Содержание
- 2. Definition of Radiation “Radiation is an energy in the form of electro-magnetic waves or particulate matter,
- 3. Forces: There are many interactions among nuclei. It turns out that there are forces other than
- 4. Radioactivity: Elements & Atoms Atoms are composed of smaller particles referred to as: Protons Neutrons Electrons
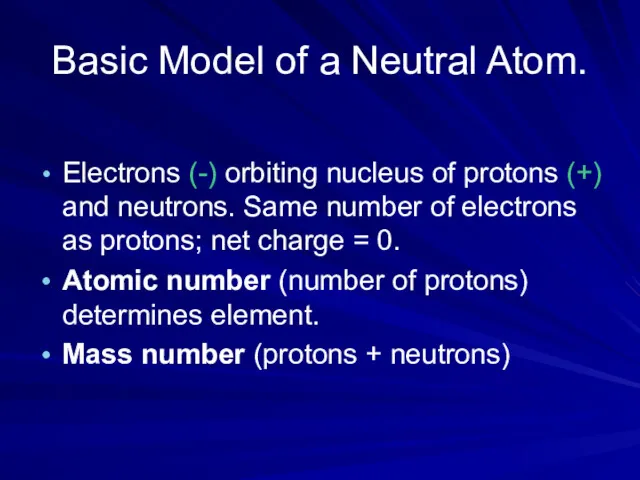
- 5. Basic Model of a Neutral Atom. Electrons (-) orbiting nucleus of protons (+) and neutrons. Same
- 7. Radioactivity If a nucleus is unstable for any reason, it will emit and absorb particles. There
- 8. Ionization Ionizing radiation is produced by unstable atoms. Unstable atoms differ from stable atoms because they
- 13. Types or Products of Ionizing Radiation β α γ or X-ray neutron
- 14. Radioactive Atom X-ray gamma ray
- 15. The electro-magnetic waves vary in their length and frequency along a very wide spectrum.
- 19. Types of Radiation Radiation is classified into: Ionizing radiation Non-ionizing radiation
- 20. Ionizing Versus Non-ionizing Radiation Ionizing Radiation Higher energy electromagnetic waves (gamma) or heavy particles (beta and
- 21. Ionizing Radiation Definition: “ It is a type of radiation that is able to disrupt atoms
- 22. Another Definition Ionizing radiation A radiation is said to be ionizing when it has enough energy
- 23. Primary Types of Ionizing Radiation Alpha particles Beta particles Gamma rays (or photons) X-Rays (or photons)
- 24. Alpha Particles: 2 neutrons and 2 protons They travel short distances, have large mass Only a
- 25. Alpha Particles (or Alpha Radiation): Helium nucleus (2 neutrons and 2 protons); +2 charge; heavy (4
- 26. Beta Particles Beta Particles: Electrons or positrons having small mass and variable energy. Electrons form when
- 27. Beta Particles: High speed electron ejected from nucleus; -1 charge, light 0.00055 AMU; Typical Energy =
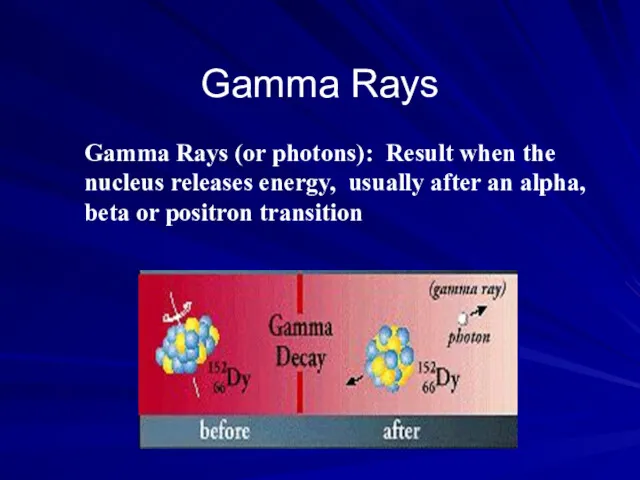
- 29. Gamma Rays Gamma Rays (or photons): Result when the nucleus releases energy, usually after an alpha,
