Содержание
- 2. Agenda Pharmacovigilance Definitions Reporting details Local literature surveillance
- 3. Pharmacovigilance Pharmacovigilance Definitions Reporting details Local literature surveillance
- 4. Why is Pharmacovigilance important? To identify: Risks and benefits of medicines to improve their safe use
- 5. Why is Pharmacovigilance important? CSL has a regulatory requirement to provide an appropriate system of Pharmacovigilance
- 6. Definitions Pharmacovigilance Definitions Reporting details Local literature surveillance
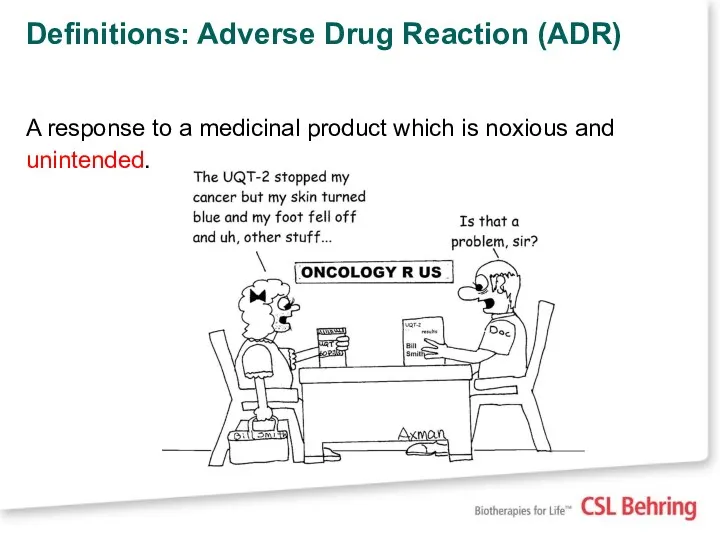
- 7. Definitions: Adverse Drug Reaction (ADR) A response to a medicinal product which is noxious and unintended.
- 8. Definitions- Adverse Event or Adverse Experience (AE) Is any untoward medical occurrence in a patient or
- 9. Definitions – Adverse Event Report In addition to Adverse events, the following events should be reported
- 10. Additional guidance on medication errors Good practice guide on recording, coding, reporting and assessment of Medication
- 11. Potential and Intercepted Medication Errors Potential medication error: Already a circumstance that may lead to a
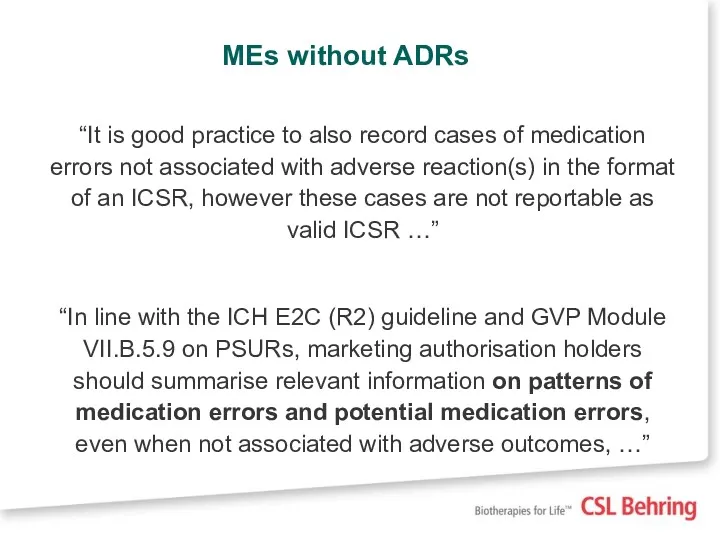
- 12. MEs without ADRs “It is good practice to also record cases of medication errors not associated
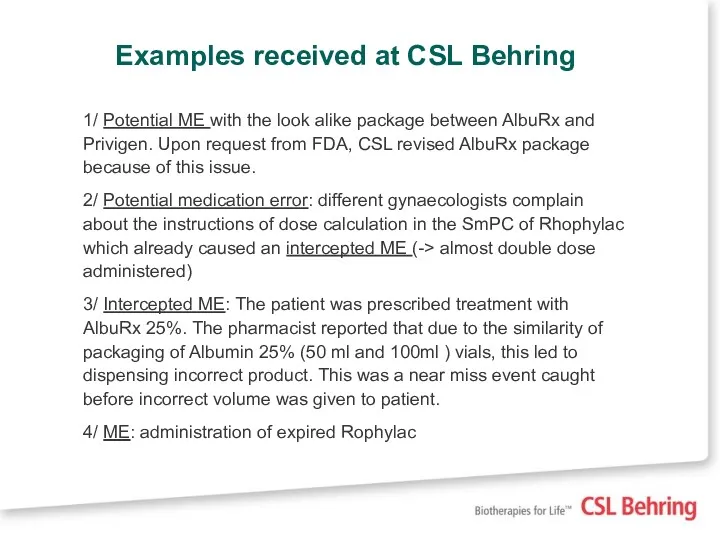
- 13. Examples received at CSL Behring 1/ Potential ME with the look alike package between AlbuRx and
- 14. EXAMPLES
- 15. Need to be reported? Midwife administered a dose of Rhophylac that had passed the expiry date
- 16. Need to be reported? Pregnant women receives Privigen Exposure during pregnancy
- 17. Need to be reported? The patient had a poor response to the drug after 2 days
- 18. Need to be reported? Treatment with Kybernin of preeclampsia in pregnant woman Exposure during pregnancy +
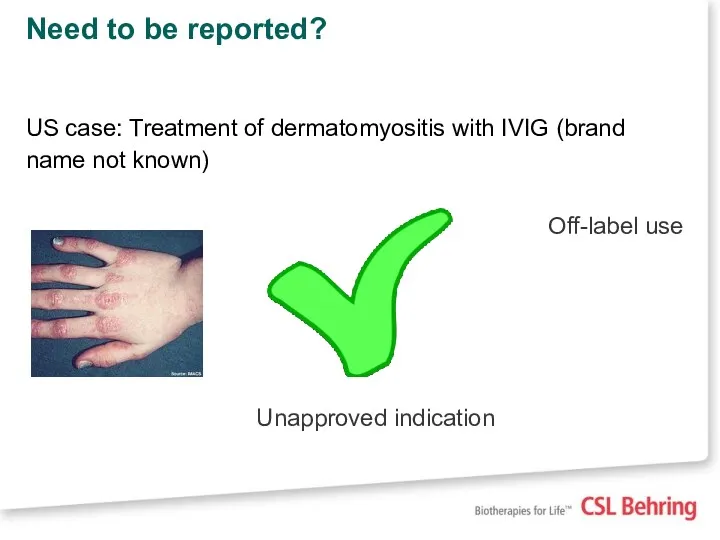
- 19. Need to be reported? US case: Treatment of dermatomyositis with IVIG (brand name not known) Off-label
- 20. Need to be reported? Breastfeeding baby was exposed to Privigen Drug exposure by mother
- 21. Need to be reported? Haemate was transferred into the syringe and kept for 8 hours until
- 22. Adverse Reactions – Some examples Non-serious – e.g. rash, headache Serious – death, hospitalisation, virus transmission,
- 23. Definitions Unexpected / unlisted ADR not defined in the Reference Safety Information (for licensed products) or
- 24. Individual Case Safety Report (ICSR) This term includes solicited AE reports (sought by CSL, e.g. clinical
