Содержание
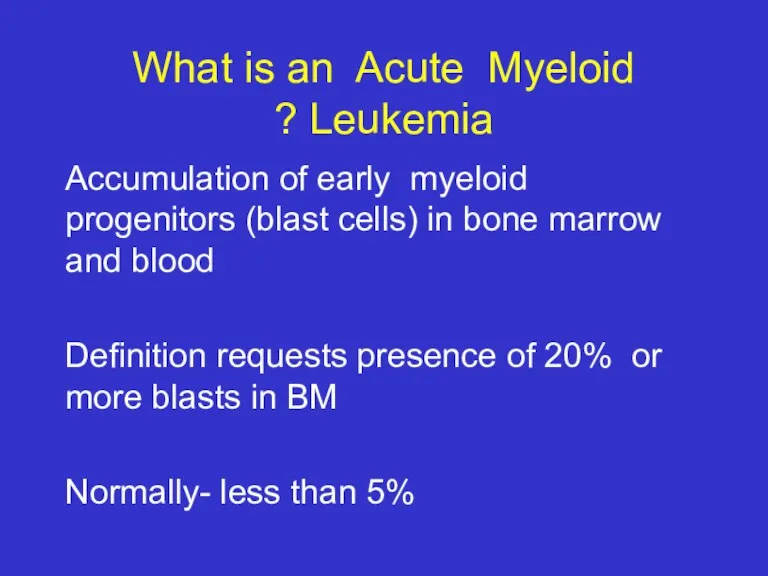
- 2. What is an Acute Myeloid Leukemia ? Accumulation of early myeloid progenitors (blast cells) in bone
- 3. ETIOLOGY Environment: irradiation, chemotherapeutic agents, organic solvents – benzene etc. Genetic diseases: neurofibromatosis, Wiscott-Aldrich synd., defective
- 5. AML Aggressive disease with an acute onset Can occur De Novo or following a known leukomogemic
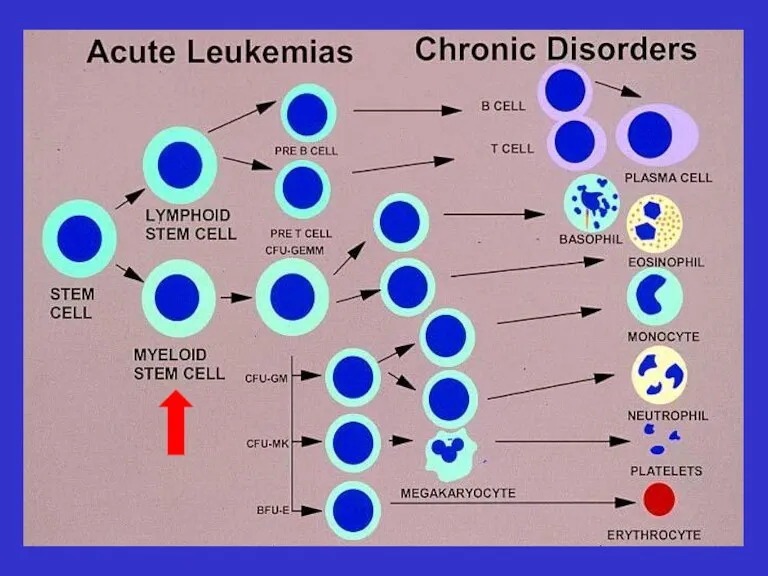
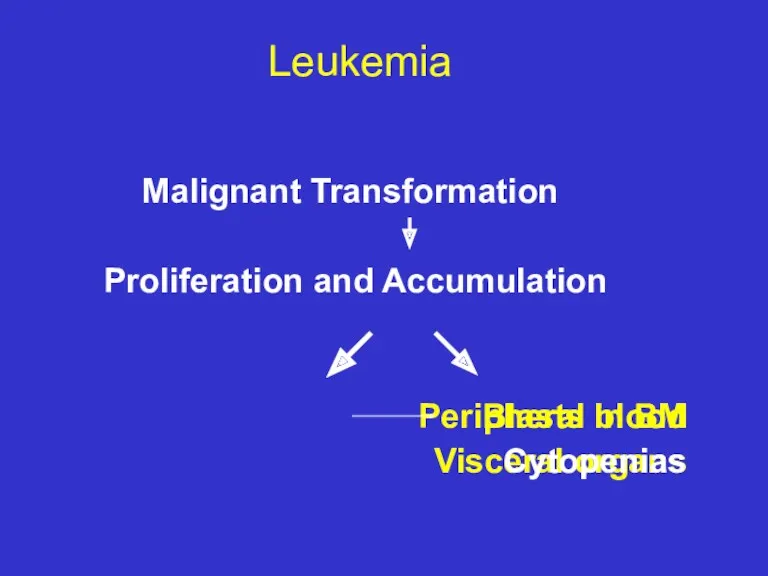
- 6. Leukemia Malignant Transformation Proliferation and Accumulation Peripheral blood Blasts in BM Visceral organs Cytopenias
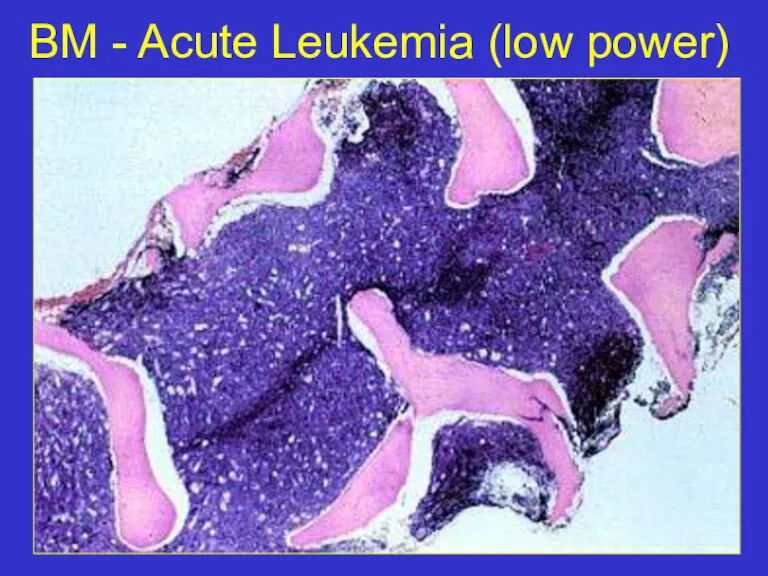
- 7. BM - Acute Leukemia (low power)
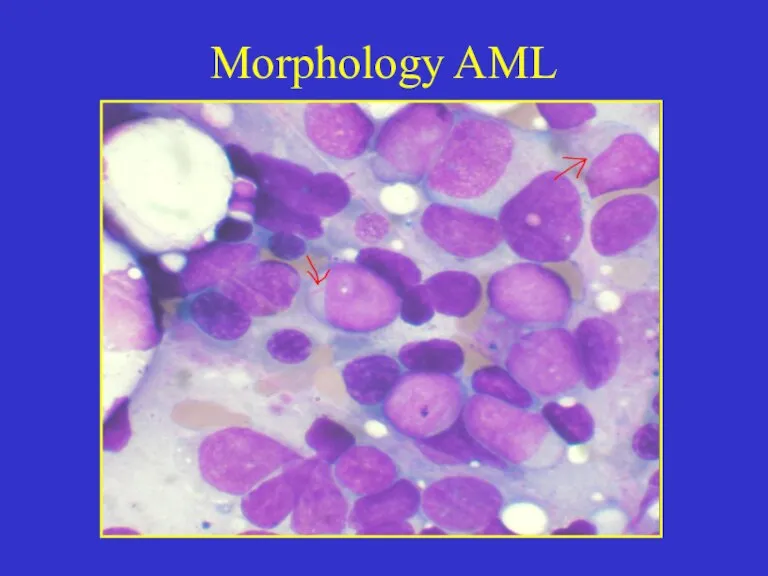
- 8. Morphology AML
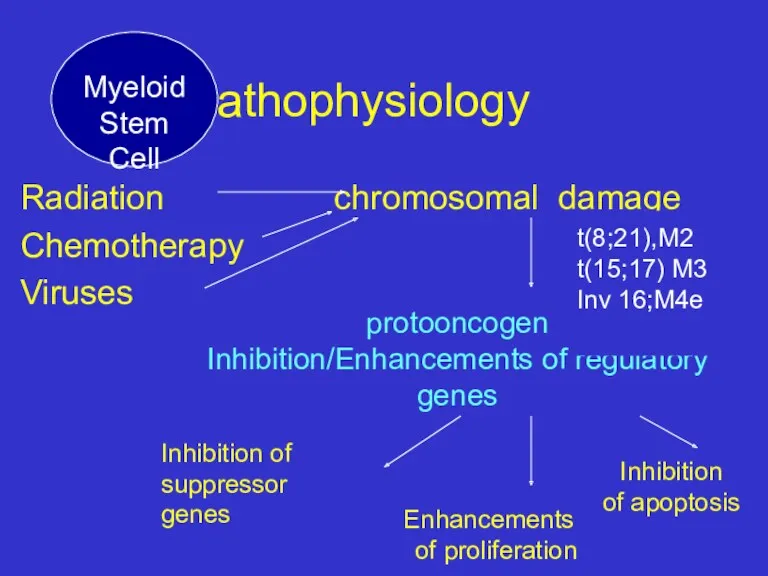
- 9. Pathophysiology Radiation chromosomal damage Chemotherapy Viruses protooncogen Inhibition/Enhancements of regulatory genes Inhibition of suppressor genes Enhancements
