Multiscale modeling of ionic liquids: combined DFT, QM/MM MD and vibrational spectroscopic study презентация
Содержание
- 2. Ionic liquids: [Emim] [BF4] + have high loading capacity ☺; + thermally stable ☺; + nonflammable
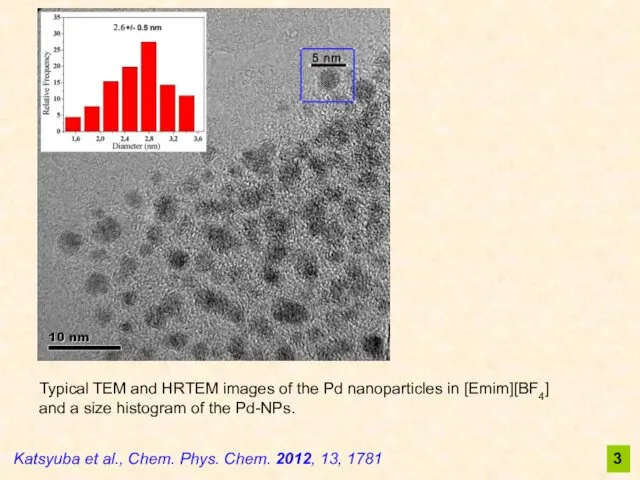
- 3. Typical TEM and HRTEM images of the Pd nanoparticles in [Emim][BF4] and a size histogram of
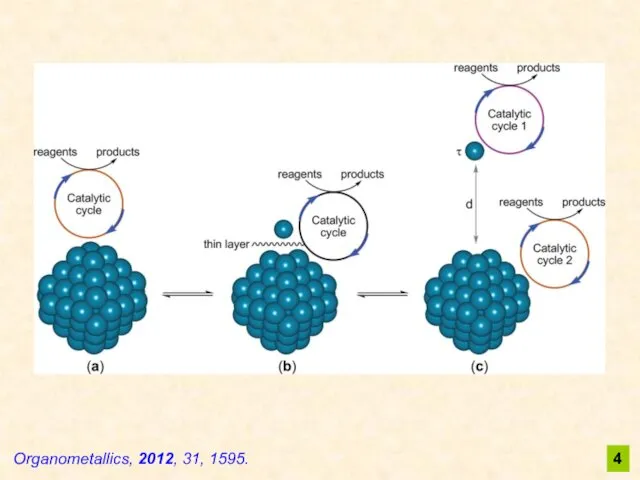
- 4. Organometallics, 2012, 31, 1595. 4
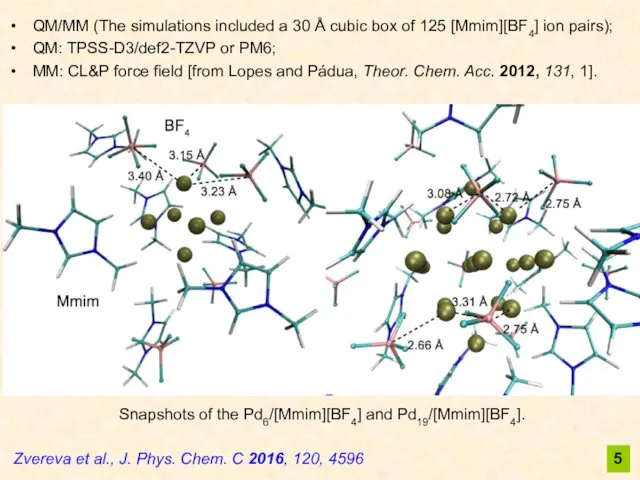
- 5. 5 Zvereva et al., J. Phys. Chem. C 2016, 120, 4596 QM/MM (The simulations included a
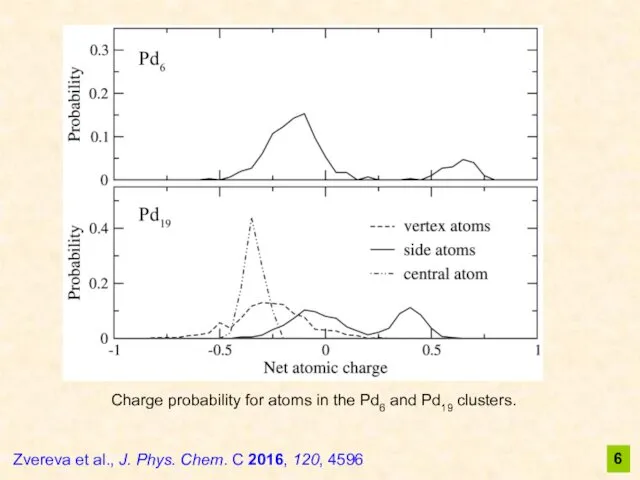
- 6. 6 Zvereva et al., J. Phys. Chem. C 2016, 120, 4596 Charge probability for atoms in
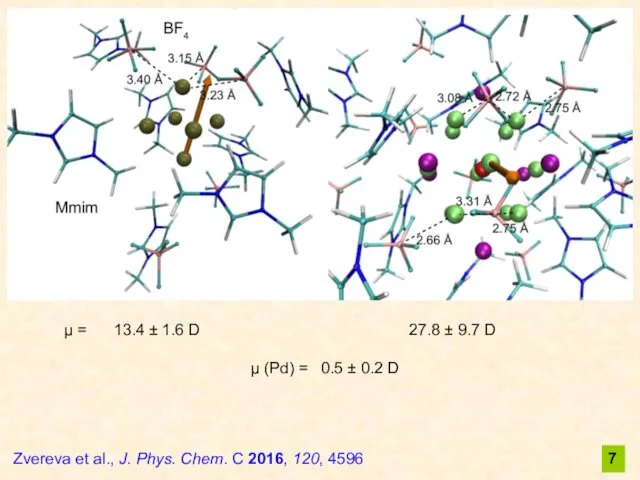
- 7. 7 μ = 13.4 ± 1.6 D 27.8 ± 9.7 D μ (Pd) = 0.5 ±
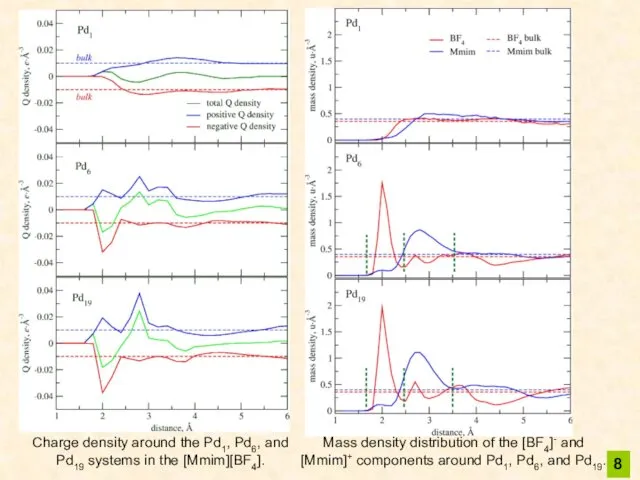
- 8. 8 Charge density around the Pd1, Pd6, and Pd19 systems in the [Mmim][BF4]. Mass density distribution
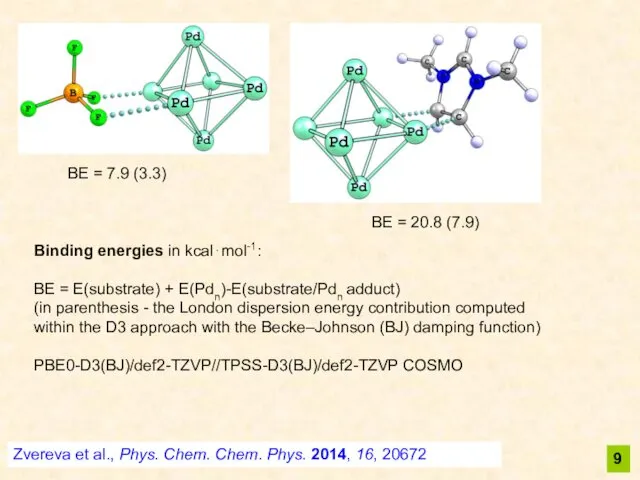
- 9. Zvereva et al., Phys. Chem. Chem. Phys. 2014, 16, 20672 Binding energies in kcal⋅mol-1: BE =
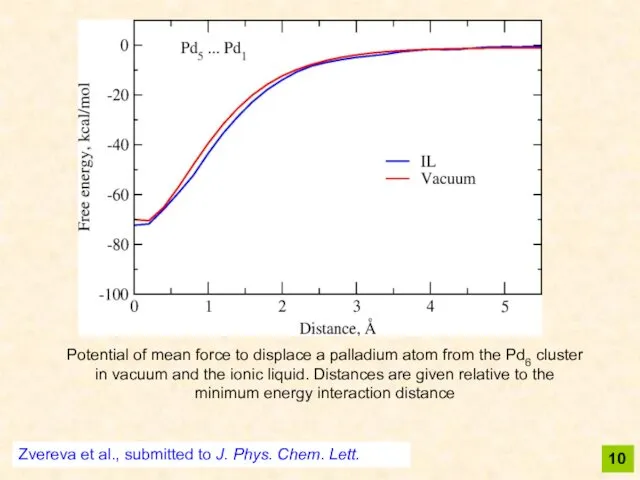
- 10. 10 Potential of mean force to displace a palladium atom from the Pd6 cluster in vacuum
