Содержание
- 2. Content Introduction (preface) History and discovery Taxonomy Biology (Morphological, physiological and biochemical features) Phenotypic (morphological and
- 3. Introduction Lactic acid bacteria are among the most important groups of microorganisms used in food fermentations.
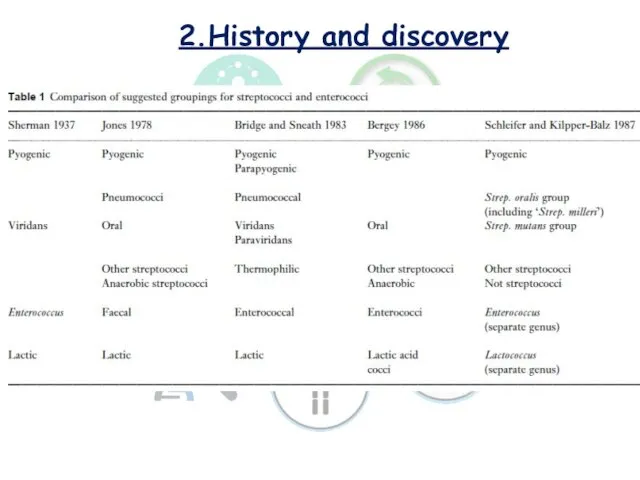
- 4. 2.History and discovery The common organism associated with the souring of milk was first described by
- 5. 2.History and discovery This group of bacteria, previously designated the lactic streptococci (Streptococcus lactis subsp. lactis
- 6. 2.History and discovery
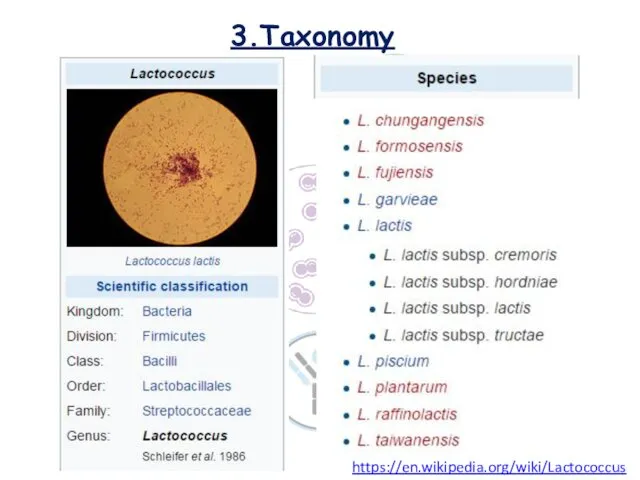
- 7. 3.Taxonomy https://en.wikipedia.org/wiki/Lactococcus
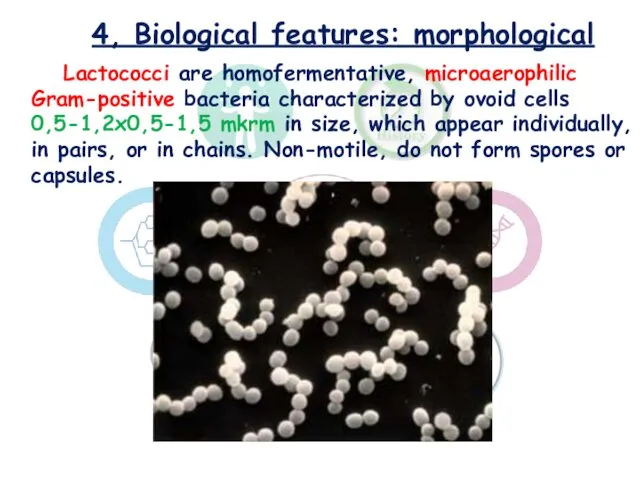
- 8. 4, Biological features: morphological Lactococci are homofermentative, microaerophilic Gram-positive bacteria characterized by ovoid cells 0,5-1,2x0,5-1,5 mkrm
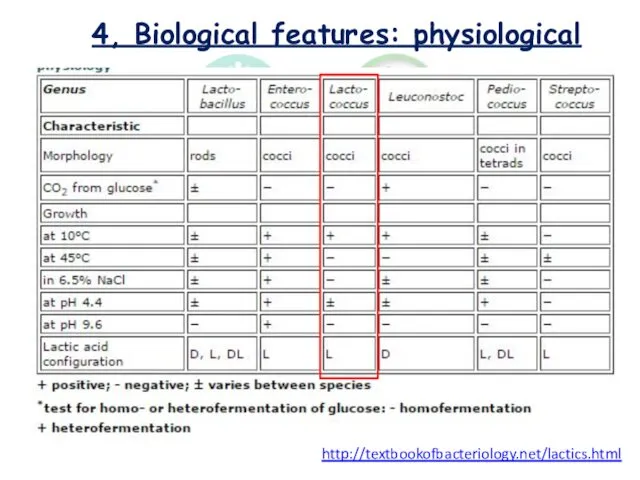
