Содержание
- 2. Buffer solutions solution which can resist the addition of a strong acid or a strong base
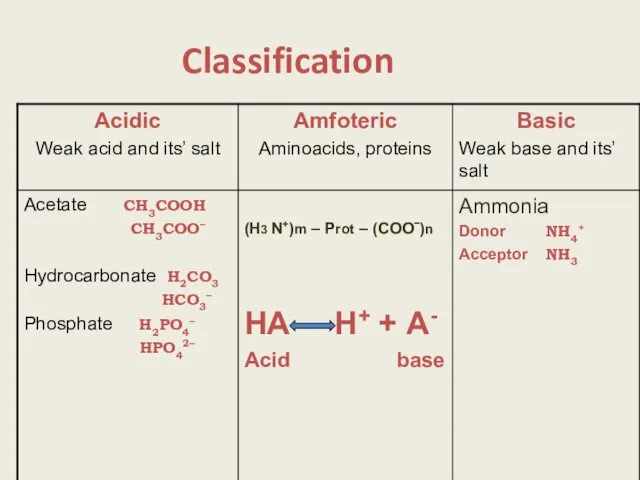
- 3. Classification
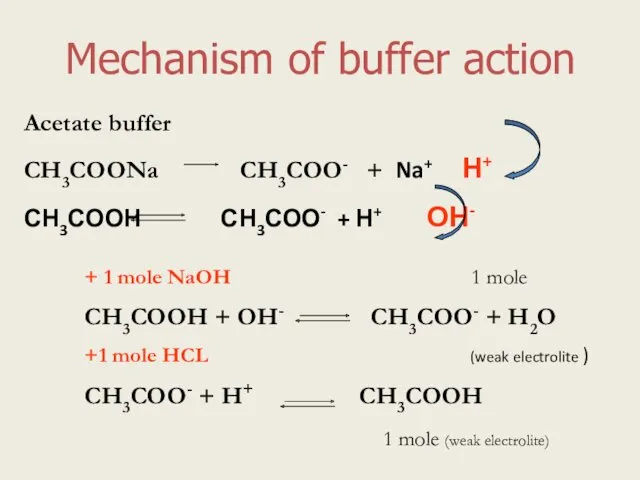
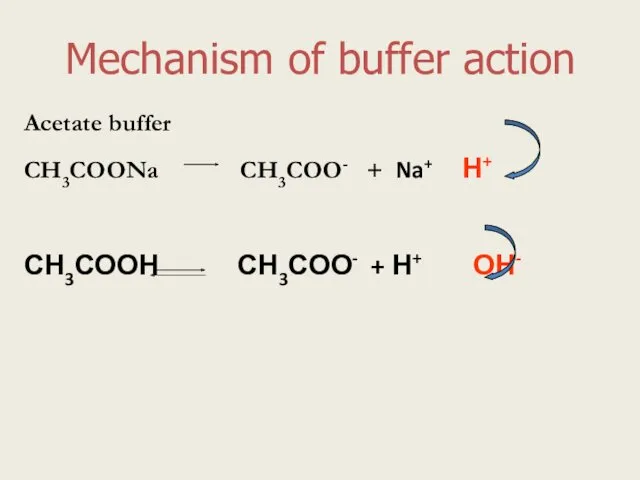
- 4. Mechanism of buffer action Acetate buffer СН3СООNa СН3СОО- + Na+ Н+ СН3СООН СН3СОО- + Н+ ОН-
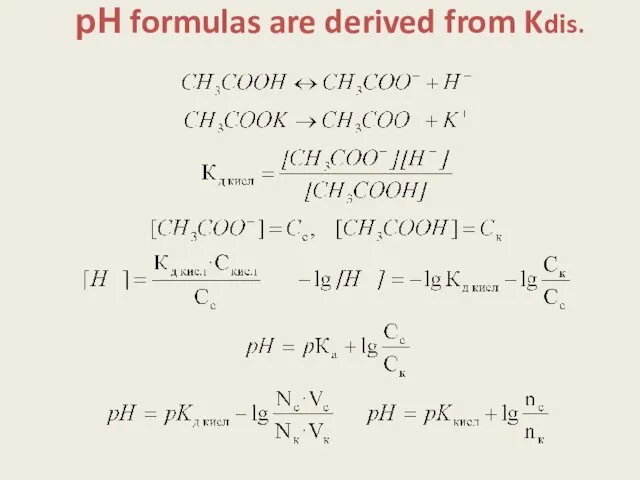
- 5. рН formulas are derived from Kdis.