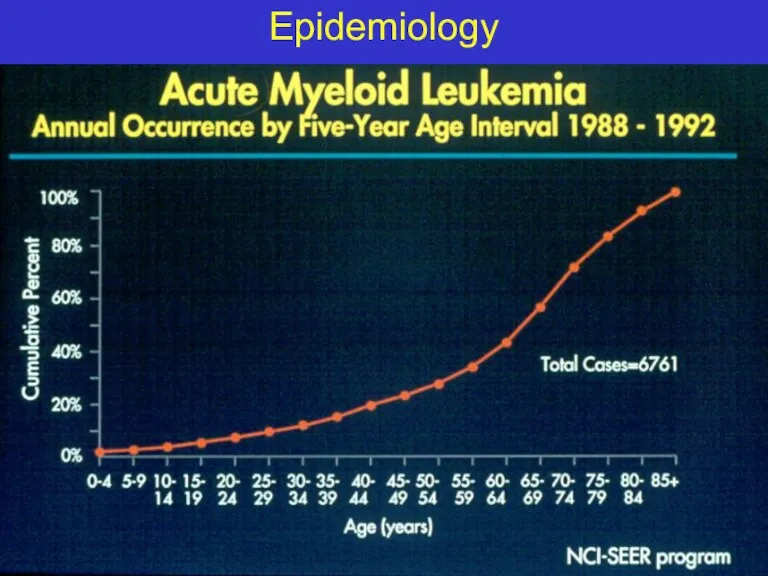
- 10. Epidemiology
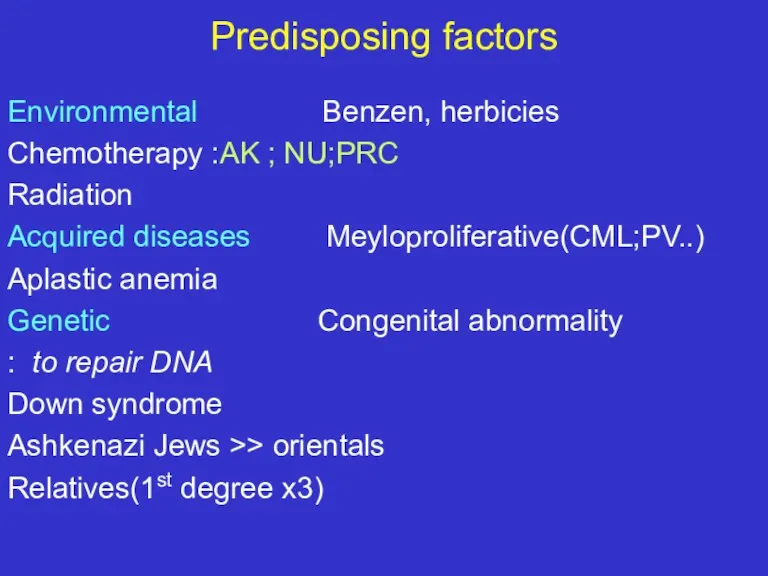
- 11. Predisposing factors Environmental Benzen, herbicies Chemotherapy :AK ; NU;PRC Radiation Acquired diseases Meyloproliferative(CML;PV..) Aplastic anemia Genetic
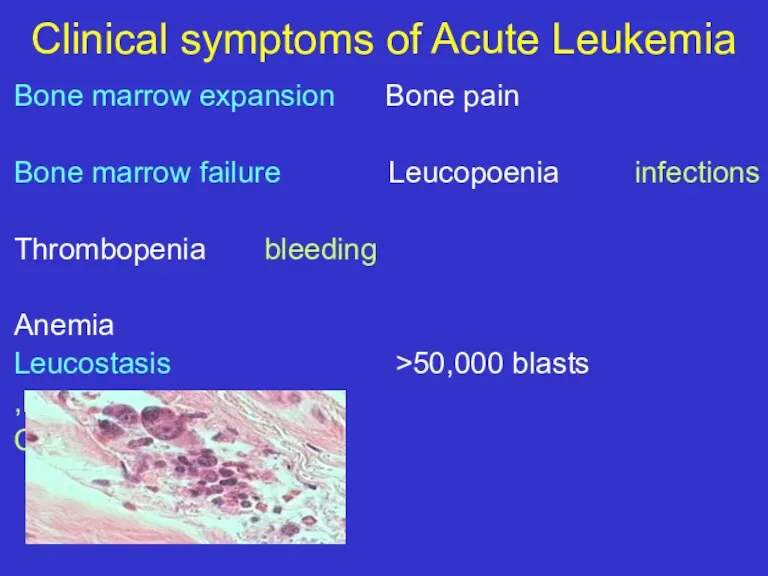
- 12. Clinical symptoms of Acute Leukemia Bone marrow expansion Bone pain Bone marrow failure Leucopoenia infections Thrombopenia
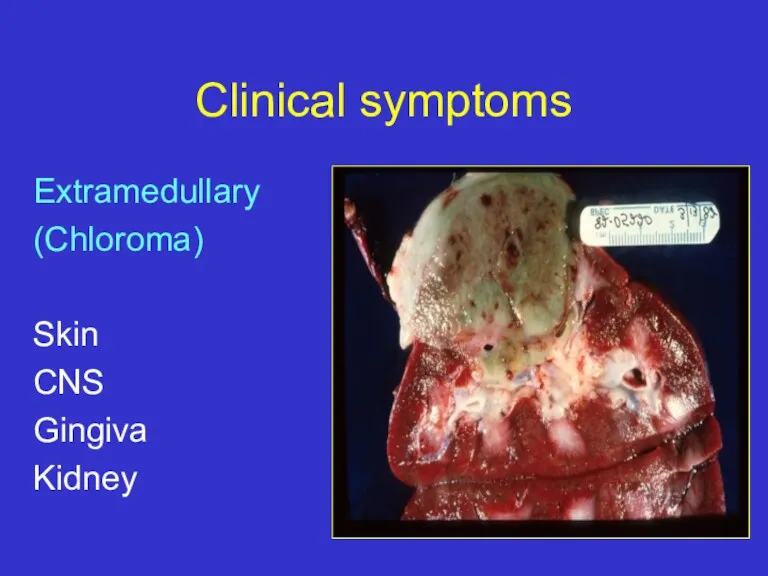
- 13. Clinical symptoms Extramedullary (Chloroma) Skin CNS Gingiva Kidney
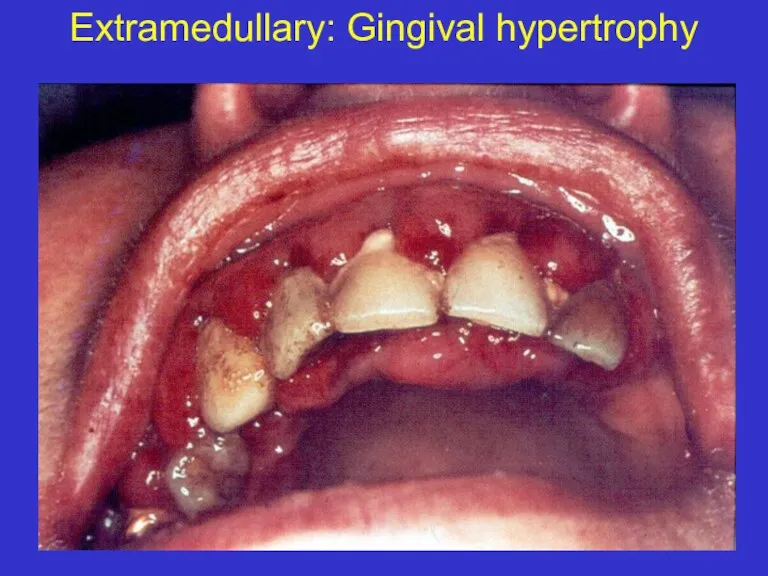
- 14. Extramedullary: Gingival hypertrophy
- 15. Clinical symptomes DIC Bleeding Thrombosis Metabolic Hyperuricemia Tumor lysis syndrome K, phosphor, Ca Uric Acid
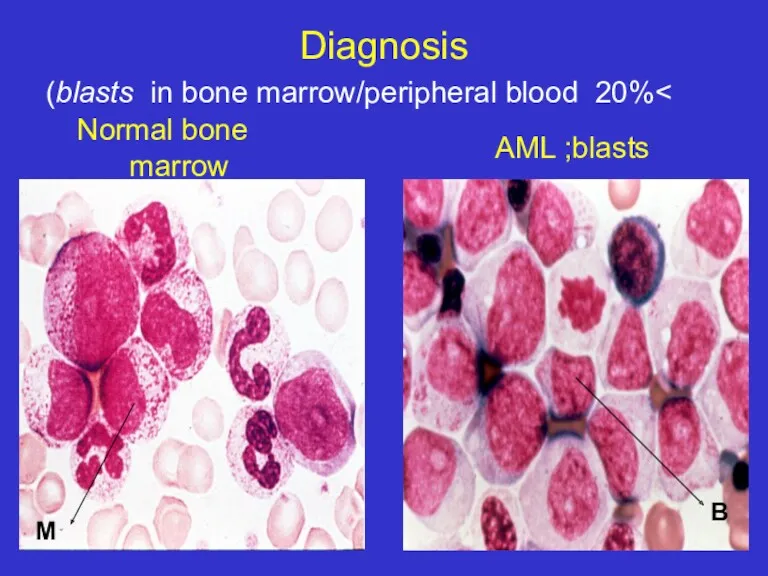
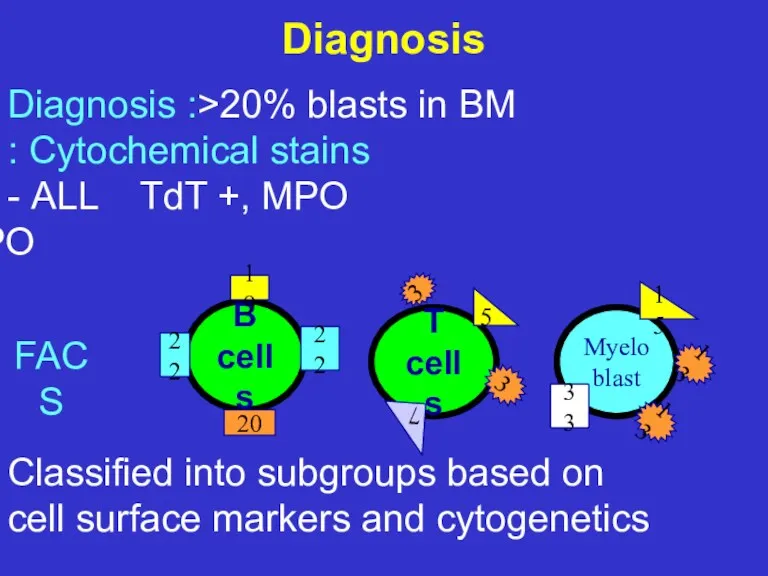