- 9. 4, Biological features: physiological http://textbookofbacteriology.net/lactics.html
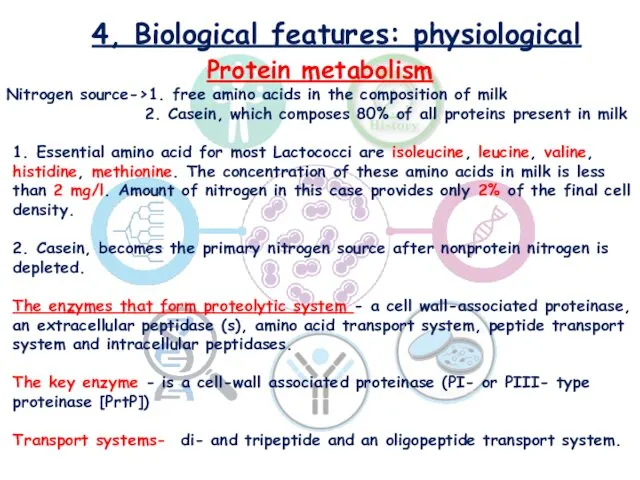
- 10. 4, Biological features: physiological Protein metabolism Nitrogen source->1. free amino acids in the composition of milk
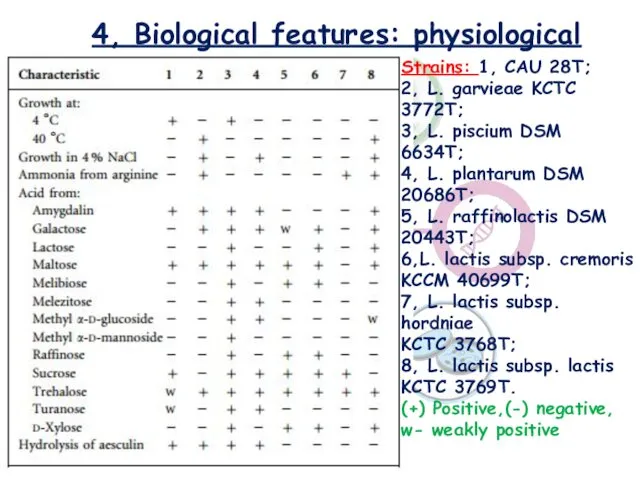
- 11. 4, Biological features: physiological Strains: 1, CAU 28T; 2, L. garvieae KCTC 3772T; 3, L. piscium
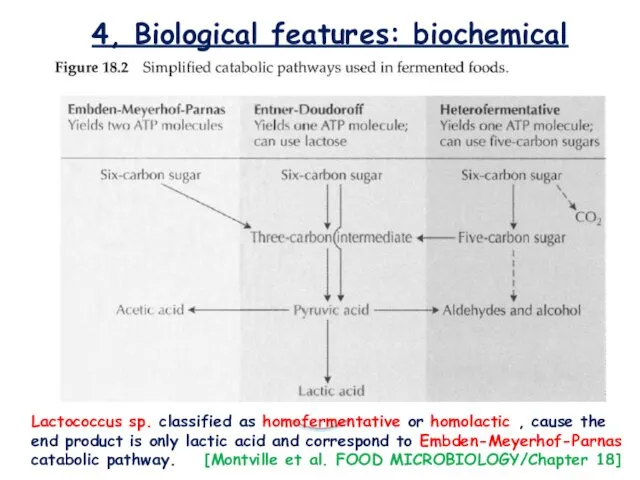
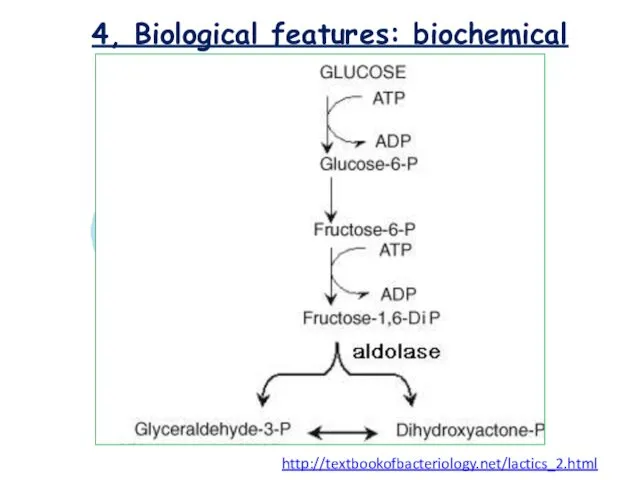
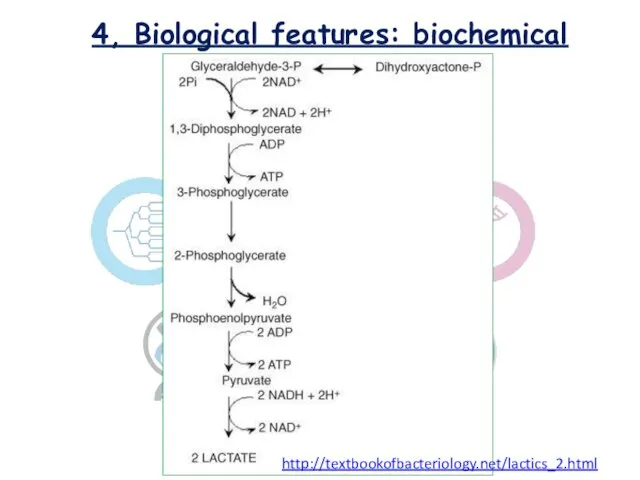
- 12. 4, Biological features: biochemical Lactococcus sp. classified as homofermentative or homolactic , cause the end product
- 13. 4, Biological features: biochemical http://textbookofbacteriology.net/lactics_2.html
- 14. 4, Biological features: biochemical http://textbookofbacteriology.net/lactics_2.html
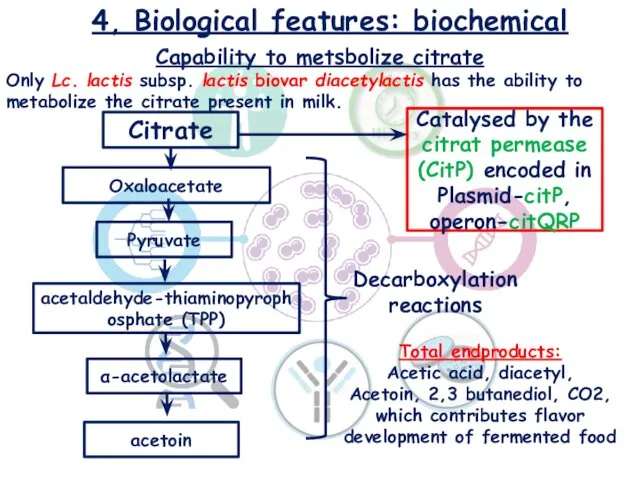
- 15. 4, Biological features: biochemical Capability to metsbolize citrate Only Lc. lactis subsp. lactis biovar diacetylactis has
