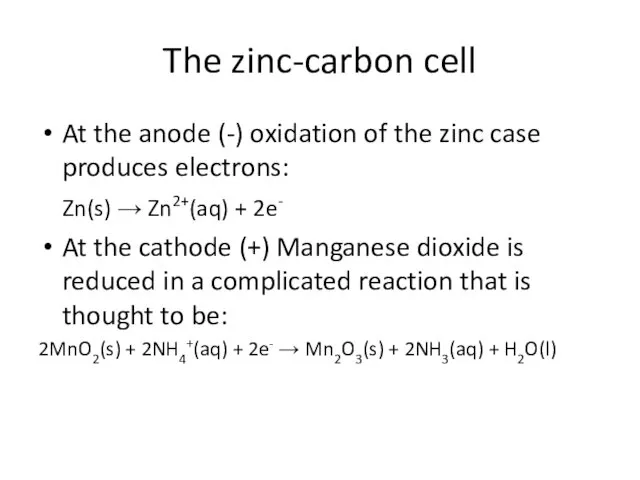
- 3. The zinc-carbon cell At the anode (-) oxidation of the zinc case produces electrons: Zn(s) →
- 4. The zinc-carbon dry cell A new cell produces about 1.5 volts, but this diminishes significantly during
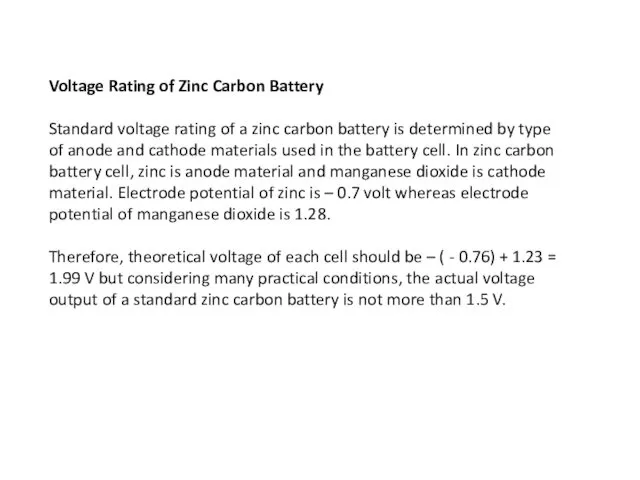
- 5. Voltage Rating of Zinc Carbon Battery Standard voltage rating of a zinc carbon battery is determined
- 6. Advantages and Disadvantages of Zinc Carbon Battery Advantages of Leclanche’ Battery The cost of this battery
- 7. Alkaline batteries Alkaline batteries and alkaline cells (a battery being a collection of multiple cells) are
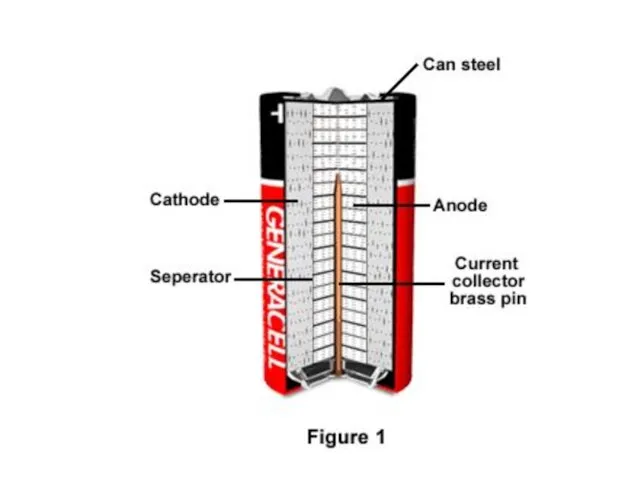
- 8. Construction A cylindrical cell is contained in a drawn steel can, which is the cathode current
- 10. Chemistry Anode : Zinc Powder Cathode : Manganese dioxide(MnO2) powder Electrolyte : Potassium hydroxide(KOH)
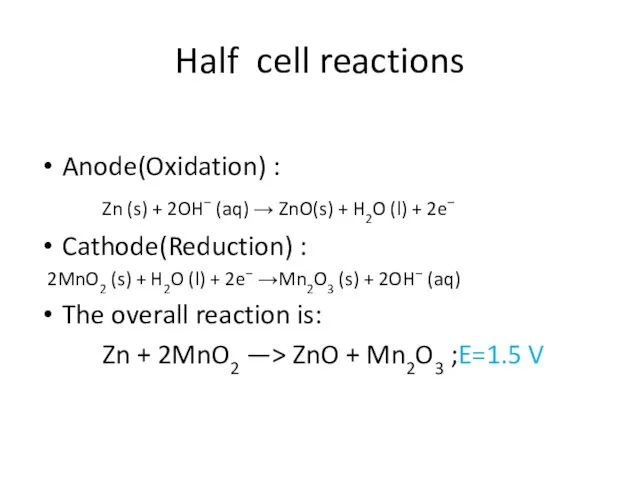
- 11. Half cell reactions Anode(Oxidation) : Zn (s) + 2OH− (aq) → ZnO(s) + H2O (l) +
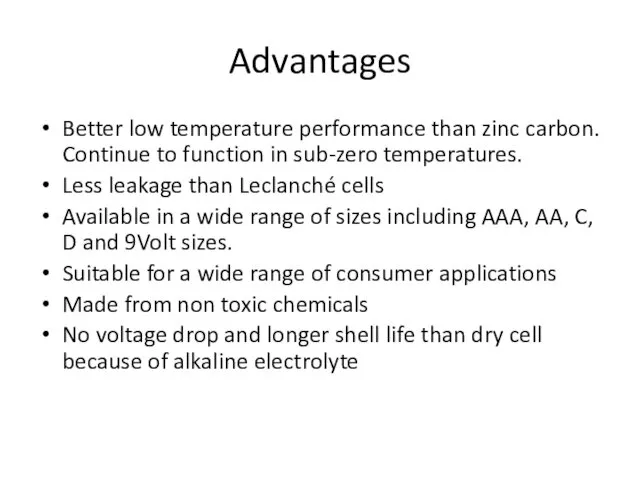
- 12. Advantages Better low temperature performance than zinc carbon. Continue to function in sub-zero temperatures. Less leakage
- 14. Скачать презентацию





 Elektricheskoe_soprotivlenie_Zakon_Oma_dlya_uchastka_tsepi
Elektricheskoe_soprotivlenie_Zakon_Oma_dlya_uchastka_tsepi Ременная передача
Ременная передача Движение электронов в электрическом, магнитном и скрещенных полях
Движение электронов в электрическом, магнитном и скрещенных полях Фотоэффект құбылысы. Эйнштейн формуласы
Фотоэффект құбылысы. Эйнштейн формуласы Архимед (287 - 212 до н.э.)
Архимед (287 - 212 до н.э.) Сила
Сила Комплексное электрофизическое воздействие на призабойную зону с целью добычи трудноизвлекаемой нефти
