Содержание
- 2. WHO’s Requirements for Drugs: Task of Pharmacotherapy Effectiveness Safety Availability for Patients Reducing Mortality Improving the
- 3. STATISTICS Mortality from Side Effects (SEs) of Drugs (excluding Medical Errors and Misusages) takes the 5
- 4. CAUSES of FATAL COMPLICATIONs Gastrointestinal Bleedings: NSAIDs, Anticoagulants, Glucocorticoids, et al. Bleedings from other organs: Cytostatics,
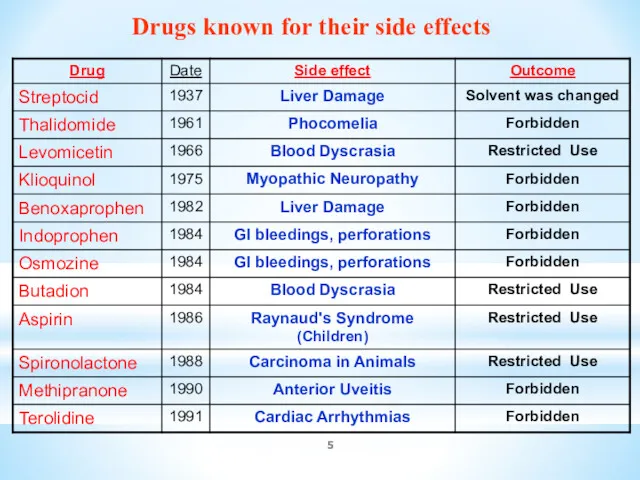
- 5. Drugs known for their side effects
- 6. Pharmacovigilance (from pharmakon - Greek for drug and vigilare - Latin for to keep watch), also
- 7. Legislative Bases of Pharmacovigilance System Functioning in Ukraine 1996 – Subdivision of the Pharmacological Committee of
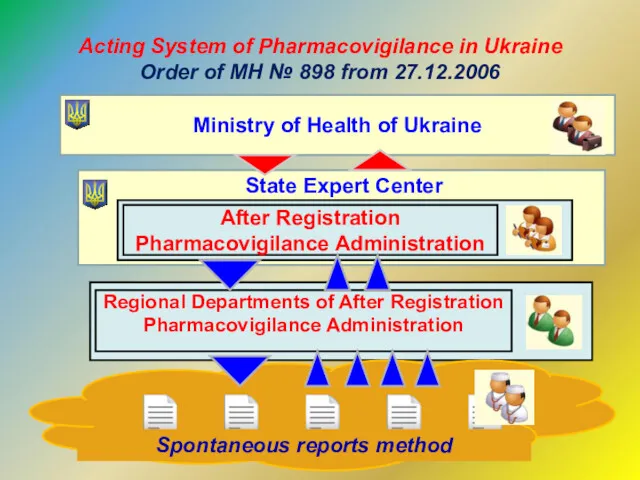
- 8. Acting System of Pharmacovigilance in Ukraine Order of MH № 898 from 27.12.2006 Ministry of Health
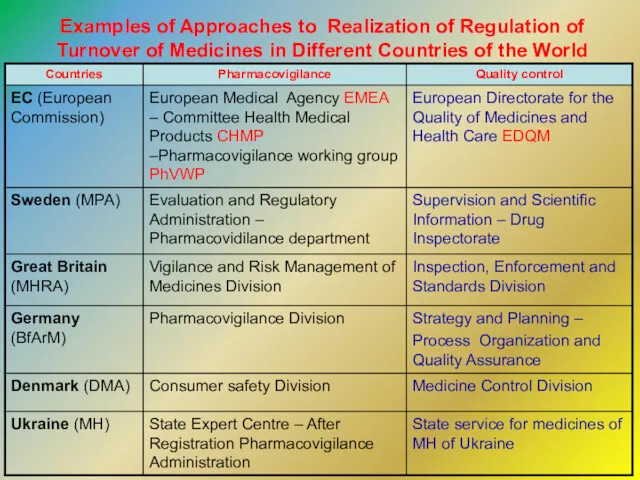
- 9. Examples of Approaches to Realization of Regulation of Turnover of Medicines in Different Countries of the
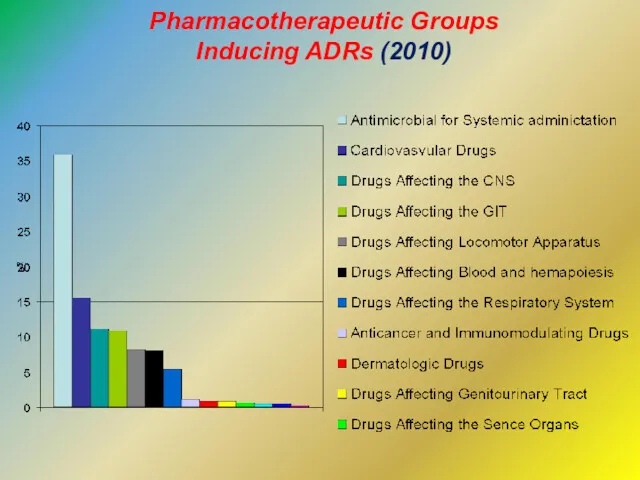
- 10. Pharmacotherapeutic Groups Inducing ADRs (2010)
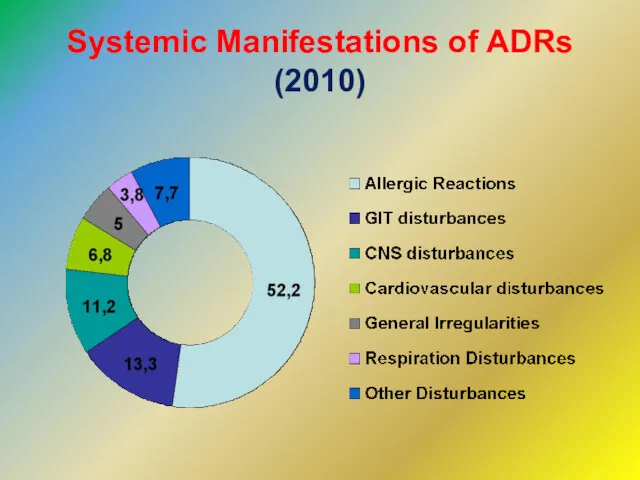
- 11. Systemic Manifestations of ADRs (2010)
- 12. Adverse Drug Reactions (ADRs) are defined as any response to a drug which is noxious and
- 13. ADVERSE DRUGS REACTIONS include: 1. Side Effects - are produced with therapeutical dose of the drug
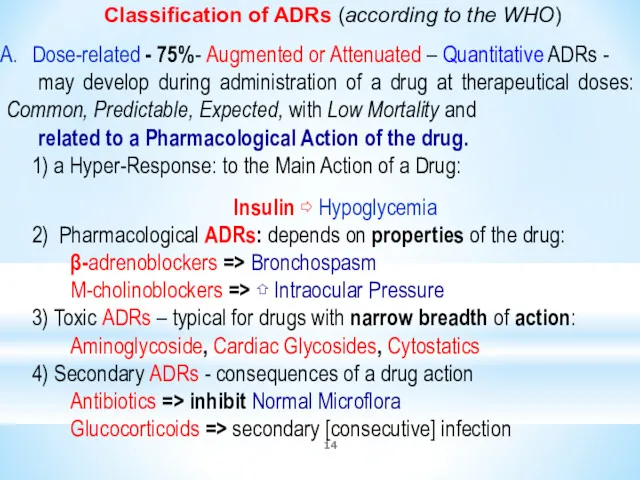
