Содержание
- 2. Factors that affect the rate of corrosion Temperature Oxygen Humidity Chemical Salts Chemicals and airborne gases
- 3. How to avoid (or control) Corrosion? Material Selection! Remember – environment key. Look at potential pH
- 4. How to avoid (or control) Corrosion? Pitting/Crevice: Watch for stagnate water/ electrolyte. Use gaskets Use good
- 5. How to avoid (or control) Corrosion? Consider organic coating (paint, ceramic, chrome, etc.) – DANGER IF
- 6. Methodes To Control Corrosion Design of structures Material selection Cathodic Protection Reduce the activity of the
- 7. DESIGN OF STRUCTURES Avoid sharp corners Complete draining of vessels Avoid sudden changes in section Avoid
- 8. Design Do’s & Don’ts Wall thickness – allowance to accommodate for corrosion effect. Avoid excessive mechanical
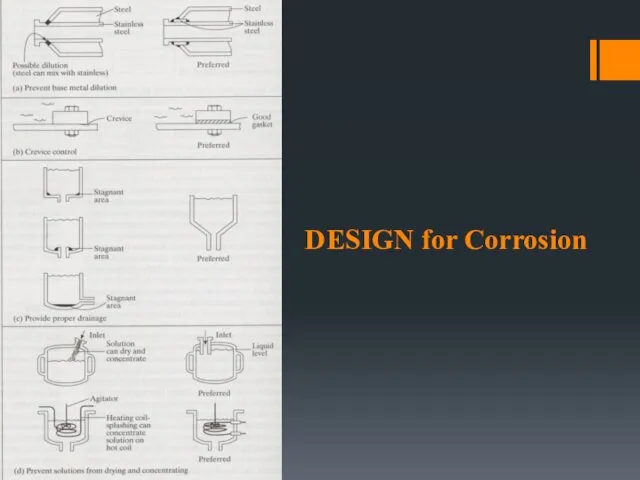
- 9. Avoid sharp corners – paint tends to be thinner at sharp corners and often starts to
- 10. DESIGN for Corrosion
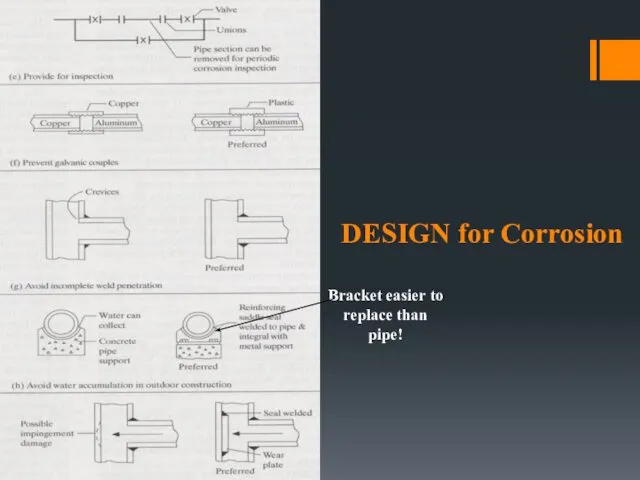
