Слайд 2Atoms
Atoms are the basic building blocks of all materials.
Слайд 3Elements
Elements are made up of one type of atom and cannot be
broken down into anything simpler.
Each element has a symbol.
Hydrogen = H
Carbon = C
Слайд 4Elements
Answers to Question 1.7:
Jewellery = Silver
Mercury = Thermometers
Copper = Plumbing
Слайд 5Molecules/Compounds
A Molecule is formed when two or more atoms join (bond) together
chemically.
A Compound is when a molecule is made up of two or more different atoms. Water (H2O) and Carbon dioxide (CO2) are compounds because they are made up of more than one type of atom.
Слайд 6Molecules/Compounds
Answers for Question 1.9:
Elements = Hydrogen, Sulfur and Oxygen
Compounds = Water, Dichloromethane, Methane
and Hydrochloric Acid
Слайд 7Structure of the Atom
The atom is made up of sub-atomic particles:
protons
neutrons
electrons.
These three particles are different from each other.
Слайд 8Atomic and Mass numbers
Each Atom has its own atomic and mass number.
Atomic
Number: This tells you how many protons (equal to number of electrons).
Mass Number: This tells you how many protons and neutrons.
Слайд 9How electrons are arranged
Niels Bohr was the first person to suggest the
idea of electron shells containing electrons orbiting the nucleus
Слайд 10How electrons are arranged
Solution to Question 1.18:
 Методический семинар Создание персонального сайта учителя
Методический семинар Создание персонального сайта учителя Гибкий производственный модуль (ГПМ)
Гибкий производственный модуль (ГПМ) Религии мира
Религии мира Язвенная болезнь желудка
Язвенная болезнь желудка Ветераны ,спасибо вам за победу!
Ветераны ,спасибо вам за победу! Возбудители бактериальных кишечных инфекций. (Лекция 13)
Возбудители бактериальных кишечных инфекций. (Лекция 13) Урок, как основная форма организации учебного процесса. Типы и структуры уроков
Урок, как основная форма организации учебного процесса. Типы и структуры уроков тех.мальч
тех.мальч Мигель Де Сервантес Сааведра
Мигель Де Сервантес Сааведра Презентация Как птицы и звери к зиме готовятся
Презентация Как птицы и звери к зиме готовятся Математика в моей профессии
Математика в моей профессии Презентация обучающего занятия для родителей (коррекция звука Ш)
Презентация обучающего занятия для родителей (коррекция звука Ш) Применение ИКТ в дошкольных учреждениях.
Применение ИКТ в дошкольных учреждениях. Роль воспитателя в организации игр детей раннего возраста
Роль воспитателя в организации игр детей раннего возраста Шекспир на все времена
Шекспир на все времена Оценка и аттестация персонала. Высвобождение персонала. Тема 5
Оценка и аттестация персонала. Высвобождение персонала. Тема 5 Health Care systems using machine learning algorithms
Health Care systems using machine learning algorithms Орфография в таблицах. Орфограммы в корне
Орфография в таблицах. Орфограммы в корне Франшиза сети кинотеатров Люмен фильм. Коммерческое предложение
Франшиза сети кинотеатров Люмен фильм. Коммерческое предложение Biochemistry
Biochemistry Работа с бумагой. Прямое плетение из полос бумаги. Коврик
Работа с бумагой. Прямое плетение из полос бумаги. Коврик Электрическая цепь и ее составные части. Решение задач
Электрическая цепь и ее составные части. Решение задач Оноре де Бальзак. Бальзак і Україна
Оноре де Бальзак. Бальзак і Україна Всероссийская акция Привет солдату
Всероссийская акция Привет солдату Работа в microsoft powerpoint 2007
Работа в microsoft powerpoint 2007 Определение режима эксплуатации аэродромного покрытия по результатам обследования и испытания
Определение режима эксплуатации аэродромного покрытия по результатам обследования и испытания Мастерская Деда Мороза
Мастерская Деда Мороза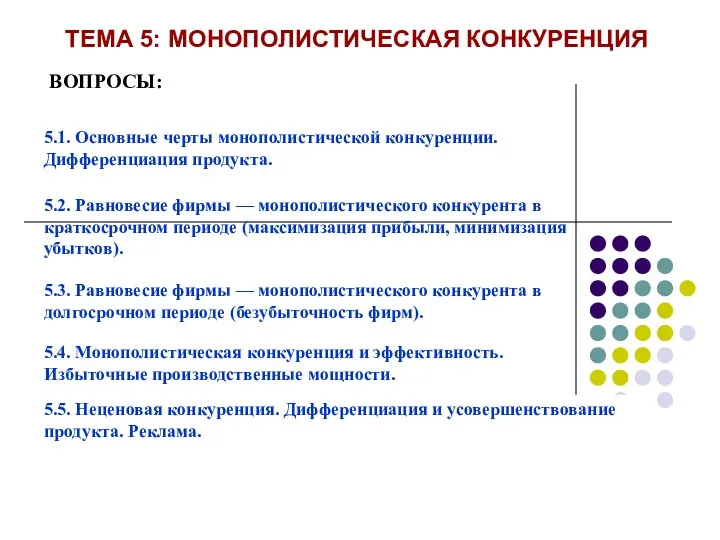 Тема 5: Монополистическая конкуренция
Тема 5: Монополистическая конкуренция