Комплексное электрофизическое воздействие на призабойную зону с целью добычи трудноизвлекаемой нефти Отражение и преломление света. Презентация.
Отражение и преломление света. Презентация. Применение закона равновесия рычага к блоку. Золотое правило механики
Применение закона равновесия рычага к блоку. Золотое правило механики Радиотехническая отрасль, ее состав и значение для развития современного общества. Системы радиосвязи и радиовещания
Радиотехническая отрасль, ее состав и значение для развития современного общества. Системы радиосвязи и радиовещания Уравнение движения системы с переменной массой
Уравнение движения системы с переменной массой Реактивний рух в природі
Реактивний рух в природі Теория и практика групповой работы на уроках физики
Теория и практика групповой работы на уроках физики Водомер Вентури
Водомер Вентури вов 2
вов 2 Техническое обслуживание и ремонт тормозной системы ВАЗ 2105
Техническое обслуживание и ремонт тормозной системы ВАЗ 2105 Решение задач на применение законов Ньютона
Решение задач на применение законов Ньютона 10 кл - Подготовка к контрольной работе по теме Основы термодинамики
10 кл - Подготовка к контрольной работе по теме Основы термодинамики Качественные задачи
Качественные задачи Полное внутреннее отражение
Полное внутреннее отражение М.В. Ломоносов
М.В. Ломоносов Урок викторина по физике
Урок викторина по физике Высота. Тембр и громкость звука
Высота. Тембр и громкость звука Ременные передачи
Ременные передачи Сила трения (7 класс)
Сила трения (7 класс) Неединичные обратные связи и инвариантность системы к задающему воздействию
Неединичные обратные связи и инвариантность системы к задающему воздействию Термоядерная реакция
Термоядерная реакция Кинематические элементы процесса точения
Кинематические элементы процесса точения