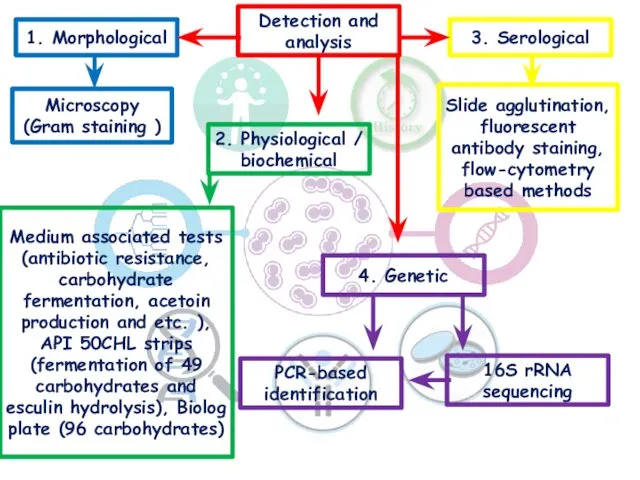
- 16. Detection and analysis 1. Morphological 2. Physiological / biochemical 3. Serological 4. Genetic Microscopy (Gram staining
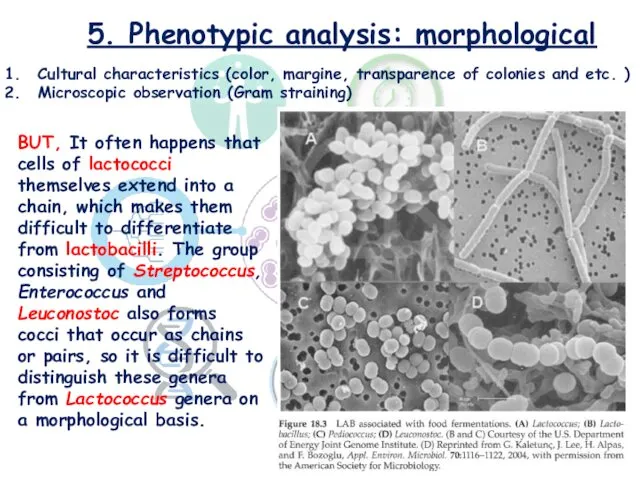
- 17. 5. Phenotypic analysis: morphological Cultural characteristics (color, margine, transparence of colonies and etc. ) Microscopic observation
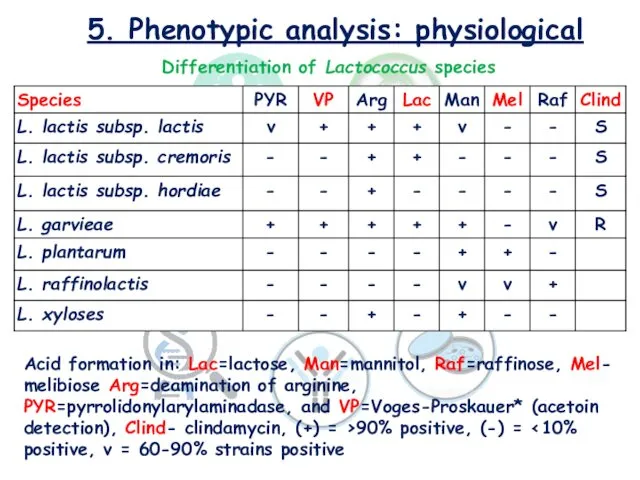
- 18. 5. Phenotypic analysis: physiological Differentiation of Lactococcus species Acid formation in: Lac=lactose, Man=mannitol, Raf=raffinose, Mel- melibiose
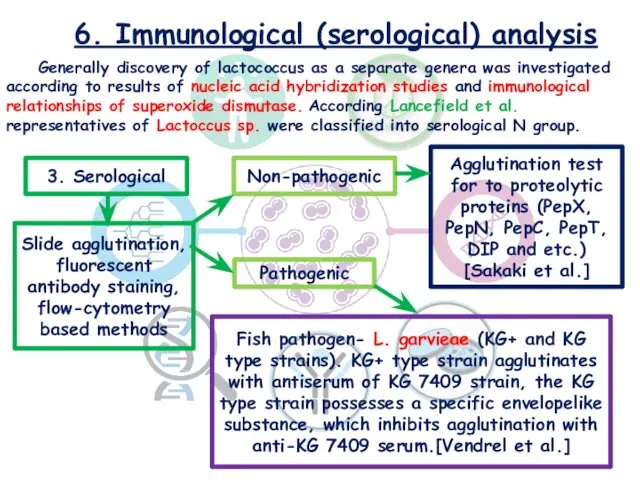
- 19. 6. Immunological (serological) analysis Generally discovery of lactococcus as a separate genera was investigated according to
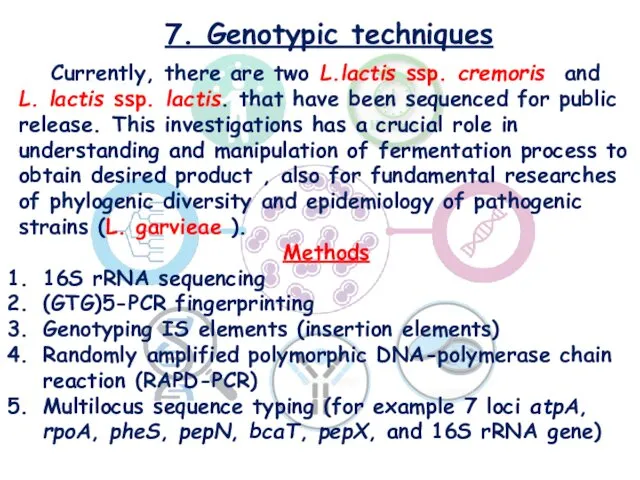
- 20. 7. Genotypic techniques Currently, there are two L.lactis ssp. cremoris and L. lactis ssp. lactis. that
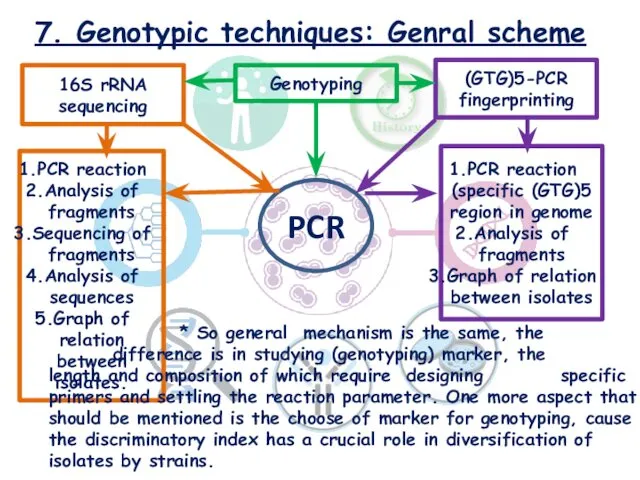
