Содержание
- 2. Vocabulary electron arrangement alkali metals (electron configuration) alkaline-earth metals shell transition metals outer shell halchogens valence
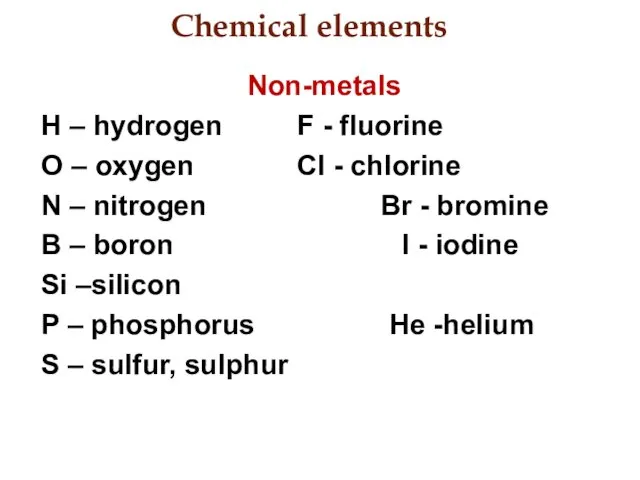
- 3. Chemical elements Non-metals H – hydrogen F - fluorine O – oxygen Cl - chlorine N
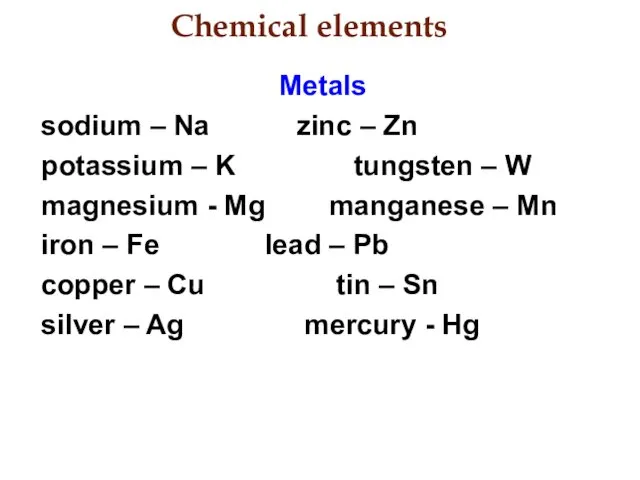
- 4. Chemical elements Metals sodium – Na zinc – Zn potassium – K tungsten – W magnesium
- 5. The Periodic Table of Chemical Elements What is the Periodic table ? What information is obtained
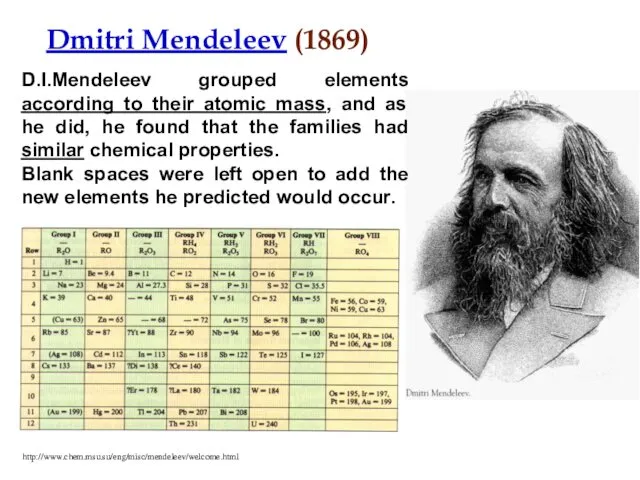
- 6. Dmitri Mendeleev (1869) D.I.Mendeleev grouped elements according to their atomic mass, and as he did, he
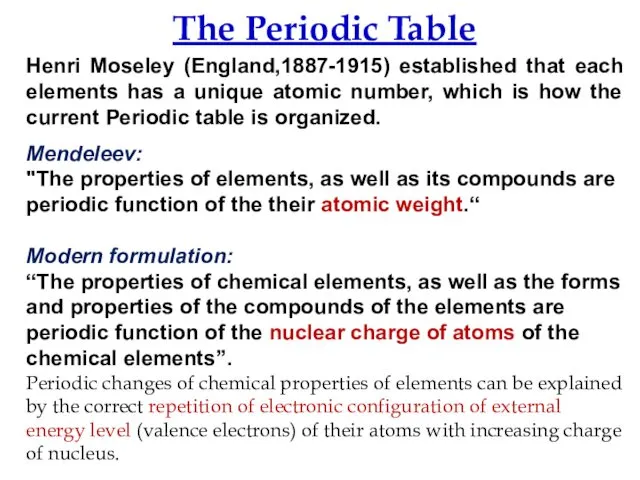
- 7. The Periodic Table Henri Moseley (England,1887-1915) established that each elements has a unique atomic number, which
- 8. The latest version The International Union of Pure and Applied Chemistry has announced the names of
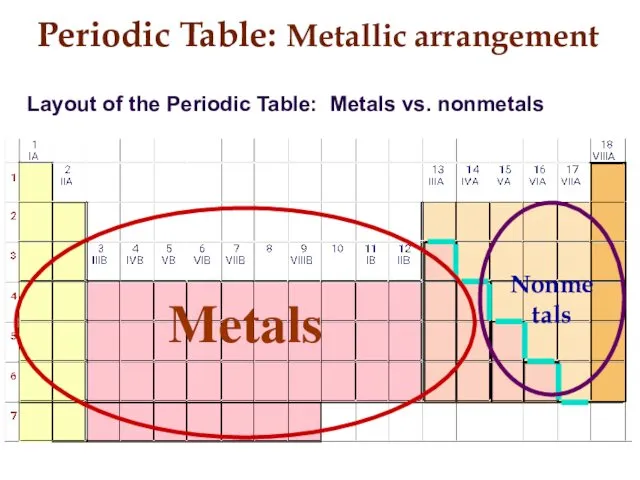
- 9. Periodic Table: Metallic arrangement Layout of the Periodic Table: Metals vs. nonmetals Metals Nonmetals
