Содержание
- 2. Learning Objectives Recognise that halogenoalkanes will react with nucleophiles Understand the mechanism of nucleophilic substitution reactions
- 3. Success Criteria Define the term nucleophilic substitution. Explain the differences between SN1 and SN2 mechanisms. Write
- 4. Keywords Nucleophile Substitution Nucleophilic substitution Nucleophilic substitution unimolecular (SN1) Nucleophilic substitution bimolecular (SN2) rate-determining step (slowest
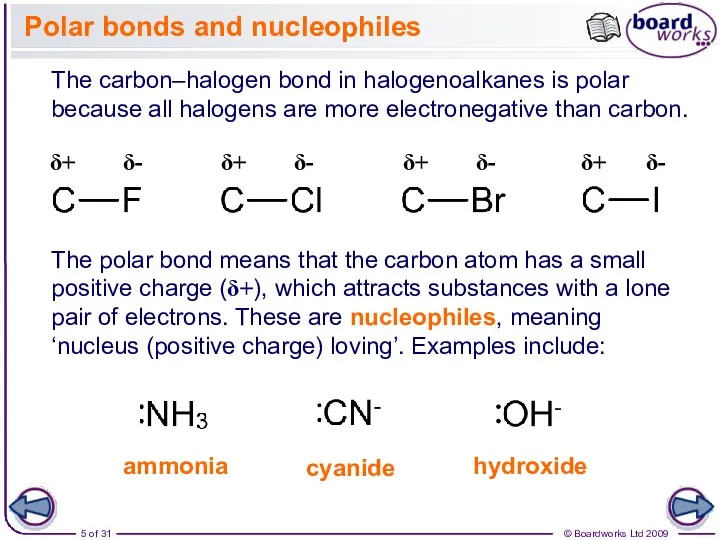
- 5. Polar bonds and nucleophiles The carbon–halogen bond in halogenoalkanes is polar because all halogens are more
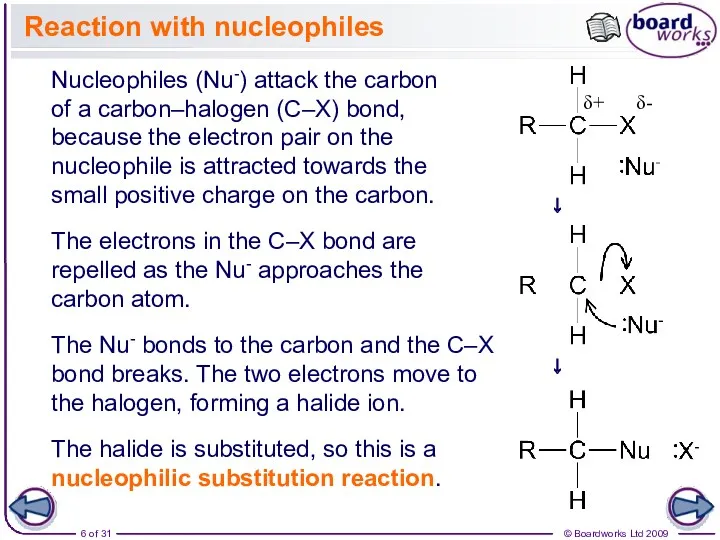