- 14. Classification of ADRs (according to the WHO) Dose-related - 75%- Augmented or Attenuated – Quantitative ADRs
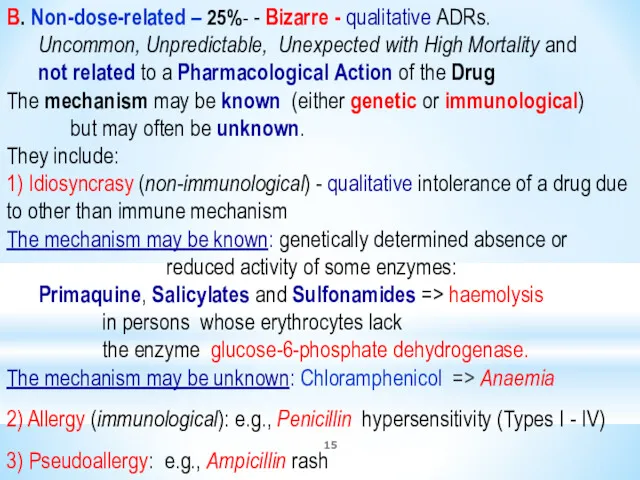
- 15. B. Non-dose-related – 25%- - Bizarre - qualitative ADRs. Uncommon, Unpredictable, Unexpected with High Mortality and
- 16. Classification of ADRs (according to the WHO) C. Dose-related and time-related - Chronic : Uncommon, Related
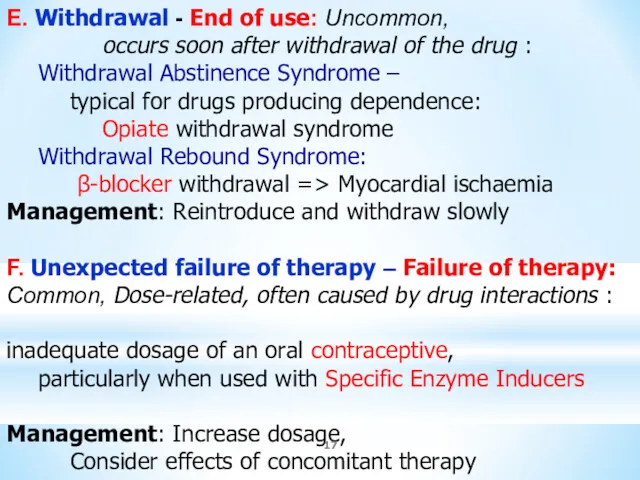
- 17. E. Withdrawal - End of use: Uncommon, occurs soon after withdrawal of the drug : Withdrawal
- 18. Complications of Drug Therapy 1. Disturbances of Functions of Organs and Systems: Neurotoxic, Hepatotoxic, Nephrotoxic, Hematotoxic,
- 19. Types of Hypersensitivity Reactions: A. Humoral type: Type I - Anaphylactic reactions – Immediate IgE mediated:
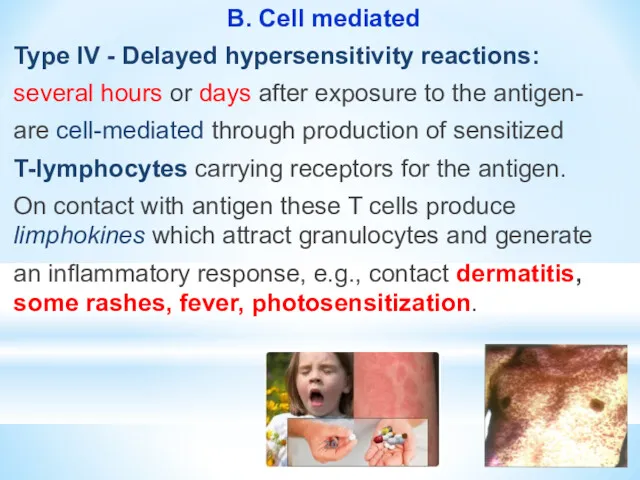
- 20. B. Cell mediated Type IV - Delayed hypersensitivity reactions: several hours or days after exposure to
- 21. Causality assessment of suspected ADRs 1. Certain ADRs - a clinical event, including a laboratory test
- 22. 3. Possible ADRs – a clinical event, including a laboratory test abnormality, that occurs in a
- 23. 5. Conditional / Unclassified - a clinical event, including a laboratory test abnormality, reported as an
- 24. Seriousness Determination An adverse event is considered serious if it meets one or more of the
- 25. Testosterone propionate => Seborrhea with akne-like skin rash after cancellation of the drug without consequences. Moderate