- 16. Diagnosis >20% blasts in bone marrow/peripheral blood) AML ;blasts B M Normal bone marrow
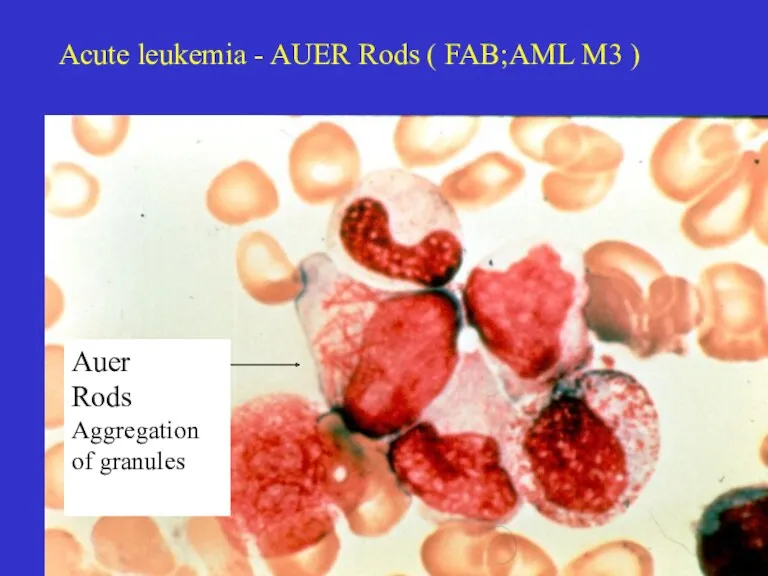
- 17. Acute leukemia - AUER Rods ( FAB;AML M3 ) Auer Rods Aggregation of granules
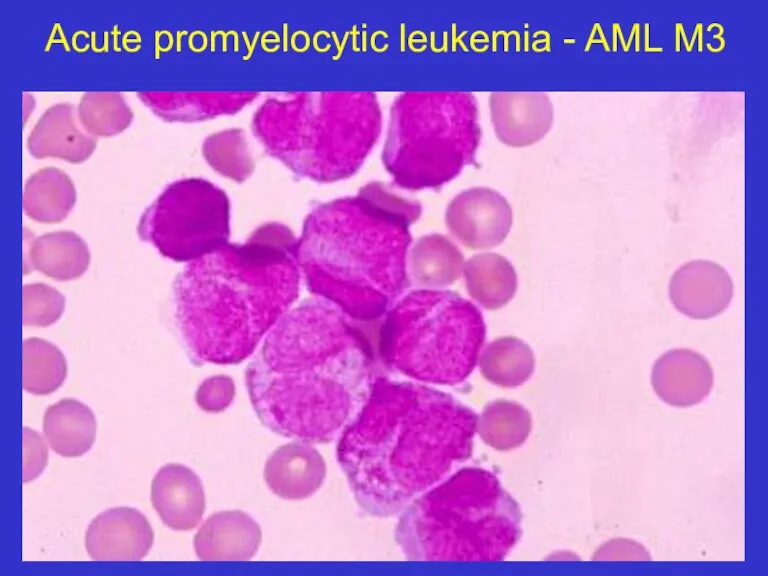
- 18. Acute promyelocytic leukemia - AML M3
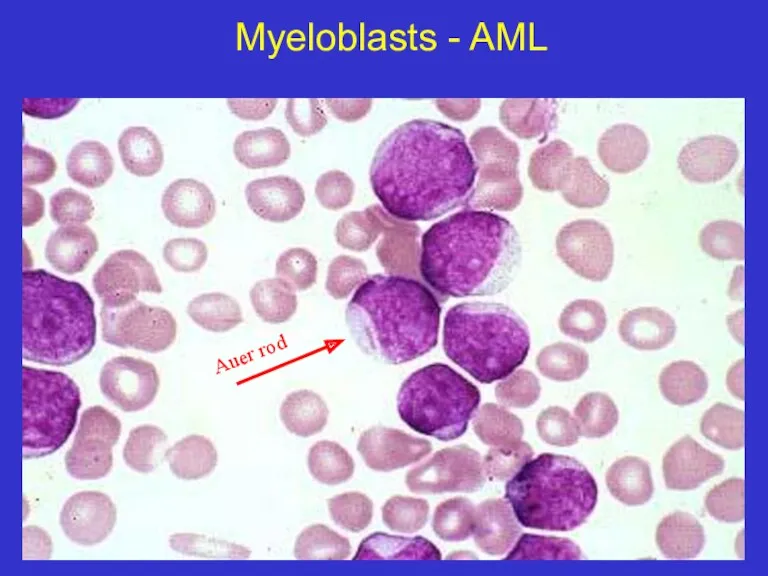
- 19. Myeloblasts - AML Auer rod
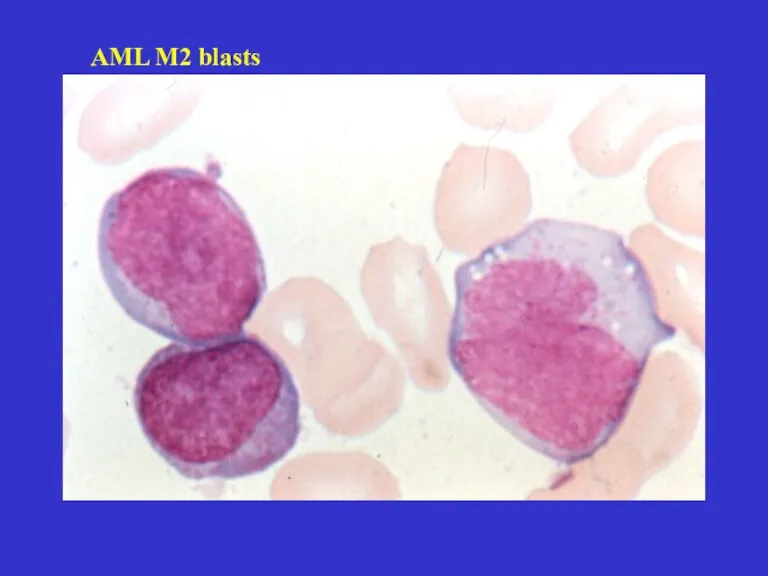
- 20. AML M2 blasts
- 21. French American British (FAB) classification -Based on morphology and staining (cytochemistry) -Divides patients into 7 AML
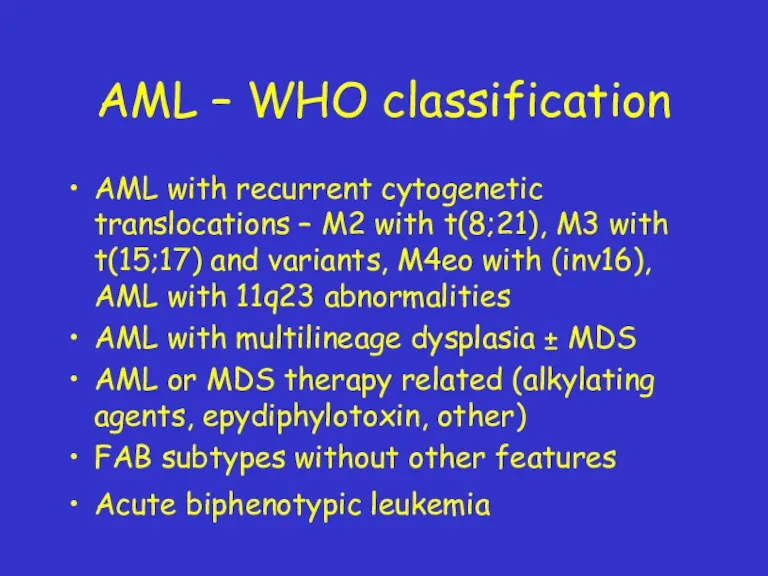
- 22. AML – WHO classification AML with recurrent cytogenetic translocations – M2 with t(8;21), M3 with t(15;17)
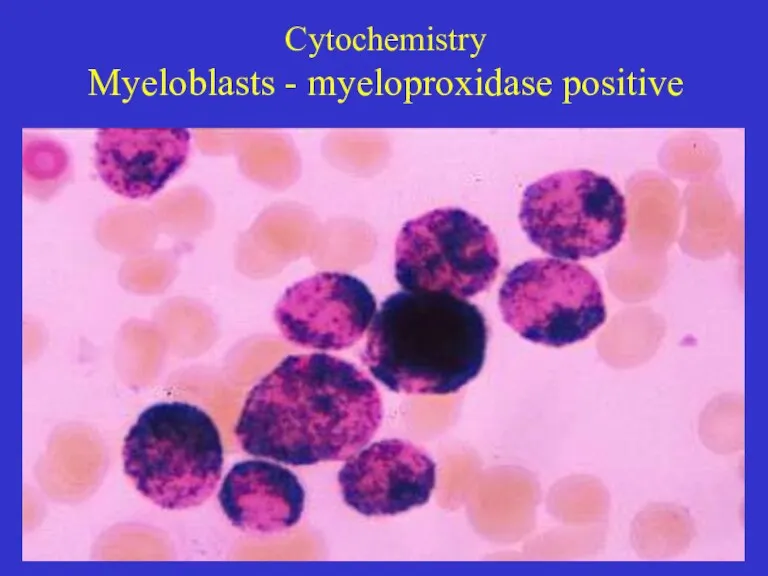
- 23. Cytochemistry Myeloblasts - myeloproxidase positive
- 24. Diagnosis Diagnosis :>20% blasts in BM Cytochemical stains : ALL TdT +, MPO - AML TdT
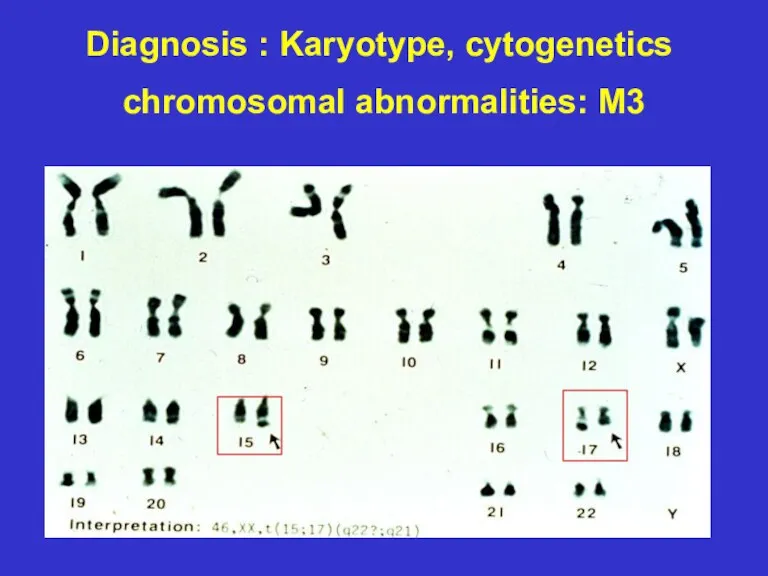