- 6. Nucleophiles (Nu-) attack the carbon of a carbon–halogen (C–X) bond, because the electron pair on the
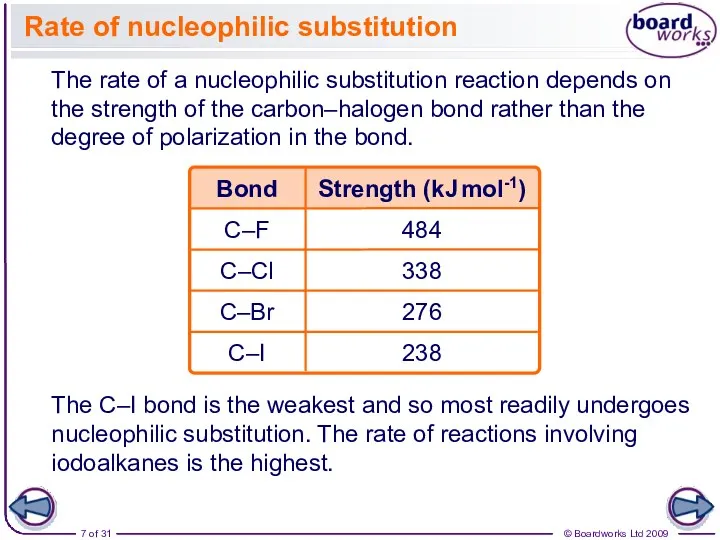
- 7. Rate of nucleophilic substitution The rate of a nucleophilic substitution reaction depends on the strength of
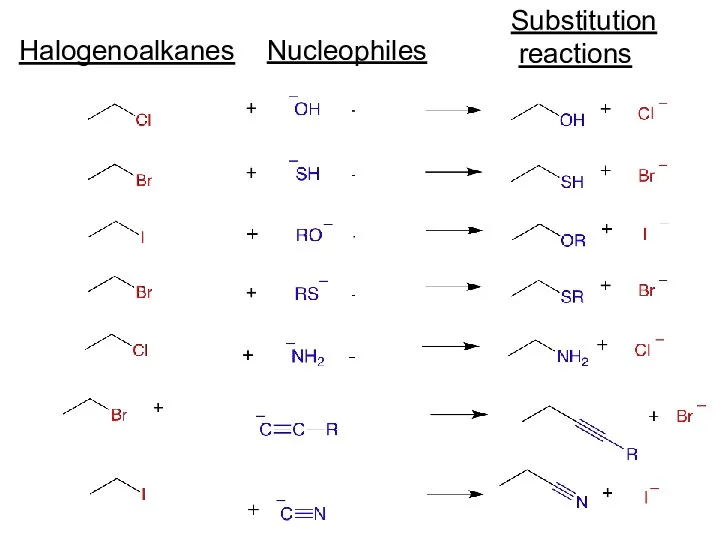
- 8. Nucleophiles Substitution reactions Halogenoalkanes
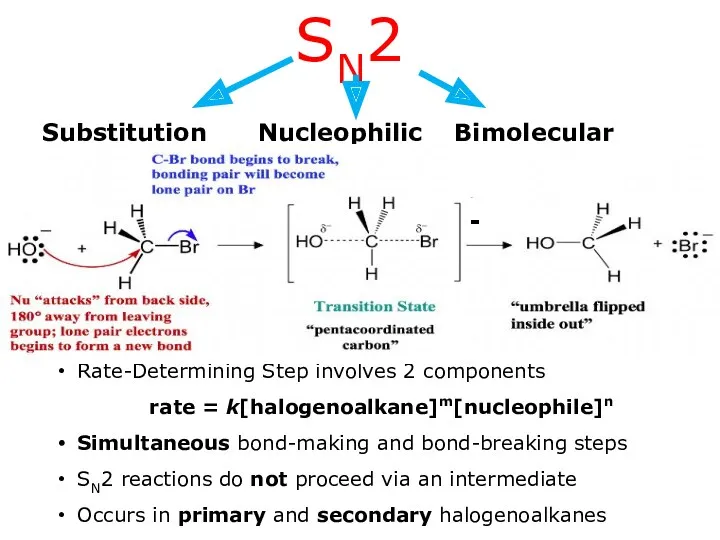
- 9. Substitution Nucleophilic Bimolecular SN2 Rate-Determining Step involves 2 components rate = k[halogenoalkane]m[nucleophile]n Simultaneous bond-making and bond-breaking
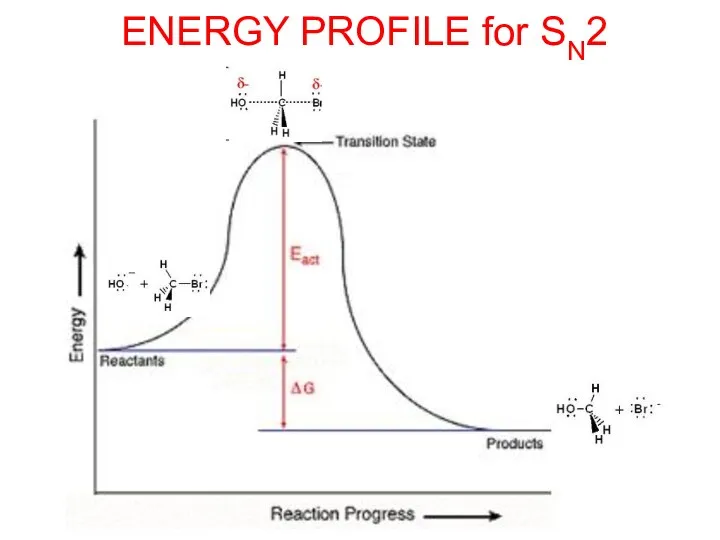
- 10. ENERGY PROFILE for SN2
- 11. SN2 MECHANISM
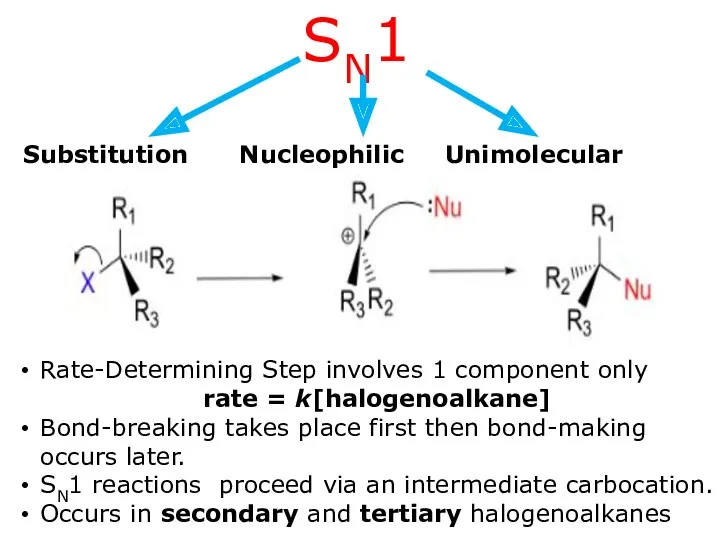