- 26. Counterfeit Medicines The WHO estimates that 10% of the global market is counterfeit and gives the
- 27. The number of confiscated fake medicines at European customs has skyrocketed, according to the current customs
- 28. Counterfeited Drugs Unmasked in Ukraine in 2006 – 2010 years: Cephasoline-KMP , for injections - ВАТ
- 29. The State Quality Control Inspection of Medicinal Agents of MPH of Ukraine withdrew from circulation (marketing
- 30. Criminal Liability for falsification of medicines: Producing, Buying, Transporting, Sending, Storing, Possession with intent to sell
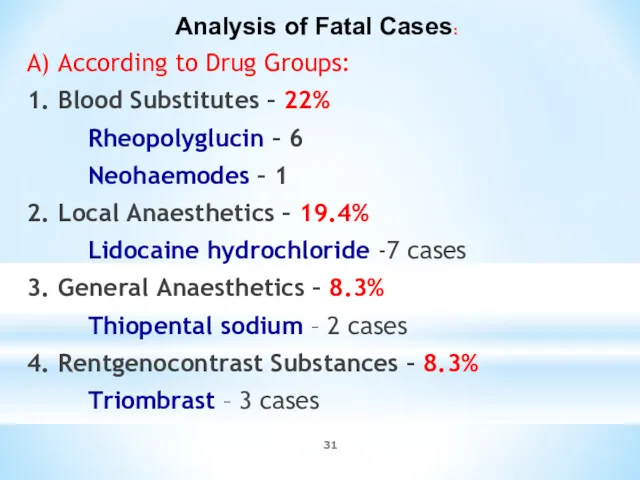
- 31. Analysis of Fatal Cases: A) According to Drug Groups: 1. Blood Substitutes – 22% Rheopolyglucin –
- 32. B) By manufacturers: I. Domestic: 1. “Health” - 6 cases (Lidocaine) 2. “KЖР” – 4 cases
- 33. C) By the cause of death: I - Anaphylactic Shock – 77.8% (28 cases: 8 –
- 34. The main method of gathering of information about SE of drugs in Ukraine is the Method
- 35. Some Drugs withdrawn from the pharmaceutical market: Rofecoxib - was approved by the FDA in 1999
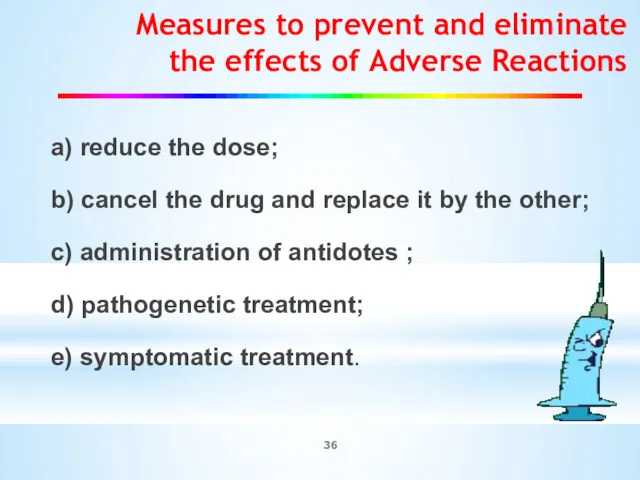
- 36. Measures to prevent and eliminate the effects of Adverse Reactions а) reduce the dose; b) cancel
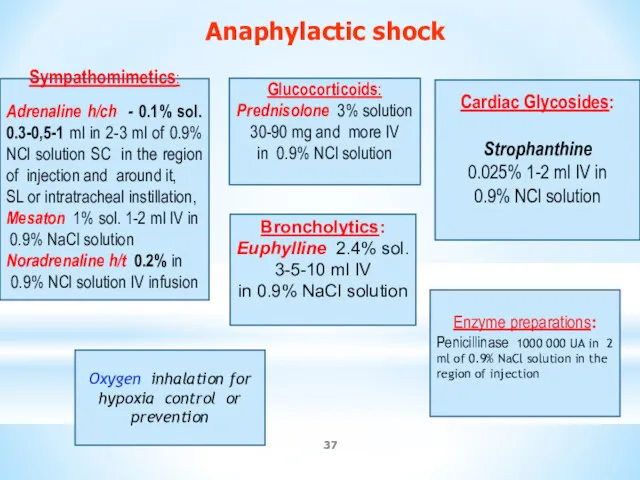
- 37. Sympathomimetics: Adrenaline h/ch - 0.1% sol. 0.3-0,5-1 ml in 2-3 ml of 0.9% NCl solution SC