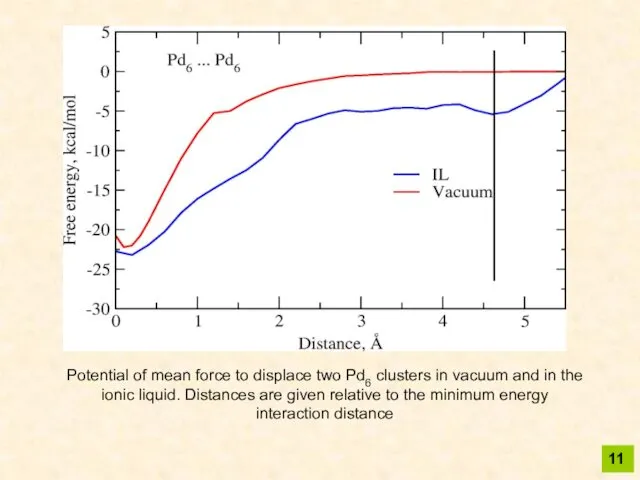
- 11. 11 Potential of mean force to displace two Pd6 clusters in vacuum and in the ionic
- 12. Experiment [EMIM][Br] νCH ~ 3070 cm-1 Zvereva et al., Russ. Chem. Bull., 2009, 9, 1812. 3087
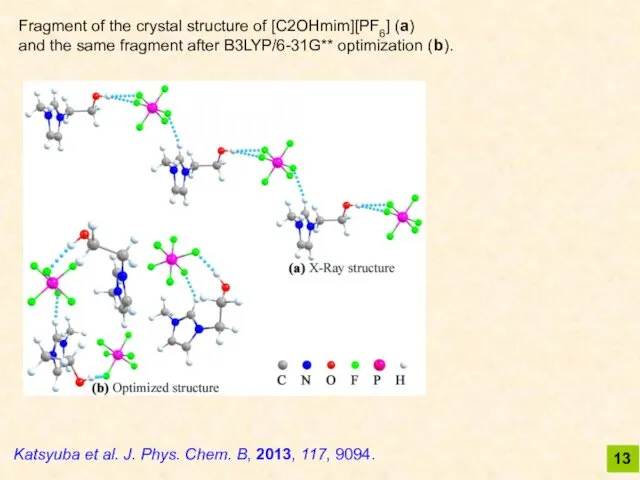
- 13. 13 Katsyuba et al. J. Phys. Chem. B, 2013, 117, 9094. Fragment of the crystal structure
- 14. 14 Katsyuba et al. J. Phys. Chem. Lett., 2015, 6, 4431. [C2OHmim][OAc]
- 15. −ΔHHB [kcal⋅mol-1] = 0.29 (I1/2 – I01/2) −ΔHHB [kcal⋅mol-1] = 0.33 (νOHfree - νOHbonded )1/2 EHB
- 16. 16
- 17. Conclusions The IL induces a strong polarization in palladium clusters The clusters have large induced dipole
- 18. Acknowledgment Prof. Paul J. Dyson, Dr. Zhaofu Fei, Dr. Rosario Scopellitti Grant 15-03-01058 A
- 20. Скачать презентацию
![Ionic liquids: [Emim] [BF4] + have high loading capacity ☺;](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/37810/slide-1.jpg)
![Experiment [EMIM][Br] νCH ~ 3070 cm-1 Zvereva et al., Russ.](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/37810/slide-11.jpg)
![14 Katsyuba et al. J. Phys. Chem. Lett., 2015, 6, 4431. [C2OHmim][OAc]](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/37810/slide-13.jpg)