- 11. DESIGN for Corrosion Bracket easier to replace than pipe!
- 14. Material Selection
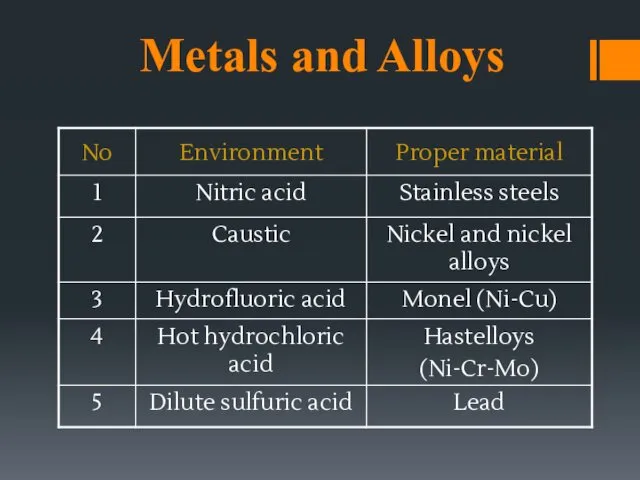
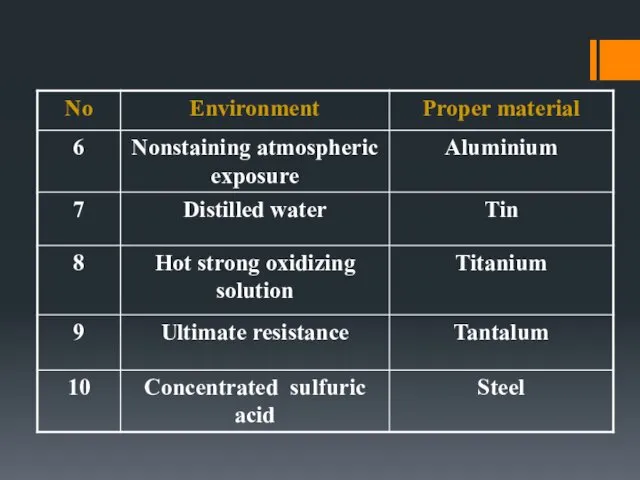
- 15. MATERIAL SELECTION (selection of proper material for a particular corrosive service) Metallic : [metal and alloy]
- 16. IMPROVEMENTS OF MATERIALS Purification of metals: Al , Zr Making more noble, e.g. Pt in Ti
- 17. Material Selection - Galvanic Series [Seawater at 77⁰ F.] Magnesium Zinc Aluminum Mild Steel Cast Iron
- 18. Combining dissimilar metals can result in corrosion. It may be very rapid or it may be
- 19. Metals and Alloys
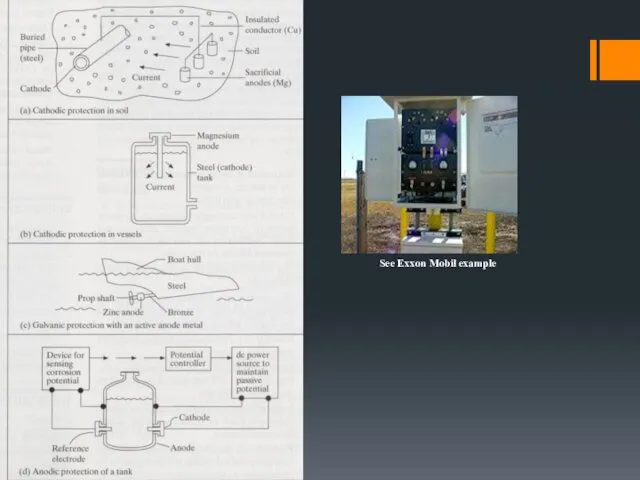
- 21. Cathodic Protection (CP) Cathodic protection (CP) is a technique to control the corrosion of a metal
- 22. CATHODIC & ANODIC PROTECTION Cathodic protection: Make the structure more cathodic by Use of sacrificial anodes
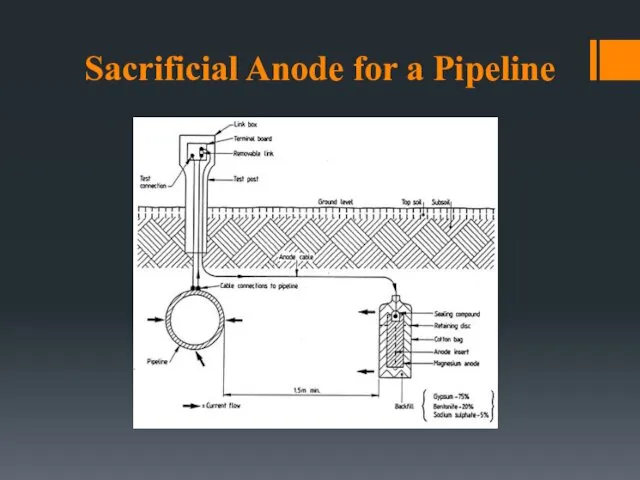
- 23. Sacrificial Anodes Galvanization of Steel Dip steel sheet in molten zinc. Get a pretty thin coating.