- 21. 7. Genotypic techniques: Genral scheme Genotyping 16S rRNA sequencing PCR PCR reaction Analysis of fragments Sequencing
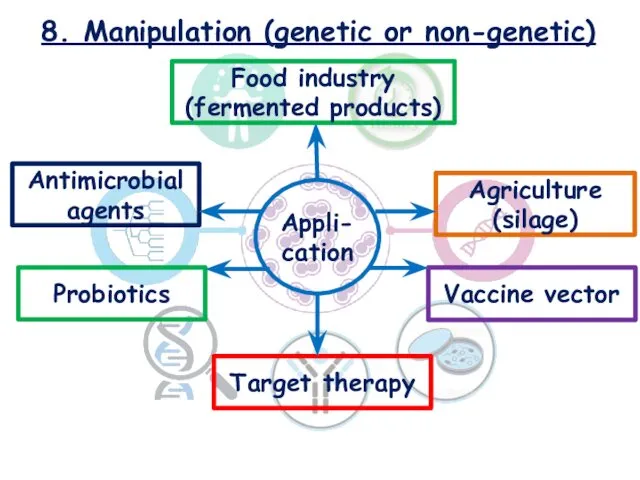
- 22. 8. Manipulation (genetic or non-genetic) Food industry (fermented products) Agriculture (silage) Antimicrobial agents Probiotics Target therapy
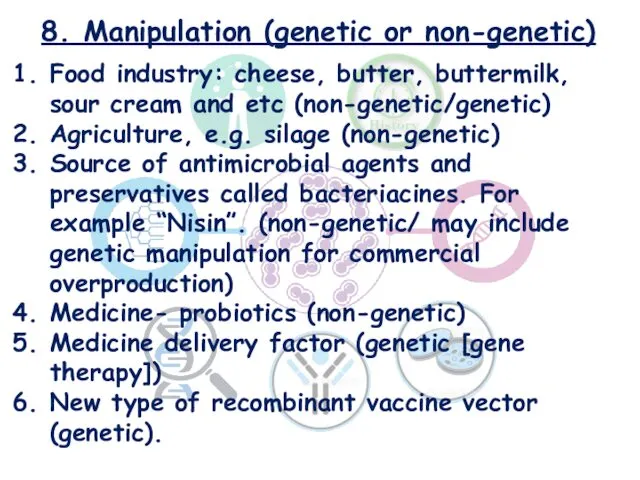
- 23. 8. Manipulation (genetic or non-genetic) Food industry: cheese, butter, buttermilk, sour cream and etc (non-genetic/genetic) Agriculture,
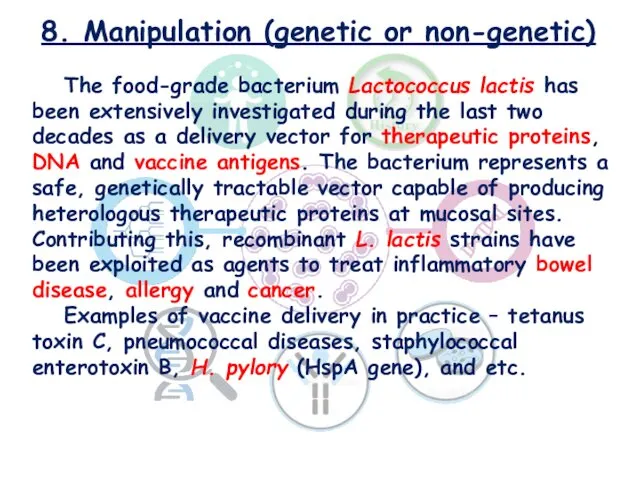
- 24. 8. Manipulation (genetic or non-genetic) The food-grade bacterium Lactococcus lactis has been extensively investigated during the
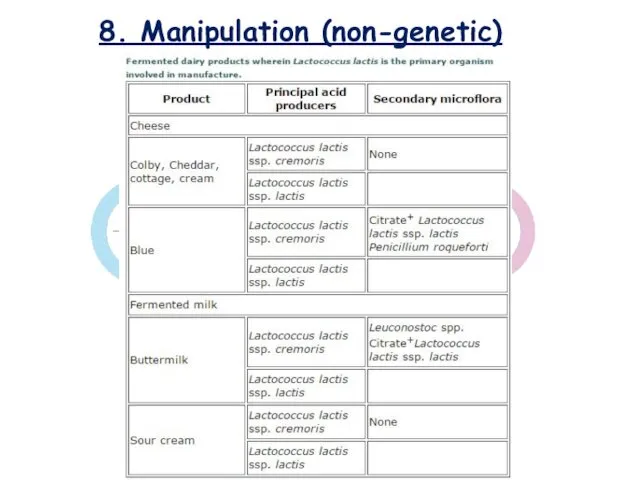
- 25. 8. Manipulation (non-genetic)
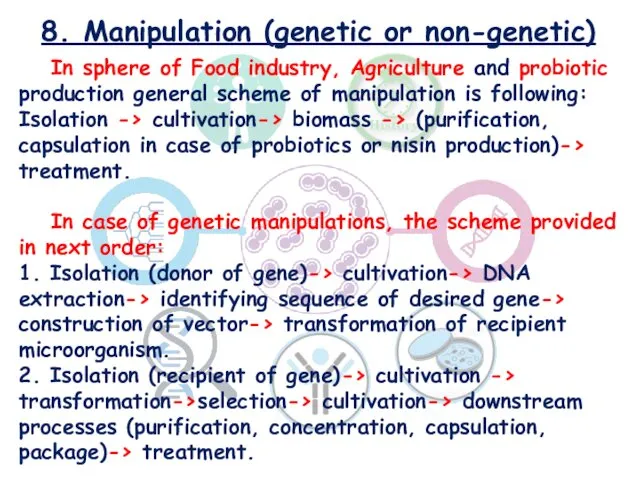
- 26. 8. Manipulation (genetic or non-genetic) In sphere of Food industry, Agriculture and probiotic production general scheme
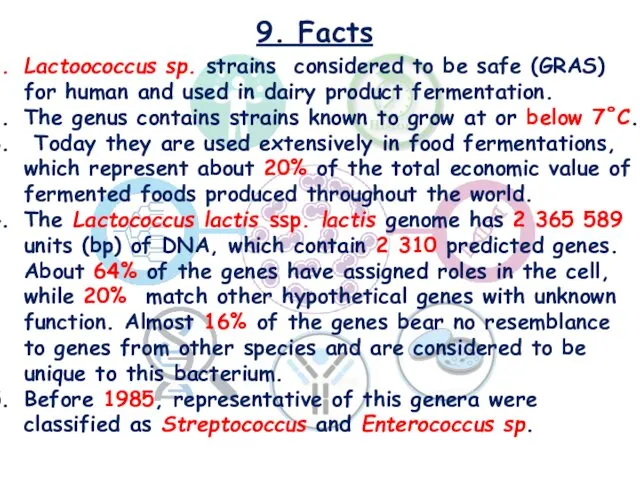