![−ΔHHB [kcal⋅mol-1] = 0.29 (I1/2 – I01/2) −ΔHHB [kcal⋅mol-1] =](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/37810/slide-14.jpg)



 экспериментальная деятельность
экспериментальная деятельность Составление технологической карты урока
Составление технологической карты урока Символы древних славян
Символы древних славян ПРЕЗЕНТАЦИЯ Использование информационно-коммуникационных технологий в работе музыкального руководителя
ПРЕЗЕНТАЦИЯ Использование информационно-коммуникационных технологий в работе музыкального руководителя Дошколятам о профессиях
Дошколятам о профессиях дизайн-концепт для сети кинотеатров города
дизайн-концепт для сети кинотеатров города Презентация Такие же как мы
Презентация Такие же как мы Не хочу делать уроки!
Не хочу делать уроки! Спортивная гимнастика
Спортивная гимнастика Творческий проект Поликлиника: какой она должна быть
Творческий проект Поликлиника: какой она должна быть Питание и пищеварение
Питание и пищеварение Новая экономическая политика
Новая экономическая политика Студенттердің тамақтану ерекшелігі мен ұйымдастырылуын бағалау. Ғылыми жоба
Студенттердің тамақтану ерекшелігі мен ұйымдастырылуын бағалау. Ғылыми жоба Методы исследования и диагностики при заболеваниях височно-нижнечелюстного сустава