- 24. Another Example Zinc is attached to the steel hull of the vessel. Attachment points
- 26. Sacrificial Anode for a Pipeline
- 27. Aluminium anodes mounted on a steel jacket structure – using galvanic corrosion for corrosion control! Called
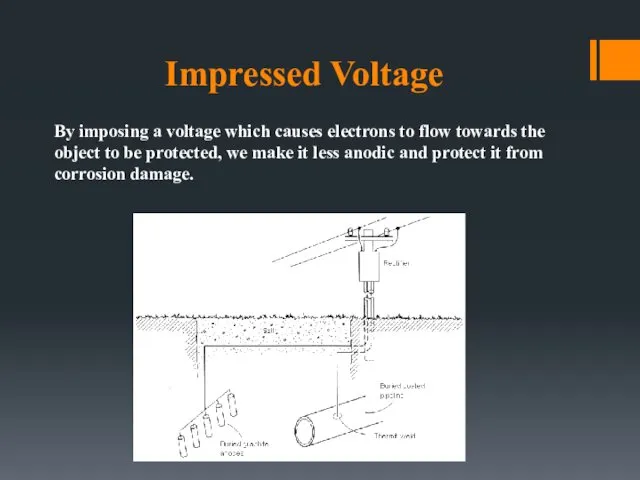
- 28. Impressed Voltage By imposing a voltage which causes electrons to flow towards the object to be
- 30. See Exxon Mobil example
- 31. Polarization This is an effect which reduces the actual chemical potential driving of the cell. If
- 32. Passivation of the anode We have two examples already. Stainless and aluminum. A thin oxide layer
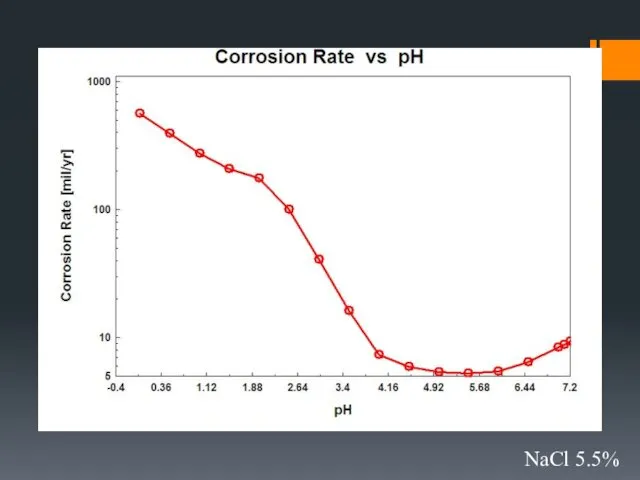
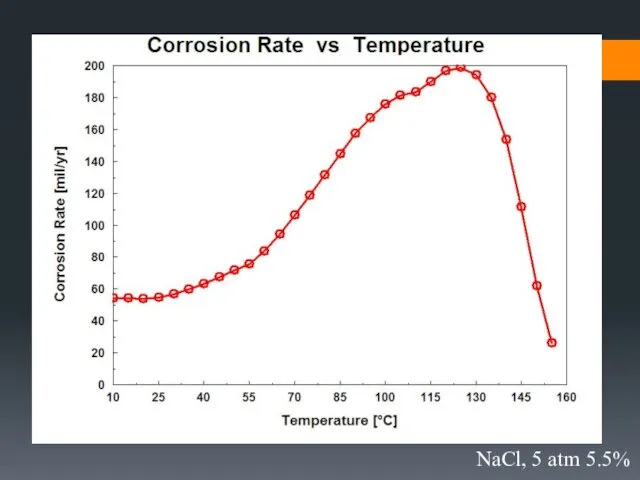
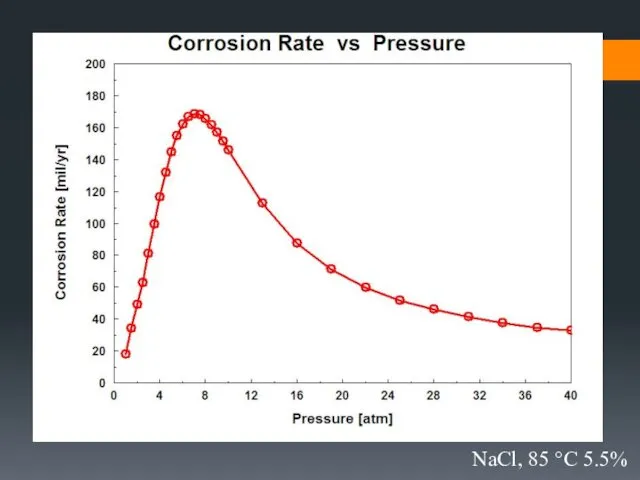
- 33. Effect of environmental parameters on the rate of corrosion
- 34. 5.5% NaCl
- 35. 5.5% NaCl, 5 atm
- 36. 5.5% NaCl, 85 °C
- 37. 5.5% NaCl, 10 °C, 15 atm
- 38. Alternation of Environment Lower temperature and velocity Remove oxygen/oxidizers Change concentration Add Inhibitors Adsorption type, e.g.