- 30. X-Rays X-Rays: Occur whenever an inner shell orbital electron is removed and rearrangement of the atomic
- 31. X- and Gamma Rays: X-rays are photons (Electromagnetic radiations) emitted from electron orbits. Gamma rays are
- 32. Neutrons Neutrons: Have the same mass as protons but are uncharged
- 35. QUANTIFICATION OF RADIATION A. Quantifying Radioactive Decay B. Quantifying Exposure and Dose
- 36. A. Quantifying Radioactive Decay Measurement of Activity in disintegrations per second (dps); 1 Becquerel (Bq) =
- 37. B. Quantifying Exposure and Dose Exposure: Roentgen 1 Roentgen (R) = amount of X or gamma
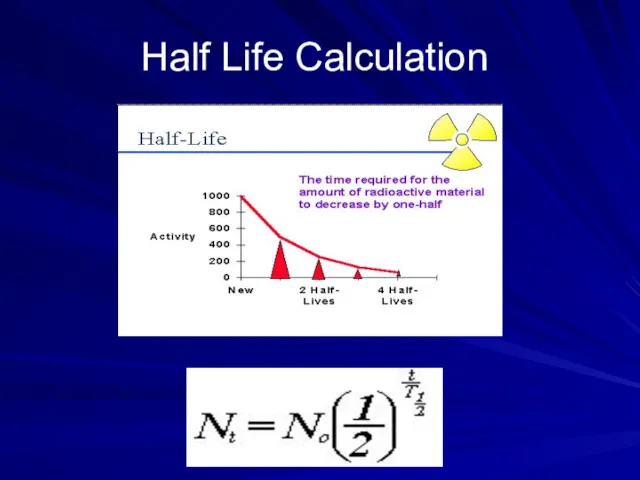
- 38. Half Life Calculation
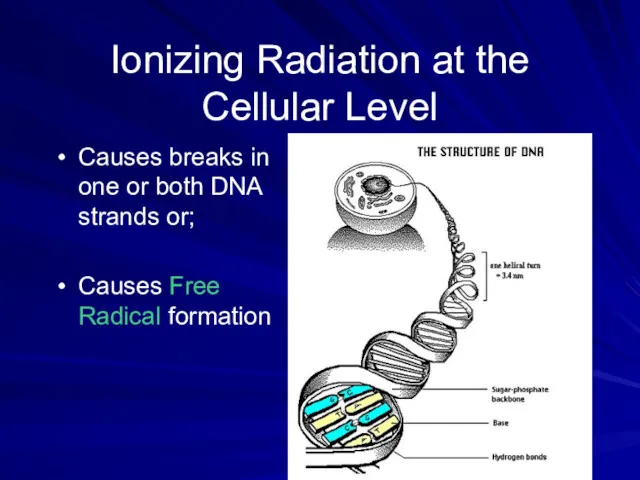
- 39. Ionizing Radiation at the Cellular Level Causes breaks in one or both DNA strands or; Causes
- 40. Exposure Limits OSHA Limits: Whole body limit = 1.25 rem/qtr or 5 rem (50 mSv) per
- 41. External/Internal Exposure Limits for Occupationally Exposed Individuals Annual Dose Limits *Effective dose equivalent
- 43. Community Emergency Radiation Hazardous Waste Sites: Radiation above background (0.01-0.02 m rem/hr) signifies possible presence which
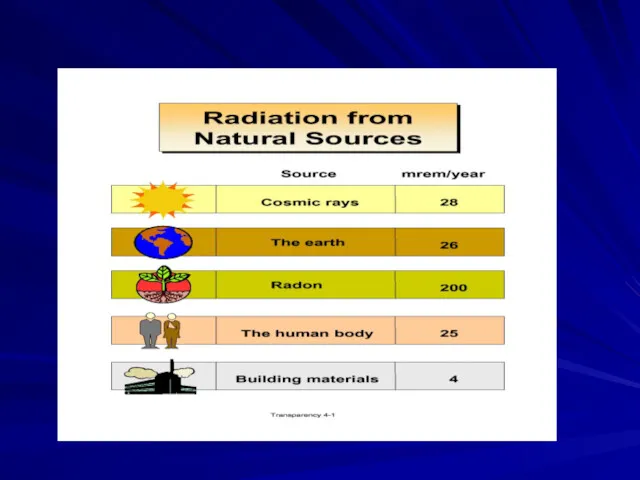
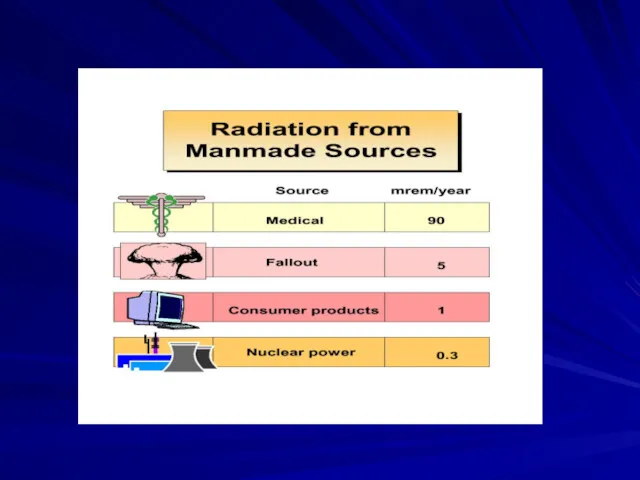
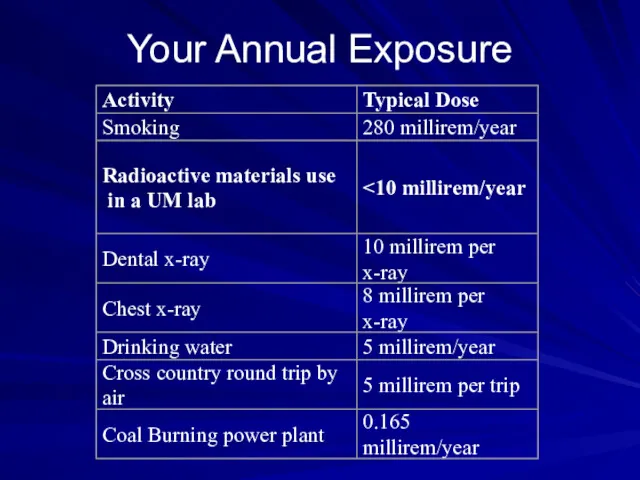
- 44. Your Annual Exposure
- 45. HEALTH EFFECTS Generalizations: Biological effects are due to the ionization process that destroys the capacity for
- 46. ACUTE DOSE(RAD) EFFECT
- 47. Delayed Somatic Effects: Delayed effects to exposed person include: Cancer, leukemia, cataracts, life shortening from organ
- 48. Critical Organs: Organs generally most susceptible to radiation damage include: Lymphocytes, bone marrow, gastro-intestinal, gonads, and
- 49. Non-ionizing Radiation Definition: “ They are electromagnetic waves incapable of producing ions while passing through matter,
- 50. All earth surface system components emit radiation---the sun and the earth are the components we are
- 52. Path of incoming solar radiation
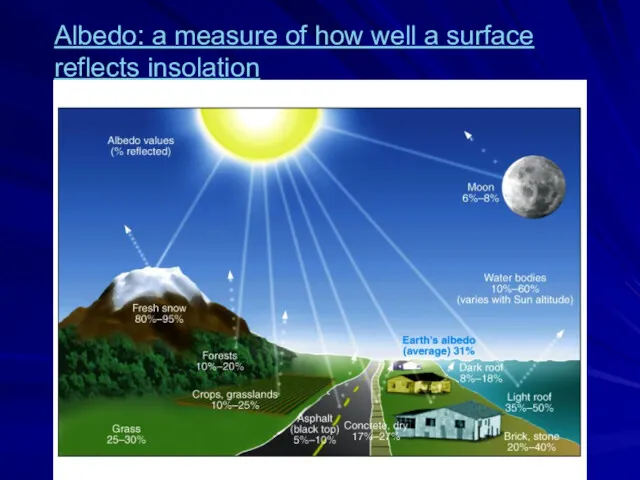