- 25. Spontaneous ICSR An unsolicited communication by a healthcare professional or consumer to a company, regulatory authority
- 26. Minimum Criteria for a valid ICSR To qualify as an ICSR there must be a minimum
- 27. ICSR reporting A report that does not contain the four minimum criteria is referred to as
- 28. Reporting details Pharmacovigilance Definitions Reporting details Local literature surveillance Reconciliation process Product Technical Complaints Setting a
- 29. Pharmacovigilance – LSO/RSO Contacts Regional Safety Officer (RSO) ECI: Marta D. Puente Navazo (marta.puente@cslbehring.com) Local Safety
- 30. An Adverse Drug Reaction (ADR) – What Should I Do? Post and faxes should be scanned
- 31. Adverse Reactions – What Should I Do? If phone calls cannot be forwarded to RSO obtain
- 32. Important to know: Every information on a potential AE must be forwarded to the RSO within
- 33. Adverse Reactions – Reporting Timelines All adverse reactions (ADR) must be reported to RSO within one
- 34. Product Exposure during Pregnancy Pregnancy reports should be monitored until the pregnancy outcome is known Attempts
- 35. An example
- 36. If you suspect an ADR…
- 37. Conference: Side effects, which are described in the Patient information leaflet have to be reported Patterns
- 38. “European Journal of Neurology” Attending a conference you come across an abstract reporting an ADR or
- 39. Local Literature surveillance Pharmacovigilance Definitions Reporting details Local literature surveillance Reconciliation process Product Technical Complaints Setting
- 40. Why local literature surveillance? Information on safety relevant observations in local journals may not be missed
- 41. Local literature surveillance 3. If you find an abstract mentioning a key word, read the full
- 42. Local literature surveillance Example Rhophylac: Global literature search uses following key words: rhesus d antibody ●
- 44. Скачать презентацию





























 Медициналық сұхбаттасу техникасы
Медициналық сұхбаттасу техникасы Возбудители зоонозных инфекций. Лекция 16
Возбудители зоонозных инфекций. Лекция 16 Мейіргердің функциялық міндеттері
Мейіргердің функциялық міндеттері Treatment of pulmonary tbpatients. (Lecture 3)
Treatment of pulmonary tbpatients. (Lecture 3) Неврологический осмотр больного в коматозном состоянии
Неврологический осмотр больного в коматозном состоянии Синдром бронхиальной обструкции. Семиотика и методы диагностики бронхиальной астмы, хронического обструктивного бронхита