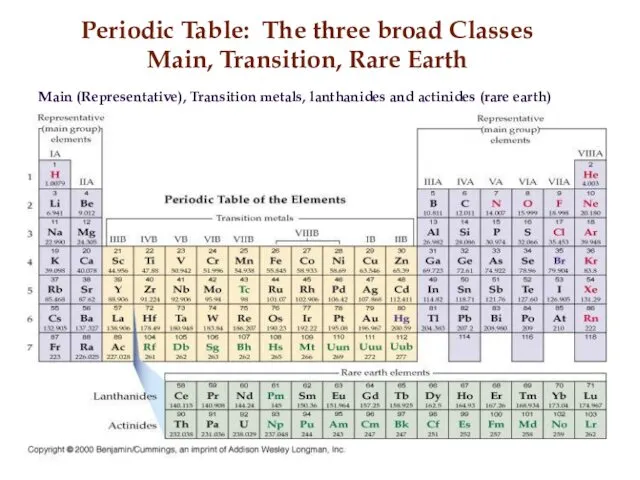
- 10. Periodic Table: The three broad Classes Main, Transition, Rare Earth Main (Representative), Transition metals, lanthanides and
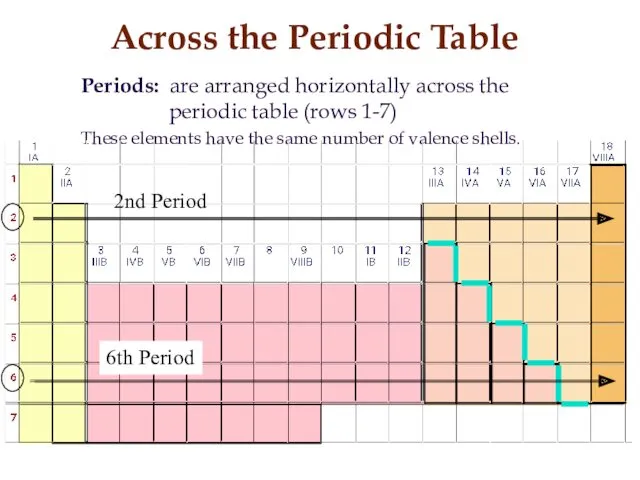
- 11. Across the Periodic Table Periods: are arranged horizontally across the periodic table (rows 1-7) These elements
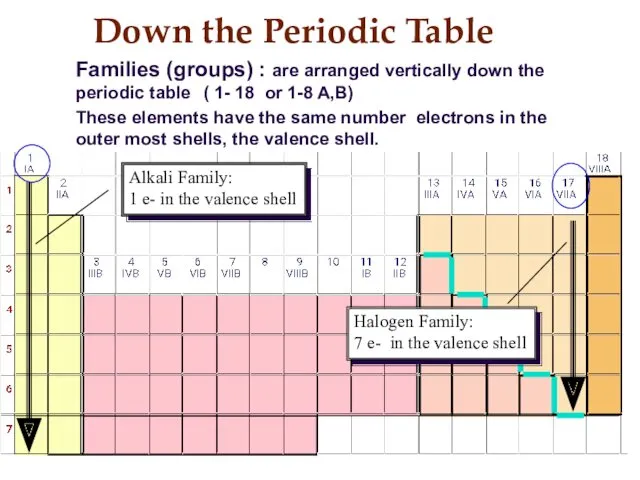
- 12. Down the Periodic Table Families (groups) : are arranged vertically down the periodic table ( 1-
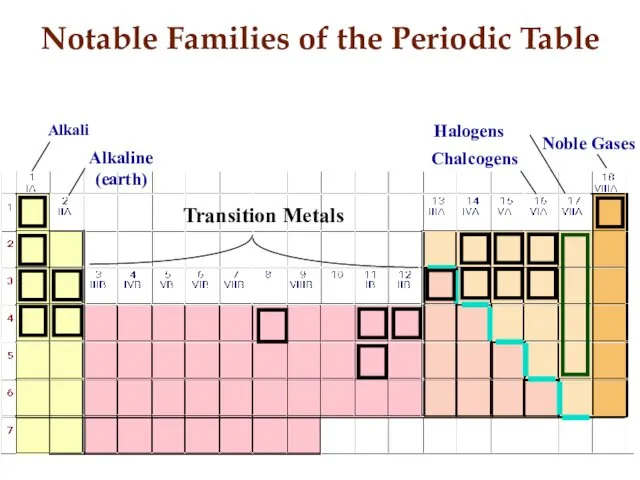
- 13. Notable Families of the Periodic Table
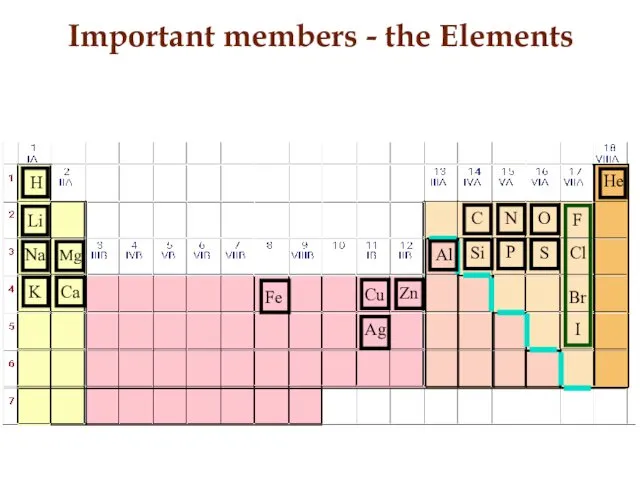
- 14. Important members - the Elements
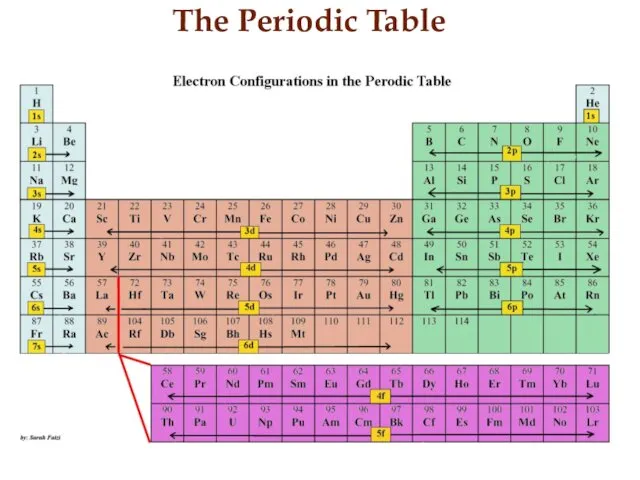
- 15. The Periodic Table
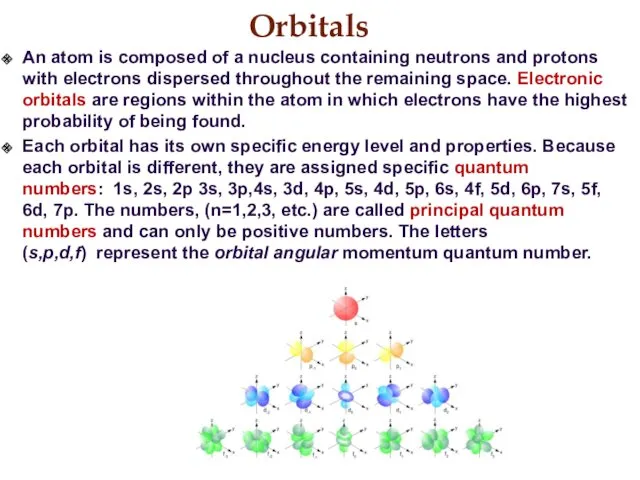
- 16. Orbitals An atom is composed of a nucleus containing neutrons and protons with electrons dispersed throughout
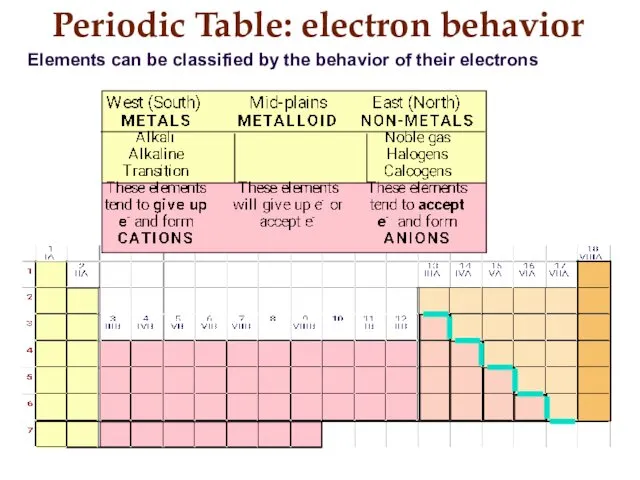
- 17. Periodic Table: electron behavior Elements can be classified by the behavior of their electrons
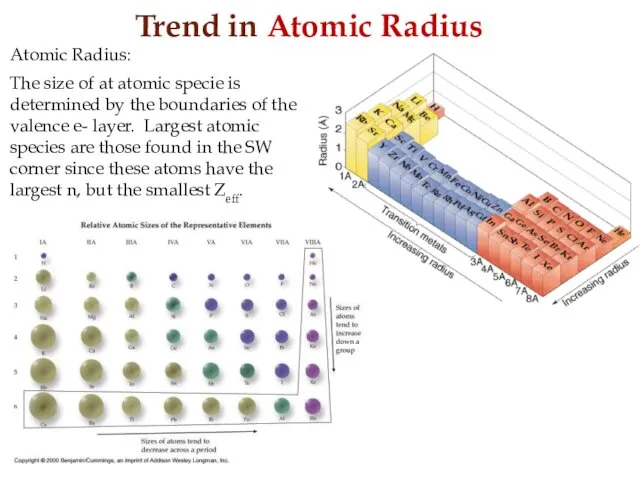