- 53. Albedo: a measure of how well a surface reflects insolation
- 54. Examples on Non-ionizing Radiation Sources Visible light Microwaves Radios Video Display Terminals Power lines Radiofrequency Diathermy
- 55. Other Manmade Sources of Non-Ionizing Radiation
- 58. Effects Radiofrequency Ranges (10 kHz to 300 GHz) Effects only possible at ten times the permissible
- 59. RADIATION CONTROLS A. Basic Control Methods for External Radiation Decrease Time Increase Distance Increase Shielding
- 60. Time: Minimize time of exposure to minimize total dose. Rotate employees to restrict individual dose. Distance:
- 62. B. Monitoring Personal Dosimeters: Normally they do not prevent exposures (no alarm), just record it. They
- 66. Direct Reading Survey Meters and Counters: Useful in identifying source of exposures recorded by personal dosimeters,
- 68. Continuous Monitors: Continuous direct reading ionization detectors (same detectors as above) can provide read-out and/or alarm
- 69. Elements of Radiation Protection Program Monitoring of exposures: Personal, area, and screening measurements; Medical/biologic monitoring. Task-Specific
- 71. Скачать презентацию




























































 Измерение давления в покоящейся жидкости
Измерение давления в покоящейся жидкости Physics basics (Unit 1)
Physics basics (Unit 1) Презентация. Гости из космоса
Презентация. Гости из космоса Властивості радіохвиль. Розподіл спектру радіохвиль. Особливості розповсюдження радіохвиль (Лекція 2.1)
Властивості радіохвиль. Розподіл спектру радіохвиль. Особливості розповсюдження радіохвиль (Лекція 2.1) Машины и оборудование для свайных работ. Лекция 6
Машины и оборудование для свайных работ. Лекция 6 Электромагнитные переходные процессы в электроэнергетических системах
Электромагнитные переходные процессы в электроэнергетических системах Открытое мероприятие Добро пожаловать в космос
Открытое мероприятие Добро пожаловать в космос Применение сообщающихся сосудов
Применение сообщающихся сосудов Презентация по теме Решение задач по кинематике с помощью квадратных уравнений.