- 6. HOW TO PREPARE BUFFER 1. Mixing the components: -for acidic buffer pH = pKa + lg
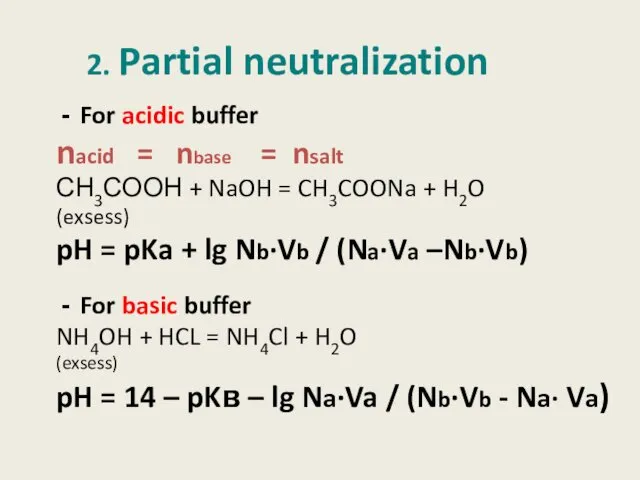
- 7. 2. Partial neutralization For acidic buffer nacid = nbase = nsalt СН3СООН + NaOH = CH3COONa
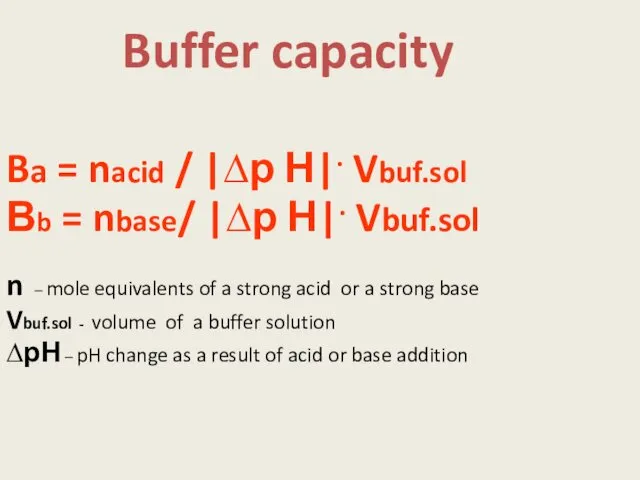
- 8. Buffer capacity Ba = nacid / |∆р Н|. Vbuf.sol Вb = nbase/ |∆р Н|. Vbuf.sol n
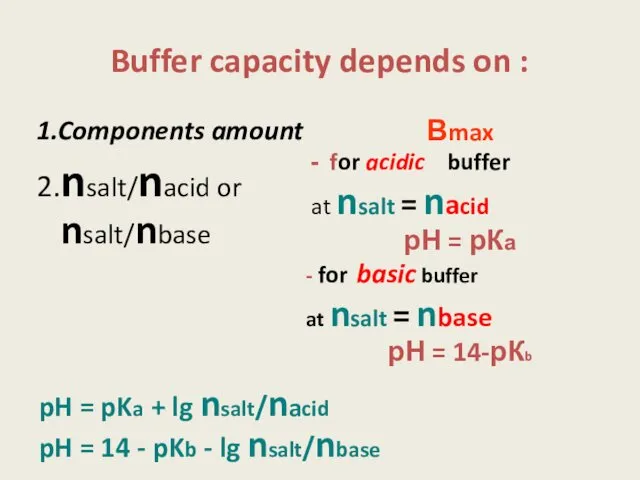
- 9. Buffer capacity depends on : pH = pKa + lg nsalt/nacid pH = 14 - pKb