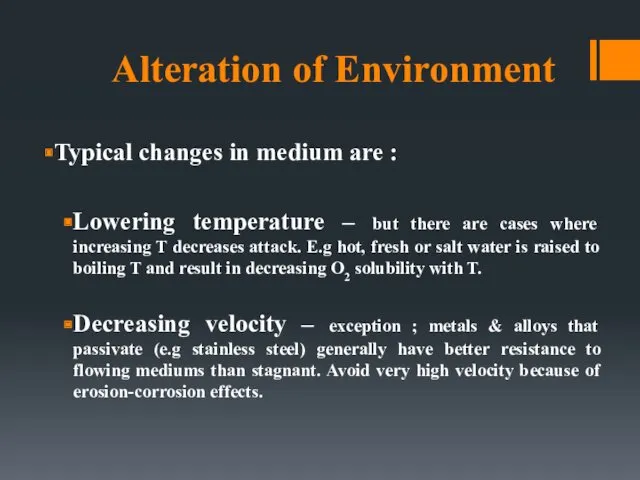
- 39. Alteration of Environment Typical changes in medium are : Lowering temperature – but there are cases
- 40. Removing oxygen or oxidizers – e.g vacuum treatment, inert gas sparging, or thru the use of
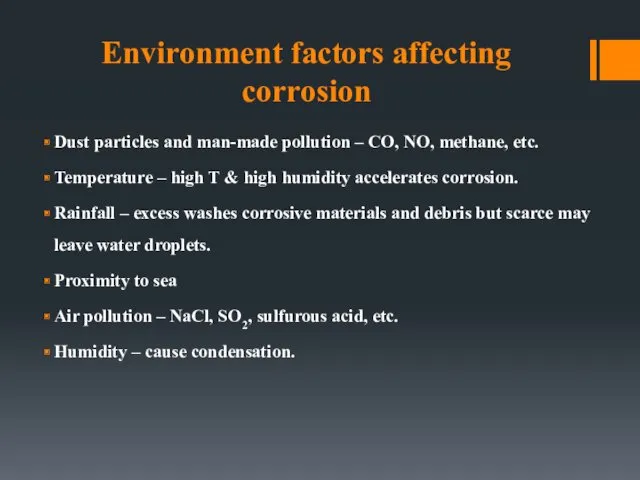
- 41. Environment factors affecting corrosion Dust particles and man-made pollution – CO, NO, methane, etc. Temperature –
- 42. Inhibitors Inhibitors are materials that may be injected into the system . They plate out on
- 43. They are sometimes injected into the water stream that may be used for the surface preparation
- 45. Скачать презентацию











![Material Selection - Galvanic Series [Seawater at 77⁰ F.] Magnesium](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/3519/slide-16.jpg)
















 Педагогика: анализ основных теоретических аспектов
Педагогика: анализ основных теоретических аспектов Любимый уголок моей республики
Любимый уголок моей республики Электроизмерительные приборы. 8 класс
Электроизмерительные приборы. 8 класс Highway construction
Highway construction Завершальний етап ліквідації поліомієліту. Перехід до бОПВ
Завершальний етап ліквідації поліомієліту. Перехід до бОПВ Профессия - стропальщик
Профессия - стропальщик Деревенское подворье: Стиль кантри в ландшафтном дизайне.
Деревенское подворье: Стиль кантри в ландшафтном дизайне. Аналоговый датчик линии
Аналоговый датчик линии Проект Фабрика загадок Диск
Проект Фабрика загадок Диск Холецистит у детей
Холецистит у детей Сигналы ограждения на железнодорожном транспорте
Сигналы ограждения на железнодорожном транспорте Столяр
Столяр Василий Андреевич Жуковский (1783-1852)
Василий Андреевич Жуковский (1783-1852) Родительское собрание на тему: Безопасность детей в Интернете
Родительское собрание на тему: Безопасность детей в Интернете Право на образование
Право на образование Учет взносов и отчислений на обязательное социальное медицинское страхование (ОСМС)
Учет взносов и отчислений на обязательное социальное медицинское страхование (ОСМС) Магия в первобытной культуре. Виды, приемы, механизмы
Магия в первобытной культуре. Виды, приемы, механизмы Построение образовательного пространства детей младшего дошкольного возраста в игровой деятельности
Построение образовательного пространства детей младшего дошкольного возраста в игровой деятельности Знаки препинания в сложном предложении
Знаки препинания в сложном предложении Фрезерование. Выбор торцевых фрез
Фрезерование. Выбор торцевых фрез Скрининговые тесты в диагностике состояния системы гемостаза
Скрининговые тесты в диагностике состояния системы гемостаза ГК Стронг. Гипсостружечная плита
ГК Стронг. Гипсостружечная плита Словарь-презентация терминов по морфологии. Именные части речи
Словарь-презентация терминов по морфологии. Именные части речи Шаблон презентации проекта
Шаблон презентации проекта Стихи собственного сочинения учащихся 3 класса Диск
Стихи собственного сочинения учащихся 3 класса Диск Талисманы Олимпиад
Талисманы Олимпиад Формирование чувства цвета у детей дошкольного возраста
Формирование чувства цвета у детей дошкольного возраста Климат Южной Америки.
Климат Южной Америки.