- 12. Substitution Nucleophilic Unimolecular SN1 Rate-Determining Step involves 1 component only rate = k[halogenoalkane] Bond-breaking takes place
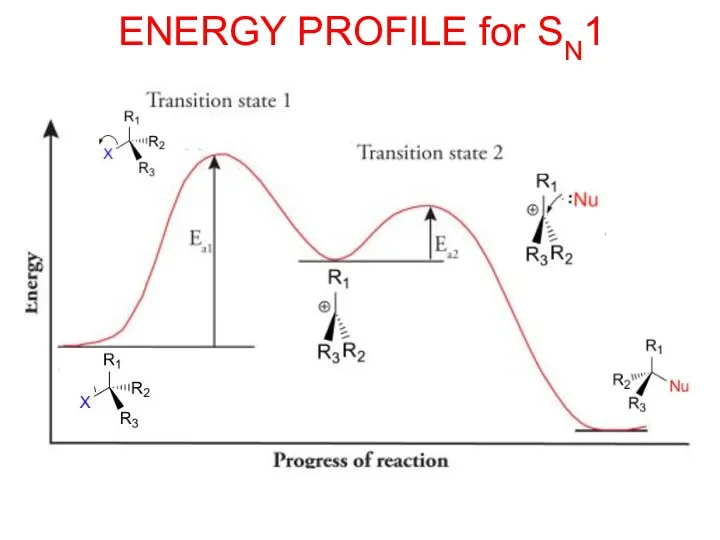
- 13. ENERGY PROFILE for SN1
- 14. SN1 MECHANISM
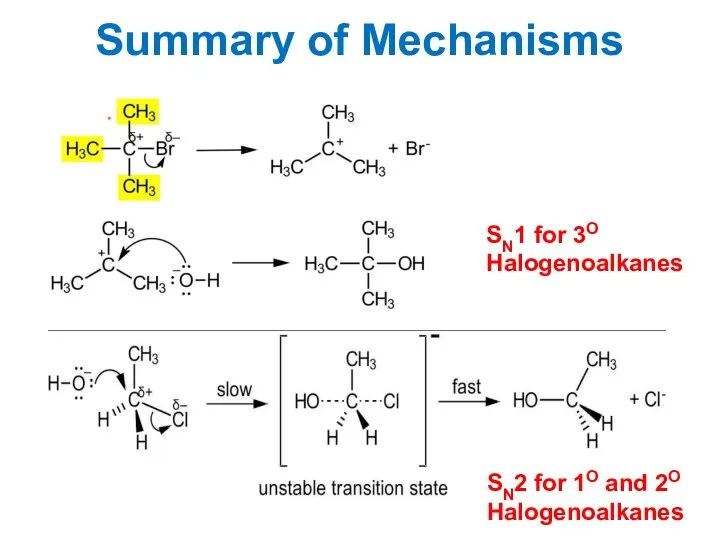
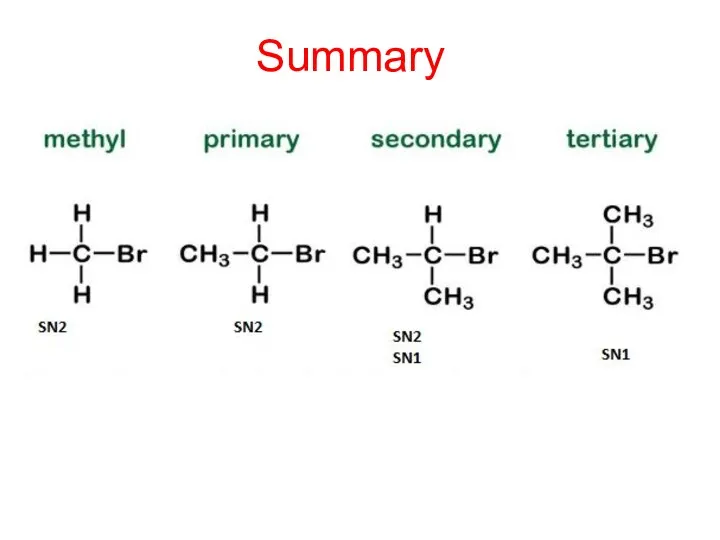
- 15. Summary of Mechanisms SN1 for 3O Halogenoalkanes SN2 for 1O and 2O Halogenoalkanes
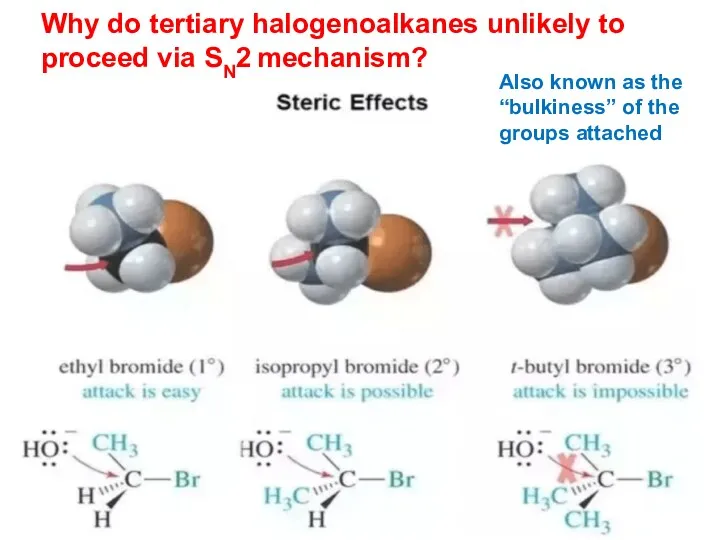
- 16. Why do tertiary halogenoalkanes unlikely to proceed via SN2 mechanism? Also known as the “bulkiness” of
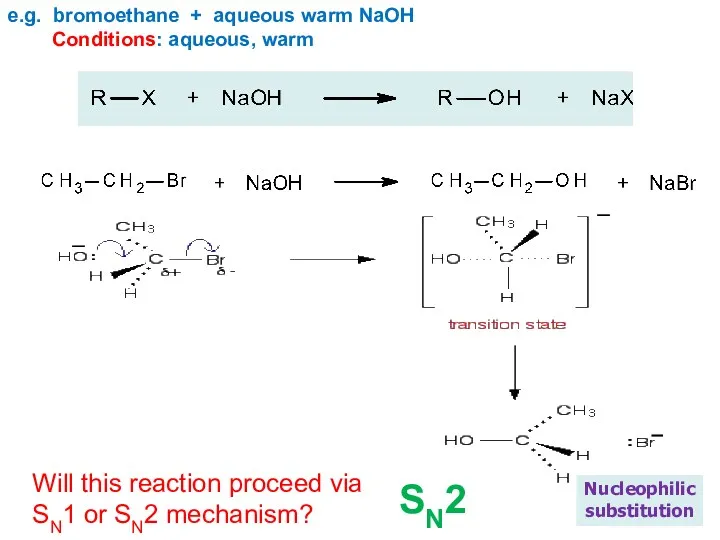
- 17. e.g. bromoethane + aqueous warm NaOH Conditions: aqueous, warm Nucleophilic substitution Will this reaction proceed via