- 25. Diagnosis : Karyotype, cytogenetics chromosomal abnormalities: M3
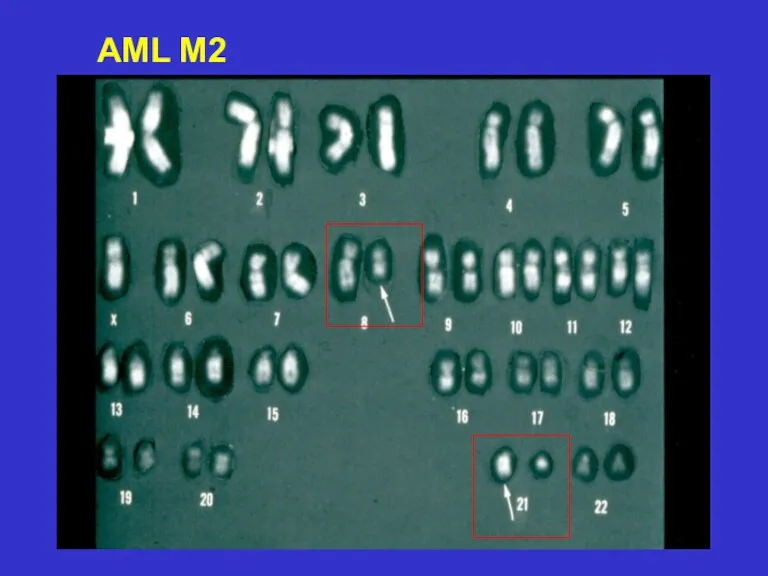
- 26. AML M2
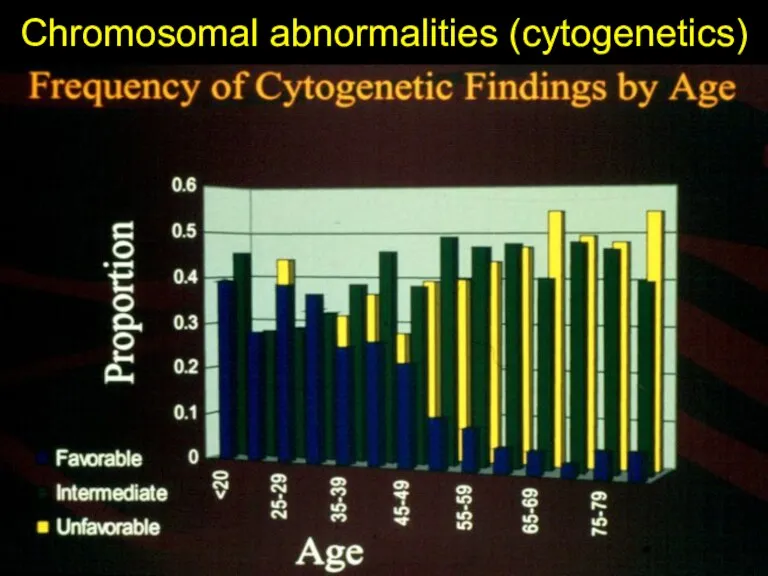
- 27. Chromosomal abnormalities (cytogenetics)
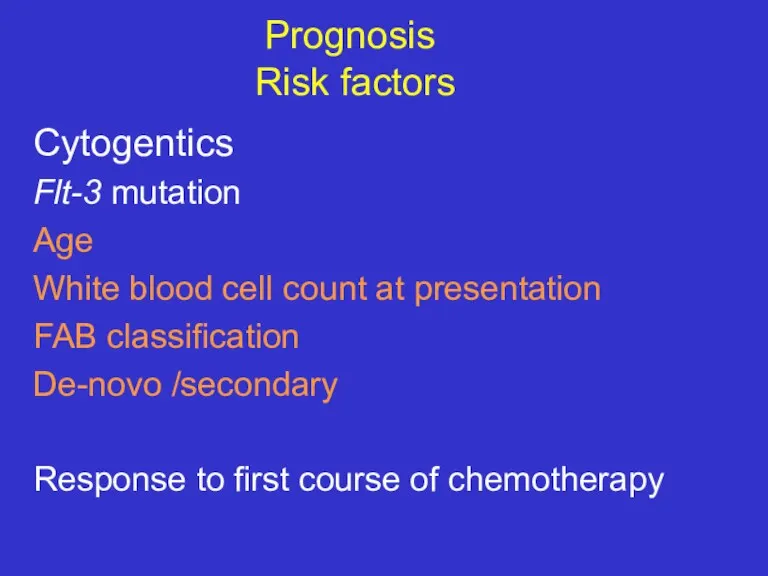
- 28. Prognosis Risk factors Cytogentics Flt-3 mutation Age White blood cell count at presentation FAB classification De-novo
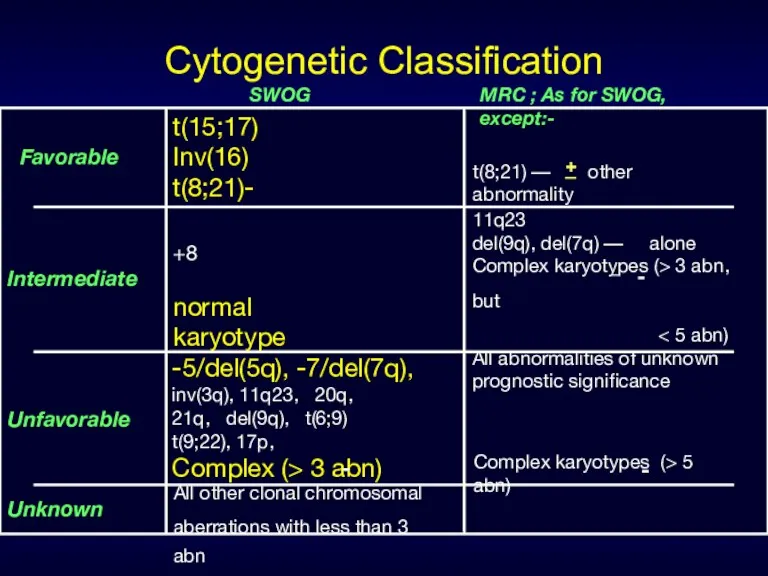
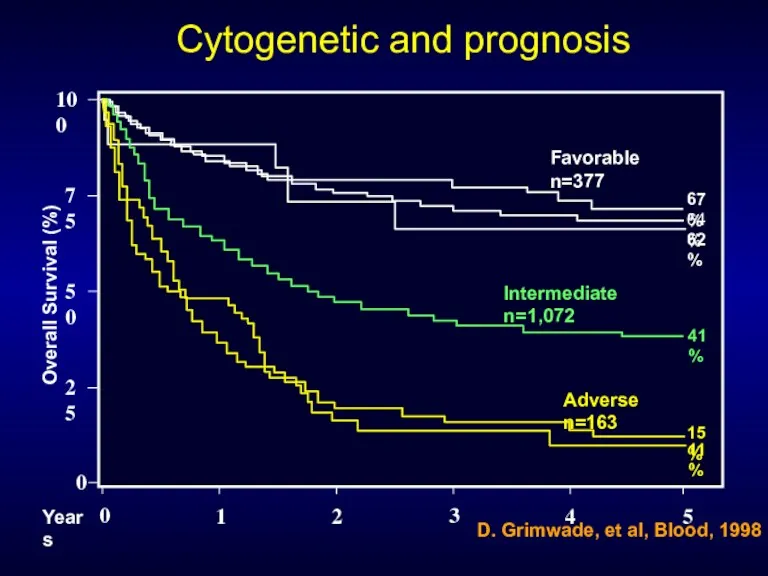
- 29. Cytogenetic Classification Favorable Intermediate SWOG Unfavorable Unknown MRC ; As for SWOG, except:- t(15;17) Inv(16) t(8;21)-
- 30. 0 50 25 75 100 0 1 Overall Survival (%) Years 2 3 4 5 67%
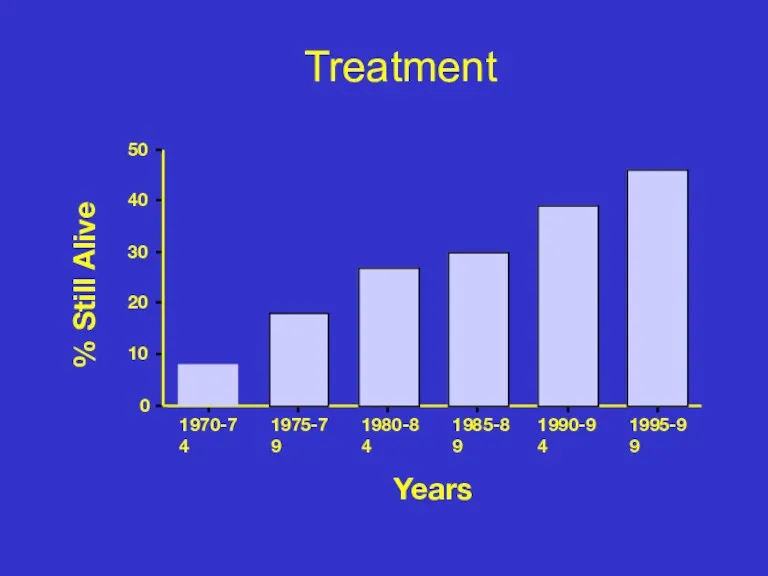
- 31. Treatment 0 10 20 30 40 50 1970-74 1975-79 1980-84 1985-89 1990-94 1995-99 % Still Alive
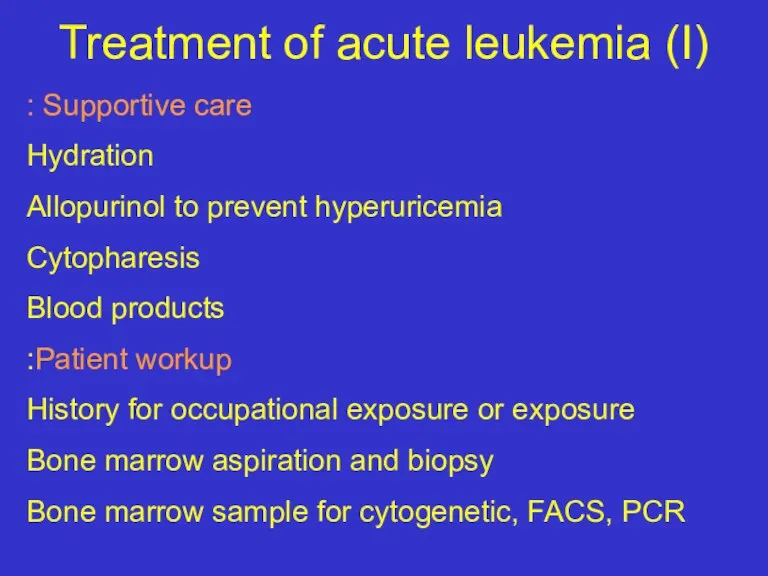
- 32. Treatment of acute leukemia (I) Supportive care : Hydration Allopurinol to prevent hyperuricemia Cytopharesis Blood products