- 18. Trend in Atomic Radius Atomic Radius: The size of at atomic specie is determined by the
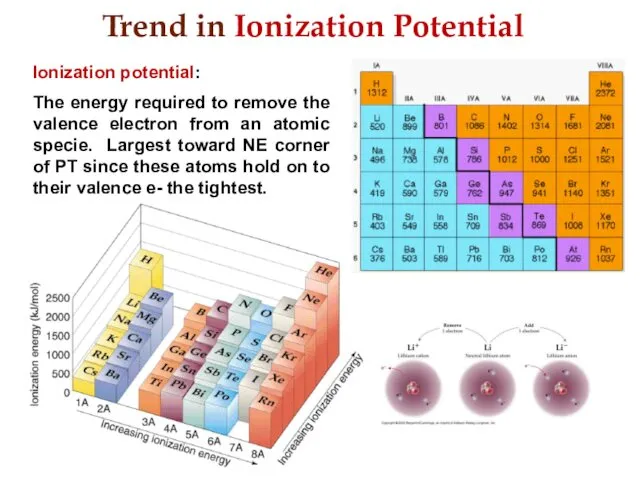
- 19. Trend in Ionization Potential Ionization potential: The energy required to remove the valence electron from an
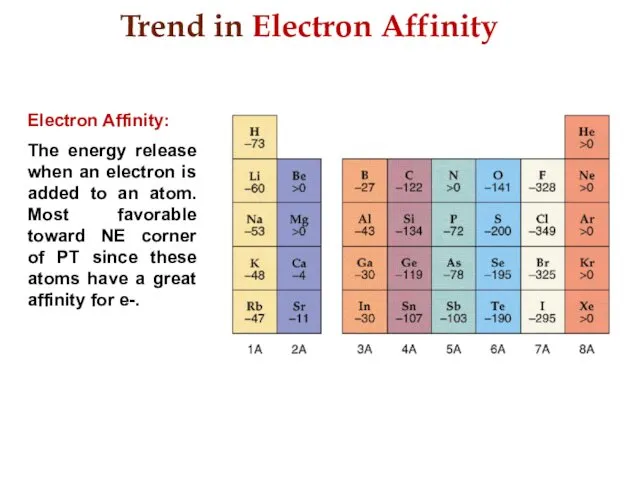
- 20. Trend in Electron Affinity Electron Affinity: The energy release when an electron is added to an
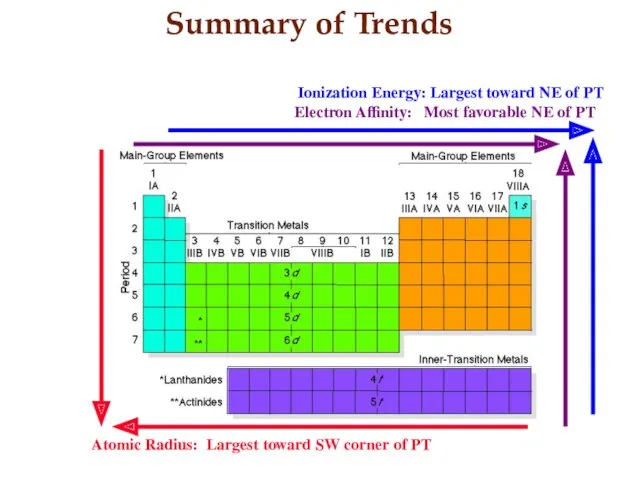
- 21. Summary of Trends Atomic Radius: Largest toward SW corner of PT Ionization Energy: Largest toward NE
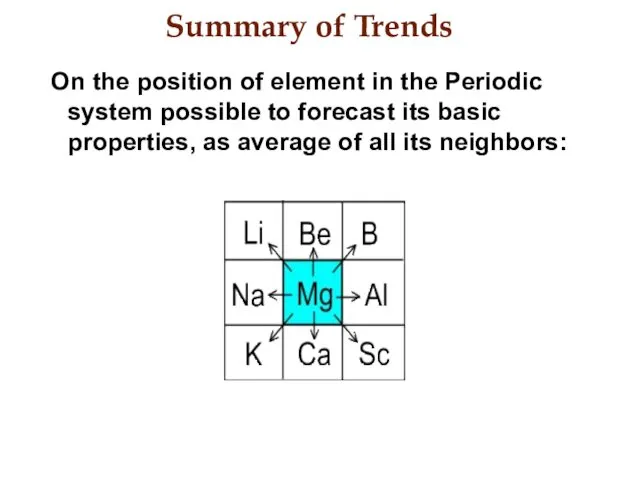
- 22. Summary of Trends On the position of element in the Periodic system possible to forecast its
- 23. Summary Periodic Table: Map of the Building blocks of matter Types: Metals and Nonmetals Families: Elements
- 24. Hydrogen The hydrogen square sits atop Family AI, but it is not a member of that
- 25. Alkali metals The alkali family is found in the first column of the Periodic table. Atoms
- 26. Alkaline Earth Metals Alkaline earth metals include magnesium and calcium, among others. They have 2 valence