- 38. The International Code of Medical Ethics declares that, " A physician shall act in the patient's
- 40. Скачать презентацию






















 Общение в сестринском деле
Общение в сестринском деле Гигиена питания
Гигиена питания Алгоритм диагностики и оказания скорой помощи при шоковых состояниях
Алгоритм диагностики и оказания скорой помощи при шоковых состояниях Диабетическая офтальмопатия
Диабетическая офтальмопатия Кишечные инфекции: ОКИ, микотические и паразитарные поражения кишечника
Кишечные инфекции: ОКИ, микотические и паразитарные поражения кишечника Травма живота у детей. Тактика лечения. Травматический разрыв печени
Травма живота у детей. Тактика лечения. Травматический разрыв печени Организация и структура системы первичной медико-санитарной помощи
Организация и структура системы первичной медико-санитарной помощи Участие медицинской сестры в лабораторных исследованиях пациента
Участие медицинской сестры в лабораторных исследованиях пациента Аллергия. Иммунопатологические реакции
Аллергия. Иммунопатологические реакции Снятие швов с послеоперационной раны
Снятие швов с послеоперационной раны Ошибки диагностики и лечения при сочетанной травме
Ошибки диагностики и лечения при сочетанной травме Ароматерапия в жизни человека
Ароматерапия в жизни человека Лучевая болезнь
Лучевая болезнь Пародонтоз. Клиникасы, диагностикасы, дифференциальды диагностикасы, пародонтоздың патологиялық анатомиясы