Презентация по теме Решение задач по кинематике с помощью квадратных уравнений. Айнымалы ток тізбегіндегі актив кедергі. (Лекция 14)
Айнымалы ток тізбегіндегі актив кедергі. (Лекция 14) Своя игра. Строения атома. (11 класс)
Своя игра. Строения атома. (11 класс) Три состояния вещества
Три состояния вещества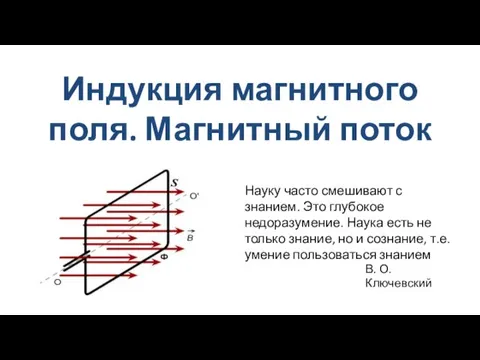 Индукция магнитного поля. Магнитный поток
Индукция магнитного поля. Магнитный поток Газовые законы
Газовые законы Автокөліктерді жөндеу технологиясы
Автокөліктерді жөндеу технологиясы Зеленый дом
Зеленый дом Энтропия в техносфере
Энтропия в техносфере Раздел 3. Оценка технического состояния, ТО и Р автомобилей. Урок №72. Тема ТО рулевого управления
Раздел 3. Оценка технического состояния, ТО и Р автомобилей. Урок №72. Тема ТО рулевого управления Ядерные реакции. Радиоактивность
Ядерные реакции. Радиоактивность Пучковые технологии. Вакуум: физические свойства, получение, измерение
Пучковые технологии. Вакуум: физические свойства, получение, измерение Тепловой расчет сферического носка ЛА. Вариант 34
Тепловой расчет сферического носка ЛА. Вариант 34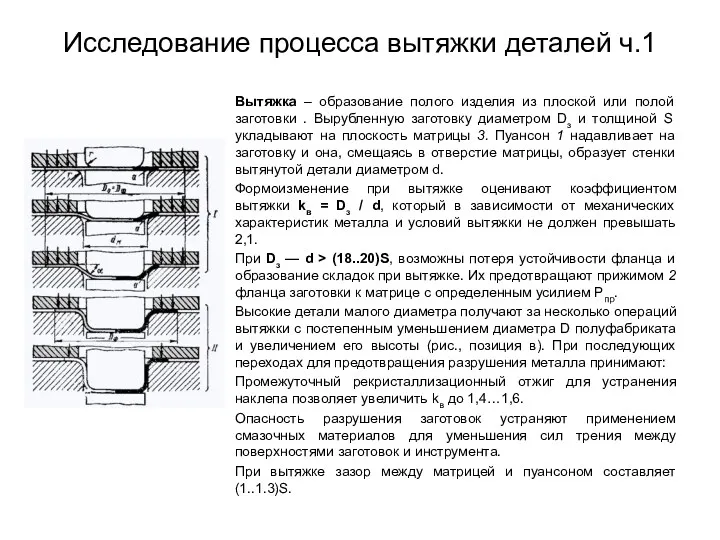 Исследование процесса вытяжки деталей ч.1
Исследование процесса вытяжки деталей ч.1 Магнитное поле
Магнитное поле Работа и мощность электрического тока
Работа и мощность электрического тока Своя игра по физике
Своя игра по физике Техническое обслуживание и ремонт сцепления автомобиля ВАЗ 2107
Техническое обслуживание и ремонт сцепления автомобиля ВАЗ 2107 Робототехника. Робот-дворник
Робототехника. Робот-дворник Коррозионные процессы
Коррозионные процессы