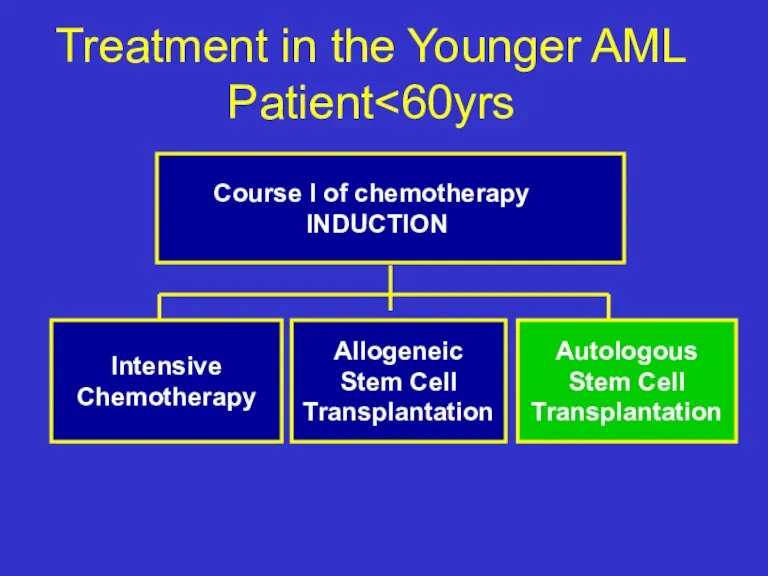
- 33. Treatment in the Younger AML Patient Course I of chemotherapy INDUCTION Intensive Chemotherapy Allogeneic Stem Cell
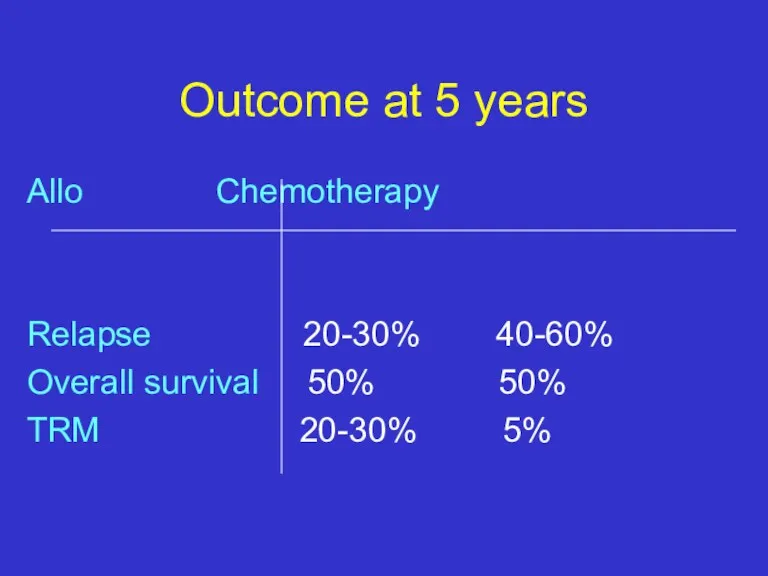
- 34. Outcome at 5 years Allo Chemotherapy Relapse 20-30% 40-60% Overall survival 50% 50% TRM 20-30% 5%
- 35. So how to choose which therapy to a specific patient? use the prognostic factors to estimate
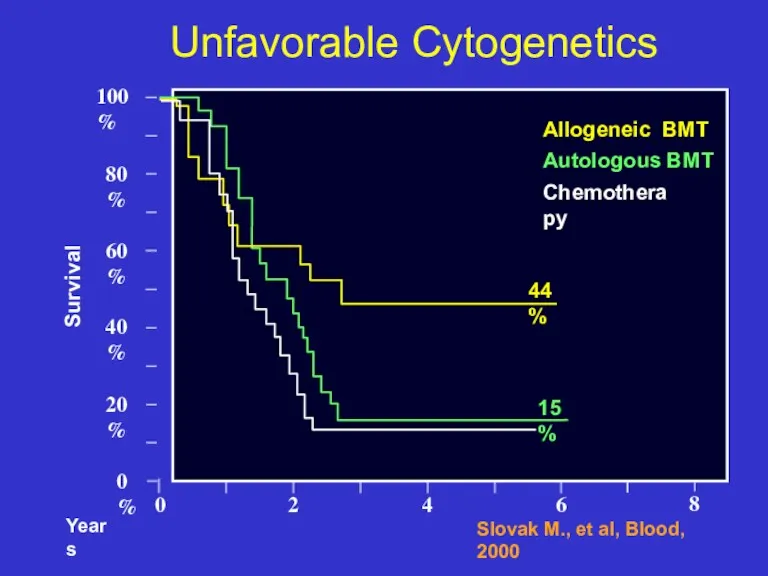
- 36. 0 0% 20% 40% Unfavorable Cytogenetics Survival 80% 60% 100% 2 4 6 Slovak M., et
- 37. What is the best treatment? Who should have a matched related Allo SCT ? Who should
- 38. AML in Elderly patients(>60 years) The majority of the patients are older than 60 Lower remission
- 39. Future directions Identify new prognostic factors New therapies : Modulation of drug resistance Biological, specific treatments:
- 40. Summary The majority of patients still die of their disease (significantly poor outcome in elderly patients)
- 42. Скачать презентацию








 Кровь. Состав крови
Кровь. Состав крови Сапонины. Строение сапонинов
Сапонины. Строение сапонинов Зондирование
Зондирование Тісжегімен және пародонттың қабыну ауруларының патогенетикалық даму механизмі
Тісжегімен және пародонттың қабыну ауруларының патогенетикалық даму механизмі Воспалительные заболевания придатков матки: диагностика и лечение
Воспалительные заболевания придатков матки: диагностика и лечение Коррекция кальций-фосфорного обмена у пациентов на гемодиализе
Коррекция кальций-фосфорного обмена у пациентов на гемодиализе Иммунологическое бесплодие
Иммунологическое бесплодие Diplomnaya_prezentatsia
Diplomnaya_prezentatsia Иммунодепрессивные болезни птиц
Иммунодепрессивные болезни птиц Лазеры в медицине. История изобретения
Лазеры в медицине. История изобретения Роль физической культуры и спорта в профилактике заболеваний и укреплении здоровья
Роль физической культуры и спорта в профилактике заболеваний и укреплении здоровья Эпилепсия. Эпилептическая реакция
Эпилепсия. Эпилептическая реакция Гис будасының тармақшалар блокадасы
Гис будасының тармақшалар блокадасы Анатомо-морфологические особенности формирующейся зубочелюстной системы у детей
Анатомо-морфологические особенности формирующейся зубочелюстной системы у детей Дихання. Анатомо-функціональні особливості дихальної системи
Дихання. Анатомо-функціональні особливості дихальної системи Дегенерация сетчатки глаза
Дегенерация сетчатки глаза Этиология, определение понятия. Причины, условия и факторы болезни. Определение понятий. Виды
Этиология, определение понятия. Причины, условия и факторы болезни. Определение понятий. Виды Лабораторная диагностика паразитарных болезней
Лабораторная диагностика паразитарных болезней Чума. Источники инфекции
Чума. Источники инфекции Естественное вскармливание ребенка первого года жизни
Естественное вскармливание ребенка первого года жизни Клиническая фармакология лекарственных средств, применяемых для лечения гастро-дуоденальной патологии
Клиническая фармакология лекарственных средств, применяемых для лечения гастро-дуоденальной патологии Рак гортани. Злокачественное новообразование гортани
Рак гортани. Злокачественное новообразование гортани Транспортировка пострадавших. Транспортные положения
Транспортировка пострадавших. Транспортные положения Абдоминальное кесарево сечение
Абдоминальное кесарево сечение Здоровый образ жизни
Здоровый образ жизни Туберкулезге қарсы іс шаралар
Туберкулезге қарсы іс шаралар Грипп и его профилактика
Грипп и его профилактика Эколого-географические особенности и этиологическая структура лептоспироза животных в Среднем Приобье
Эколого-географические особенности и этиологическая структура лептоспироза животных в Среднем Приобье