Методы исследования и диагностики при заболеваниях височно-нижнечелюстного сустава классный час для 2 класса, посв. 20-летию Конституции.
классный час для 2 класса, посв. 20-летию Конституции. Аэропорт Бен-Гурион
Аэропорт Бен-Гурион Алкоголизм. Стадии алкоголизма
Алкоголизм. Стадии алкоголизма Былина - жанр устного народного творчества. Ильины три поездочки
Былина - жанр устного народного творчества. Ильины три поездочки Город-герой Волгоград (Сталинград)
Город-герой Волгоград (Сталинград) Угол. Измерение углов
Угол. Измерение углов С днём рождения
С днём рождения Психологические особенности ребенка дошкольного возраста
Психологические особенности ребенка дошкольного возраста Взаимодействие логопеда со специалистами ДОУ
Взаимодействие логопеда со специалистами ДОУ Анна Ахматова (1889-1966)
Анна Ахматова (1889-1966) Электроснабжение очистных (подготовительных) работ участка шахты ОАО Распадская
Электроснабжение очистных (подготовительных) работ участка шахты ОАО Распадская Методы очистки сточных вод
Методы очистки сточных вод Этапы развития речи ребенка
Этапы развития речи ребенка Автомобильные дороги, аэродромы и объекты транспортной инфраструктуры
Автомобильные дороги, аэродромы и объекты транспортной инфраструктуры