- 27. Transition Metals Transition Elements include those elements in the B families. Transition elements have 1 or
- 28. Boron Family The Boron Family is named after the first element in the family. Atoms in
- 29. Carbon Family Atoms of this family have 4 valence electrons. This family includes non-metals (carbon and
- 30. Nitrogen Family The nitrogen family is named after the element that makes up 78% of our
- 31. Oxygen Family Atoms of this family have 6 valence electrons. Most elements in this family share
- 32. Halogen Family Halogens have 7 valence electrons, which explains why they are the most active non-metals.
- 33. Noble Gases Noble gases are colorless gases that are extremely un-reactive. They are inactive because their
- 34. Rare Earth Elements The thirty rare earth elements are composed of the lanthanide and actinide series.
- 36. Скачать презентацию



 Изотерапия, как одна из граней арттерапии.
Изотерапия, как одна из граней арттерапии. Раствор. Типы растворов. Способы выражения концентрации растворов. Теория электролитической диссоциации
Раствор. Типы растворов. Способы выражения концентрации растворов. Теория электролитической диссоциации Креативное программирование. Погружение в мир программирования и создание креативных проектов
Креативное программирование. Погружение в мир программирования и создание креативных проектов 275-летие со дня рождения русского полководца М.И. Кутузова (1745-1813). НТБ Люблинское
275-летие со дня рождения русского полководца М.И. Кутузова (1745-1813). НТБ Люблинское Развитие географических знаний о Земле. 6 кл.
Развитие географических знаний о Земле. 6 кл. Проект Виртуальный музей
Проект Виртуальный музей Расчёт блока вакуумной перегонки мазута, производительностью 2700000 т/год
Расчёт блока вакуумной перегонки мазута, производительностью 2700000 т/год Схема выдачи мощности Новоазовской ВЭС
Схема выдачи мощности Новоазовской ВЭС презентация к уроку Мы изучаем Японию
презентация к уроку Мы изучаем Японию Електробезпека
Електробезпека Механизированные способы добычи нефти
Механизированные способы добычи нефти Классификация химических реакций
Классификация химических реакций Старинная ярмарка.
Старинная ярмарка. Сера. Презентация к уроку химии в 9 классе.
Сера. Презентация к уроку химии в 9 классе. Оплодотворение
Оплодотворение Схема поверхностных течений
Схема поверхностных течений Проект Как погладить ёжика?
Проект Как погладить ёжика? Titania SlidesCarnival
Titania SlidesCarnival презентация проекта Встреча с будущим Диск
презентация проекта Встреча с будущим Диск Проводники, непроводники и полупроводники электричества
Проводники, непроводники и полупроводники электричества Международный женский день
Международный женский день урок с позиции здоровьесбережения
урок с позиции здоровьесбережения Карбоновые кислоты
Карбоновые кислоты презентация кружка оригами
презентация кружка оригами Духовная культура общества
Духовная культура общества Характеристики трещиностойкости фибробетона
Характеристики трещиностойкости фибробетона Отчет о прохождении учебной практики по профессиональному модулю
Отчет о прохождении учебной практики по профессиональному модулю Вес тела. Невесомость
Вес тела. Невесомость