- 27. 9. Facts Lactoococcus sp. strains considered to be safe (GRAS) for human and used in dairy
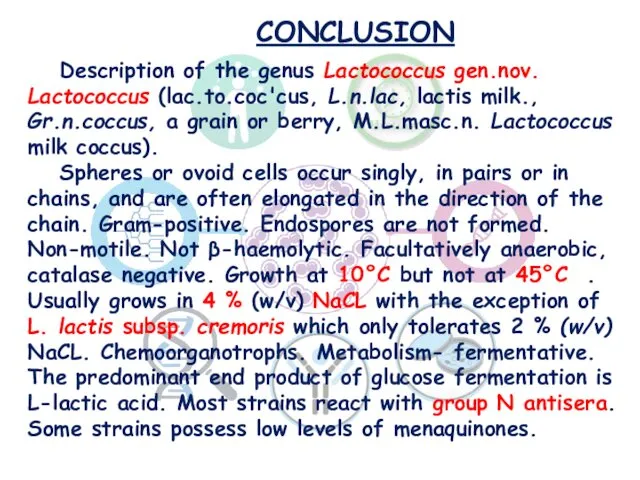
- 28. CONCLUSION Description of the genus Lactococcus gen.nov. Lactococcus (lac.to.coc'cus, L.n.lac, lactis milk., Gr.n.coccus, a grain or
- 29. CONCLUSION The major glycolipid of all strains is Glc(a1-2)Glc(a1-3)acyI2- Gro, a constant minor component is Glc(a1-2),
- 30. CONCLUSION Nucleic acid hybridization and comparative immunological studies demonstrate that members of the genus Lactococcus are
- 31. 10. References D.Samaržija, N.Antunac, J.L. Havranek. Taxonomy, physiology and growth of Lactococcus lactis: a review. Mljekarstvo
- 32. 10. References 9. M. Sakaki et al. Immunological and electrophoretic study of the proteolytic enzymes from
- 33. 10. References 15. E. Ferna´ndez et al. Comparative Phenotypic and Molecular Genetic Profiling of Wild Lactococcus