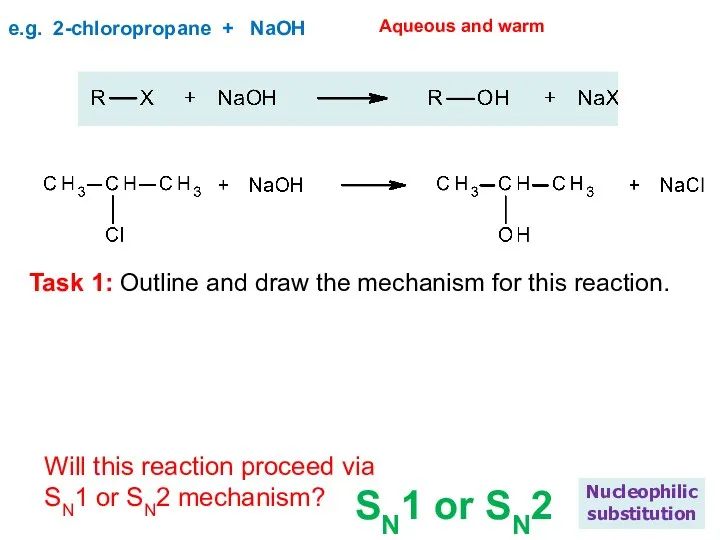
- 18. e.g. 2-chloropropane + NaOH Nucleophilic substitution Aqueous and warm Will this reaction proceed via SN1 or
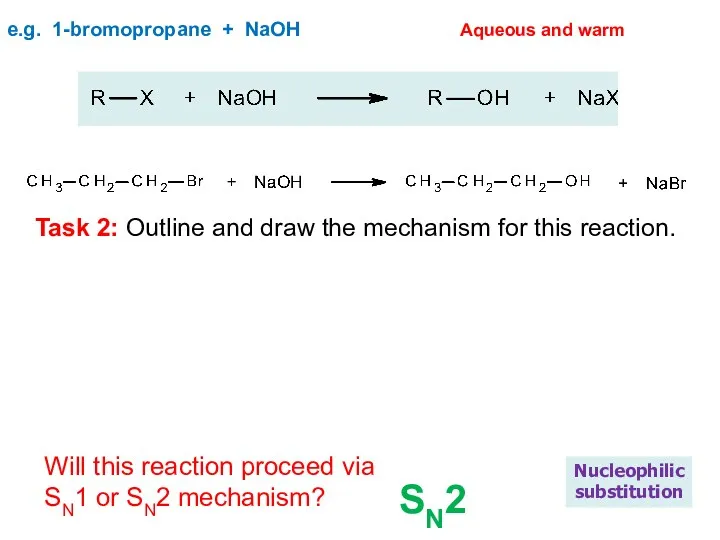
- 19. e.g. 1-bromopropane + NaOH Nucleophilic substitution Aqueous and warm Will this reaction proceed via SN1 or
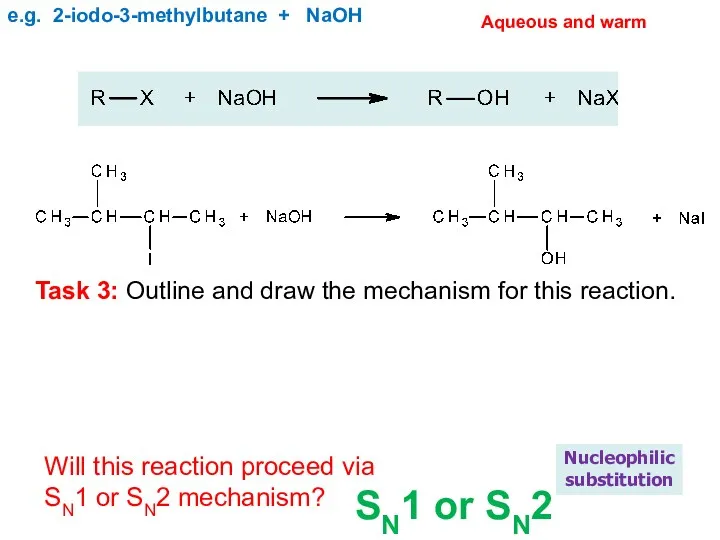
- 20. e.g. 2-iodo-3-methylbutane + NaOH Nucleophilic substitution Aqueous and warm Will this reaction proceed via SN1 or
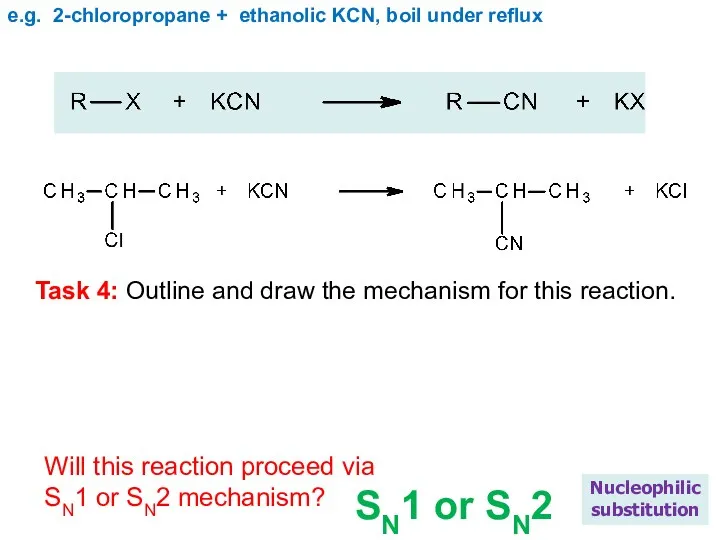
- 21. e.g. 2-chloropropane + ethanolic KCN, boil under reflux Nucleophilic substitution Task 4: Outline and draw the
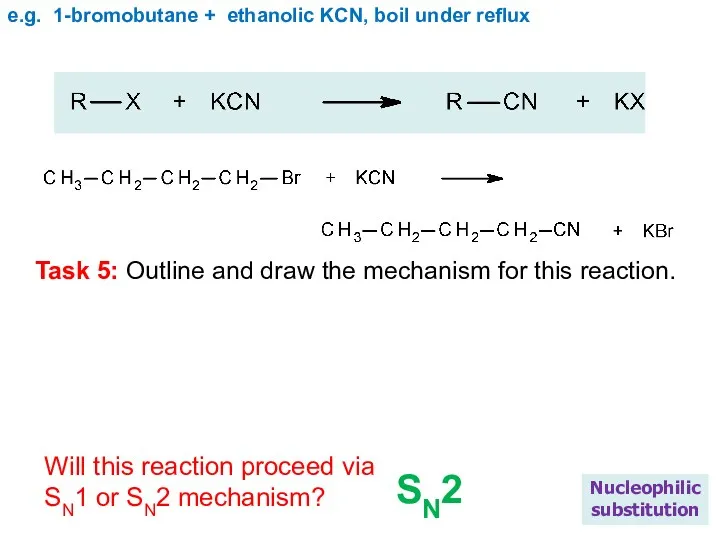