- 10. Mechanism of buffer action Acetate buffer СН3СООNa СН3СОО- + Na+ Н+ СН3СООН СН3СОО- + Н+ ОН-
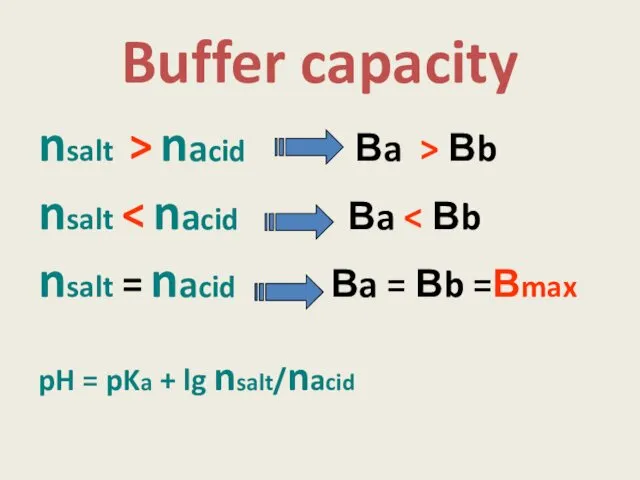
- 11. Buffer capacity nsalt > nacid Вa > Вb nsalt nsalt = nacid Вa = Вb =Вmax
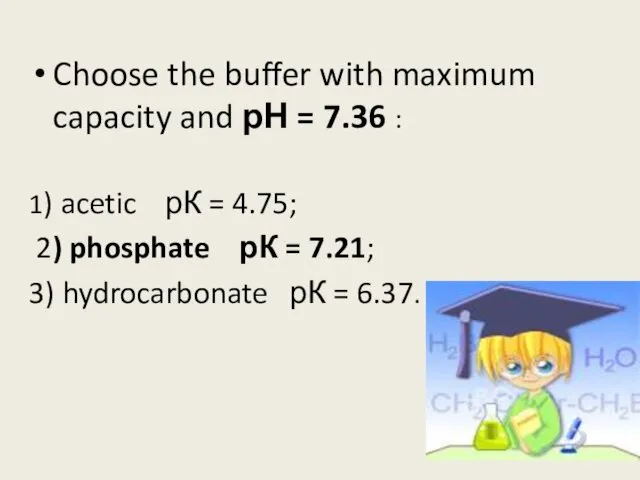
- 12. Choose the buffer with maximum capacity and рН = 7.36 : 1) acetic рК = 4.75;
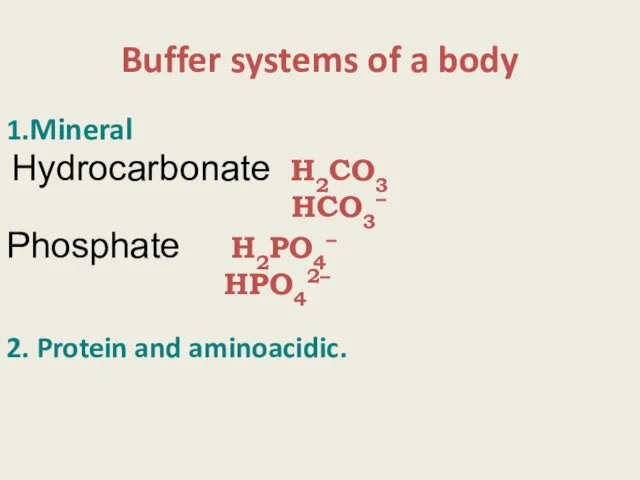
- 13. Buffer systems of a body 1.Mineral Hydrocarbonate Н2СО3 НСО3– Phosphate Н2РО4– НРO42– 2. Protein and aminoacidic.