- 34. 10. References 21. S. B. Hanniffy et al. Mucosal Delivery of a Pneumococcal Vaccine Using Lactococcus
- 36. Скачать презентацию





 Сухие строительные смеси ГЕРКУЛЕС. Шпатлевки
Сухие строительные смеси ГЕРКУЛЕС. Шпатлевки Мастер-класс Новогодняя игрушка Елочка
Мастер-класс Новогодняя игрушка Елочка ПрезентацияSlava 130420A
ПрезентацияSlava 130420A Радио Комсомольская правда. Новые программы в эфире. Рекламные возможности
Радио Комсомольская правда. Новые программы в эфире. Рекламные возможности Измельчительно-режущее оборудование
Измельчительно-режущее оборудование Компания MasterMould. Высокоскоростная штамповка
Компания MasterMould. Высокоскоростная штамповка Урок в 11 химико-биологическом классе по теме: Особенности свойств отдельных классов неорганических и органических веществ на примере лекарственных средств
Урок в 11 химико-биологическом классе по теме: Особенности свойств отдельных классов неорганических и органических веществ на примере лекарственных средств Системы обучения в начальной школе
Системы обучения в начальной школе Предприятие – основное звено рыночного хозяйствования
Предприятие – основное звено рыночного хозяйствования Талшықты лазер
Талшықты лазер С праздником 8 марта
С праздником 8 марта Свобода в деятельности человека
Свобода в деятельности человека Организация воспитательно-образовательной работы с детьми раннего возраста в группе кратковременного пребывания
Организация воспитательно-образовательной работы с детьми раннего возраста в группе кратковременного пребывания Трапеция. Свойства трапеции
Трапеция. Свойства трапеции Презентация для детей старшего дошкольного возраста Как добывают уголь
Презентация для детей старшего дошкольного возраста Как добывают уголь School days
School days Геометрические задачи С4, по материалам ЕГЭ. Подобие треугольников
Геометрические задачи С4, по материалам ЕГЭ. Подобие треугольников Расчет элементов железобетонных конструкций по предельным состояниям второй группы
Расчет элементов железобетонных конструкций по предельным состояниям второй группы Венерические заболевания
Венерические заболевания презентация вкр (2)
презентация вкр (2) Методические рекомендации по построению предметно-развивающей среды в соответствии с ФГОС в группе раннего возраста.
Методические рекомендации по построению предметно-развивающей среды в соответствии с ФГОС в группе раннего возраста. Кухни Боснии, Герцеговины, Хорватии и Сербии
Кухни Боснии, Герцеговины, Хорватии и Сербии Гордость театральной сцены Башкортостана
Гордость театральной сцены Башкортостана Сократительная функция всех типов мышц
Сократительная функция всех типов мышц Врожденная непроходимость ЖКТ у детей
Врожденная непроходимость ЖКТ у детей Трахеобронхомегалия (синдром Мунье-Куна)
Трахеобронхомегалия (синдром Мунье-Куна) Линейная алгебра. Лекционно-практические занятия
Линейная алгебра. Лекционно-практические занятия Маркетинговые исследования
Маркетинговые исследования