- 22. e.g. 1-bromobutane + ethanolic KCN, boil under reflux Nucleophilic substitution Task 5: Outline and draw the
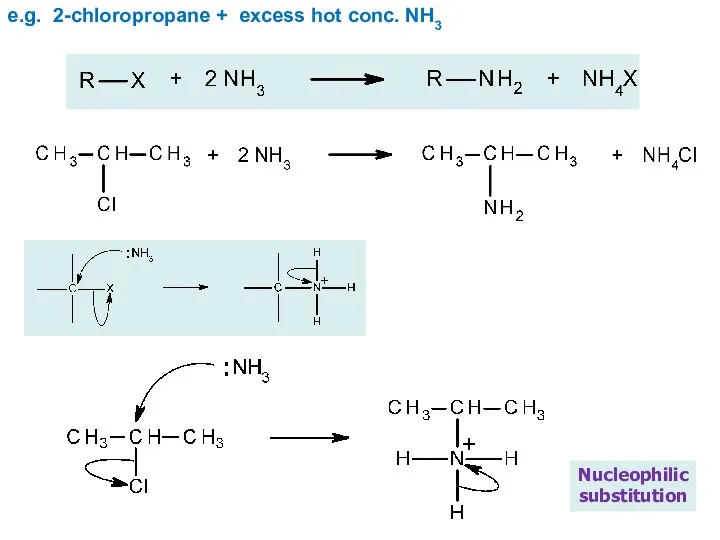
- 23. e.g. 2-chloropropane + excess hot conc. NH3 Nucleophilic substitution
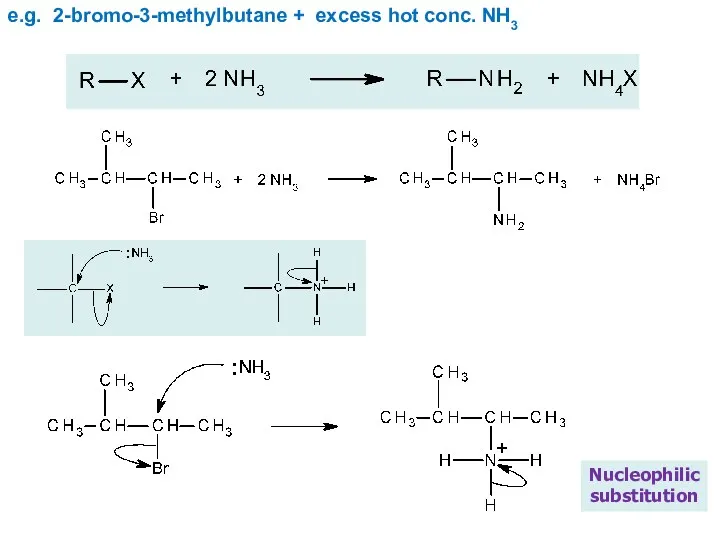
- 24. e.g. 2-bromo-3-methylbutane + excess hot conc. NH3 Nucleophilic substitution
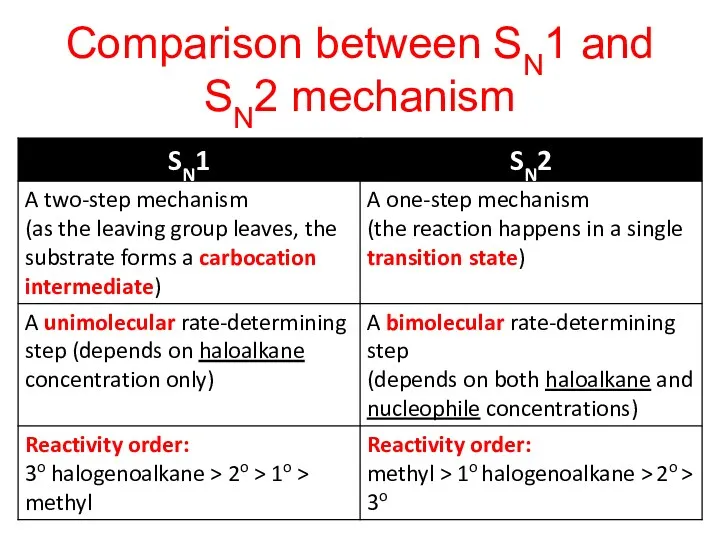
- 25. Comparison between SN1 and SN2 mechanism
- 26. Summary
- 28. Скачать презентацию


 Интеграция ФЭМП в разных образовательных областях
Интеграция ФЭМП в разных образовательных областях Презентация к уроку Шошо
Презентация к уроку Шошо Повторение. Действия с десятичными дробями. Действия с рациональными числами