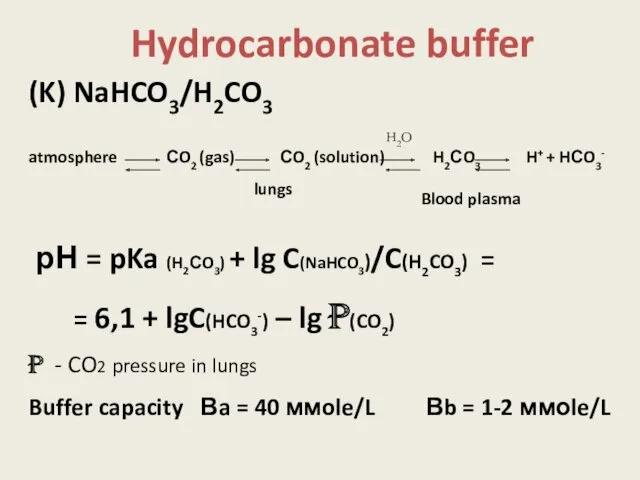
- 14. Hydrocarbonate buffer (K) NaHCO3/H2CO3 atmosphere СO2 (gas) СO2 (solution) H2СO3 H+ + HСO3- рН = pKa
- 15. [НСО3–]:[СО2] = 20:1 Вa > Вb Н2СО3 – 13 моle/ day Other acids – from 0.03
- 16. 1. A buffer consists of 0,5 moles of equivalent NH3 and 0,5 moles of equivalent NH4Cl.
- 17. 4. What volume of 0,01M NaOH should be added to 100 ml of 0,5M CH3COOH solution
- 19. Скачать презентацию


![[НСО3–]:[СО2] = 20:1 Вa > Вb Н2СО3 – 13 моle/](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/53221/slide-14.jpg)


 Short stories
Short stories Постимпрессионизм. Поль Гоген
Постимпрессионизм. Поль Гоген Серебряный век русской поэзии
Серебряный век русской поэзии Презентация на тему: Натуральный каучук.
Презентация на тему: Натуральный каучук. Управление и моделирование бизнес-процессами
Управление и моделирование бизнес-процессами Натурные испытания аэродромных покрытий
Натурные испытания аэродромных покрытий Медианы, биссектрисы и высоты треугольника
Медианы, биссектрисы и высоты треугольника Шоколад-вред или польза?
Шоколад-вред или польза? Гербарий сказочных растений
Гербарий сказочных растений Административное правонарушение и административная ответственность
Административное правонарушение и административная ответственность Способы увеличения протяженности акустического канала утечки информации
Способы увеличения протяженности акустического канала утечки информации Организационные структуры в проектах. Управление проектами
Организационные структуры в проектах. Управление проектами Предконцепция Променад-парка
Предконцепция Променад-парка Как делают бумагу
Как делают бумагу Регламент оказания услуг ранней помощи в условиях консультационных пунктов
Регламент оказания услуг ранней помощи в условиях консультационных пунктов My future plans
My future plans портфолио Диск Диск Диск Диск Диск Диск Диск
портфолио Диск Диск Диск Диск Диск Диск Диск Храмы-памятники воинской славы
Храмы-памятники воинской славы Основы теории градостроительства и районной планировки
Основы теории градостроительства и районной планировки презентация к 1 родительскому собранию по ТРИЗ
презентация к 1 родительскому собранию по ТРИЗ Терапия депрессий и профилактика суицида
Терапия депрессий и профилактика суицида Первое родительское собрание
Первое родительское собрание Обрабатывающая промышленность
Обрабатывающая промышленность Наш лучший друг - Агния Барто
Наш лучший друг - Агния Барто Dental instruments
Dental instruments Теоретические подходы к пониманию организаций и их положения
Теоретические подходы к пониманию организаций и их положения Работа цеха покрытий в августе 2017 года
Работа цеха покрытий в августе 2017 года 20230816_prezentatsiya_istoriya_raketostroeniya
20230816_prezentatsiya_istoriya_raketostroeniya