Пародонтоз. Клиникасы, диагностикасы, дифференциальды диагностикасы, пародонтоздың патологиялық анатомиясы Сучасні проблеми молекулярної біології. Генна терапія. (Лекція 8)
Сучасні проблеми молекулярної біології. Генна терапія. (Лекція 8) Гипоксически-ишемические поражения головного мозга у новорожденных
Гипоксически-ишемические поражения головного мозга у новорожденных Особенности анестезиологии и интенсивной терапии в педиатрической практике
Особенности анестезиологии и интенсивной терапии в педиатрической практике Фортранс. Рекомендации по приёму препарата фортранс
Фортранс. Рекомендации по приёму препарата фортранс Ультразвук для терапии боли
Ультразвук для терапии боли Клинический случай
Клинический случай Логоритмика как средство коррекции речевых и неречевых нарушений
Логоритмика как средство коррекции речевых и неречевых нарушений Клинические проявления бронхиальной астмы
Клинические проявления бронхиальной астмы Ожирение как глобальная проблема человечества
Ожирение как глобальная проблема человечества Зубы и уход за ними
Зубы и уход за ними Дыхательная гимнастика для детей дошкольного возраста
Дыхательная гимнастика для детей дошкольного возраста Влияние радиации на здоровье человека
Влияние радиации на здоровье человека Современные подходы в лечении сахарного диабета 2 типа
Современные подходы в лечении сахарного диабета 2 типа Влияние компьютера на здоровье человека
Влияние компьютера на здоровье человека