Синдром бронхиальной обструкции. Семиотика и методы диагностики бронхиальной астмы, хронического обструктивного бронхита Відмороження та його періоди. Класифікація відмороження. Домедична допомога. 11 клас
Відмороження та його періоди. Класифікація відмороження. Домедична допомога. 11 клас Past medical and family history
Past medical and family history Гигиена детских и образовательных учреждений
Гигиена детских и образовательных учреждений Медицинское страхование и медицинское обслуживание АО СК Sinoasia B&R (Синоазия БиЭндАр)
Медицинское страхование и медицинское обслуживание АО СК Sinoasia B&R (Синоазия БиЭндАр) Дисциркуляторная энцефалопатия. Болезнь Альцгеймера
Дисциркуляторная энцефалопатия. Болезнь Альцгеймера Питание детей от 1 года до 3-х лет
Питание детей от 1 года до 3-х лет Интубация трахеи. Виды, техника, осложнения. Алгоритм трудной интубации
Интубация трахеи. Виды, техника, осложнения. Алгоритм трудной интубации Пре-и постнатальный онтогенез. Развитие сердца
Пре-и постнатальный онтогенез. Развитие сердца Корь, её симптомы и профилактика
Корь, её симптомы и профилактика Геморрагиялық шок
Геморрагиялық шок Иммунология на службе здоровья человека
Иммунология на службе здоровья человека Действие ионизирующих излучений на критические системы организма
Действие ионизирующих излучений на критические системы организма Заболевания щитовидной железы. Гипотиреоз
Заболевания щитовидной железы. Гипотиреоз Тар жамбас
Тар жамбас Возрастные особенности ротовой полости и формирования зубов. Нарушения. Гигиена
Возрастные особенности ротовой полости и формирования зубов. Нарушения. Гигиена Ангина, лечение
Ангина, лечение Etude générale de la maladie. Hérédité, réactivité et résistence en pathologie. (Lection 1)
Etude générale de la maladie. Hérédité, réactivité et résistence en pathologie. (Lection 1) Артериальная гипертензия и беременность
Артериальная гипертензия и беременность Патофизиология желудочно-кишечного тракта. Процессы, протекающие в кишечнике
Патофизиология желудочно-кишечного тракта. Процессы, протекающие в кишечнике Гострі кишкові інфекції у дітей (1-ша частина)
Гострі кишкові інфекції у дітей (1-ша частина) Синдром желудочной диспепсии. Гастроэзофагеальная рефлюксная болезнь. Хронические гастриты
Синдром желудочной диспепсии. Гастроэзофагеальная рефлюксная болезнь. Хронические гастриты Мезенхималық майлы дистрофиялар
Мезенхималық майлы дистрофиялар