Повторение. Действия с десятичными дробями. Действия с рациональными числами IstPit
IstPit Разработка урока Озон. Аллотропия кислорода
Разработка урока Озон. Аллотропия кислорода Введение в историю менеджмента
Введение в историю менеджмента Бизнес-план. Производство туалетной бумаги и бумажных полотенец
Бизнес-план. Производство туалетной бумаги и бумажных полотенец Творческий проект по театрализованной деятельности для детей подготовительной группы Театр и дети
Творческий проект по театрализованной деятельности для детей подготовительной группы Театр и дети У меня живет морская свинка!
У меня живет морская свинка! Smart irrigation system
Smart irrigation system Релаксація
Релаксація АО Энергия Холдинг
АО Энергия Холдинг ЭКГ при ИБС
ЭКГ при ИБС Теория эволюции. Откуда берутся новые формы живых систем?
Теория эволюции. Откуда берутся новые формы живых систем? Современные концепции естественнонаучного образования в начальной школе
Современные концепции естественнонаучного образования в начальной школе Игра в формате Своя игра
Игра в формате Своя игра Электромагнитное поле
Электромагнитное поле Моя самопрезентация к конкурсу Учитель Несветая 2013 в номинации Педагогический дебют
Моя самопрезентация к конкурсу Учитель Несветая 2013 в номинации Педагогический дебют Занятия в Сити-Клубе, г. Троицк
Занятия в Сити-Клубе, г. Троицк Развитие непрерывного инклюзивного образования как управленческая задача
Развитие непрерывного инклюзивного образования как управленческая задача Сенсорное развитие детей раннего возраста
Сенсорное развитие детей раннего возраста Мотивация в системе менеджмента
Мотивация в системе менеджмента Сказка - презентация Пых
Сказка - презентация Пых Развитие познавательной активности младших школьников
Развитие познавательной активности младших школьников Проектирование производственного процесса изготовления печатного издания
Проектирование производственного процесса изготовления печатного издания Колокольные звоны на Руси
Колокольные звоны на Руси City Tour Tbilisi 35 lari
City Tour Tbilisi 35 lari Финансовое управление администрации МОГО Ухта
Финансовое управление администрации МОГО Ухта