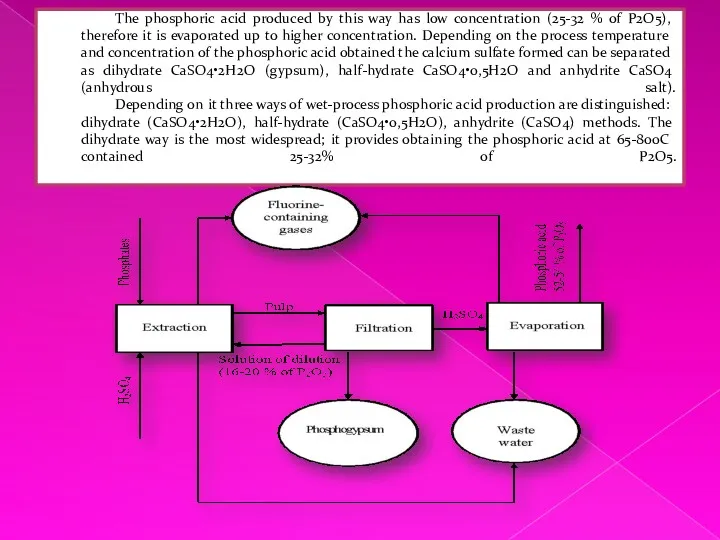
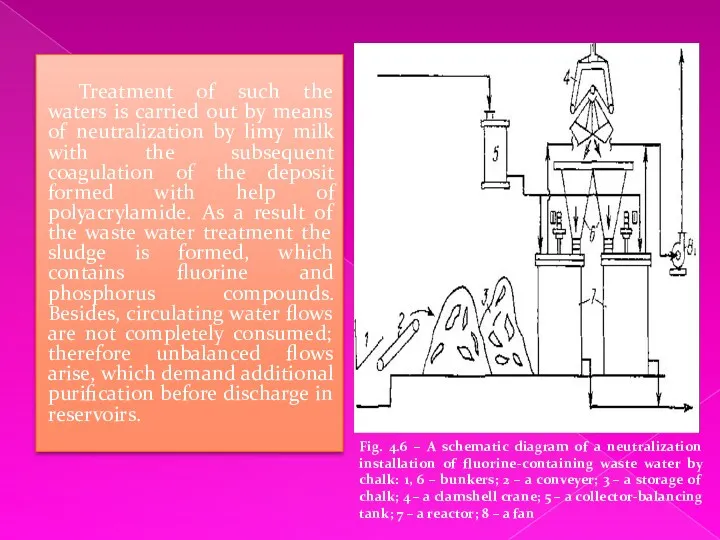
Treatment of such the waters is carried out by means of
neutralization by limy milk with the subsequent coagulation of the deposit formed with help of polyacrylamide. As a result of the waste water treatment the sludge is formed, which contains fluorine and phosphorus compounds. Besides, circulating water flows are not completely consumed; therefore unbalanced flows arise, which demand additional purification before discharge in reservoirs.
Fig. 4.6 – A schematic diagram of a neutralization installation of fluorine-containing waste water by chalk: 1, 6 – bunkers; 2 – a conveyer; 3 – a storage of chalk; 4 – a clamshell crane; 5 – a collector-balancing tank; 7 – a reactor; 8 – a fan





 Поведение на льду
Поведение на льду Вызов
Вызов Микрозаймы. Плюсы и минусы. 9 класс
Микрозаймы. Плюсы и минусы. 9 класс Методическая разработка урока.
Методическая разработка урока. Презентация по теме Синтетические моющие средства
Презентация по теме Синтетические моющие средства Растим патриотов Самары проект От истоков до современности
Растим патриотов Самары проект От истоков до современности English lexicology
English lexicology Виды литейных дефектов. Классификации и методы их устранения
Виды литейных дефектов. Классификации и методы их устранения Солнце, состав и внутреннее строение. 10-11 класс
Солнце, состав и внутреннее строение. 10-11 класс Презентация МБОУ ДОД Детская музыкальная школа г. Дзержинский Московской области
Презентация МБОУ ДОД Детская музыкальная школа г. Дзержинский Московской области Простые и сложные вещества
Простые и сложные вещества Виробництво, зберігання та використання комбікормів (лекція 16)
Виробництво, зберігання та використання комбікормів (лекція 16) Формирование универсальных учебных действий
Формирование универсальных учебных действий Товарная политика. Товар и его характеристики
Товарная политика. Товар и его характеристики Обращение. Знаки препинания при обращении
Обращение. Знаки препинания при обращении Нетрадиционные технологии как средство коррекции эмоциональных нарушений у детей дошкольного возраста
Нетрадиционные технологии как средство коррекции эмоциональных нарушений у детей дошкольного возраста Қазіргі дәстүрлі емес діни қозғалыстар және культтар. (Дәріс 7)
Қазіргі дәстүрлі емес діни қозғалыстар және культтар. (Дәріс 7) Контроль параметров, регулировка и поиск неисправностей при установки и стабилизации углов колёс автомобиля ВАЗ-2110
Контроль параметров, регулировка и поиск неисправностей при установки и стабилизации углов колёс автомобиля ВАЗ-2110 Детский травматизм. Особенности травм у детей
Детский травматизм. Особенности травм у детей Megaline
Megaline Времена года
Времена года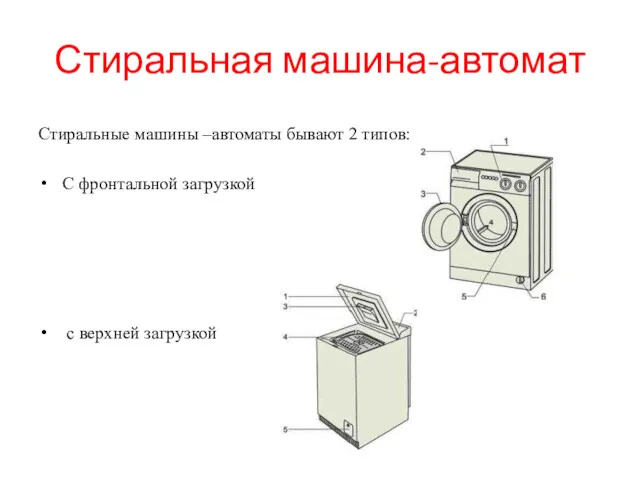 Стиральная машина-автомат
Стиральная машина-автомат Сборник стихов Н.А. Некрасова
Сборник стихов Н.А. Некрасова Презентация Памятник примирения
Презентация Памятник примирения Презентация Трагедия Беслана. 10 лет спустя.
Презентация Трагедия Беслана. 10 лет спустя. Социальная реклама. Разработка социальной кампании по защите окружающей среды от мусора
Социальная реклама. Разработка социальной кампании по защите окружающей среды от мусора Реализация задач ФГТ в образовательной области Безопасность
Реализация задач ФГТ в образовательной области Безопасность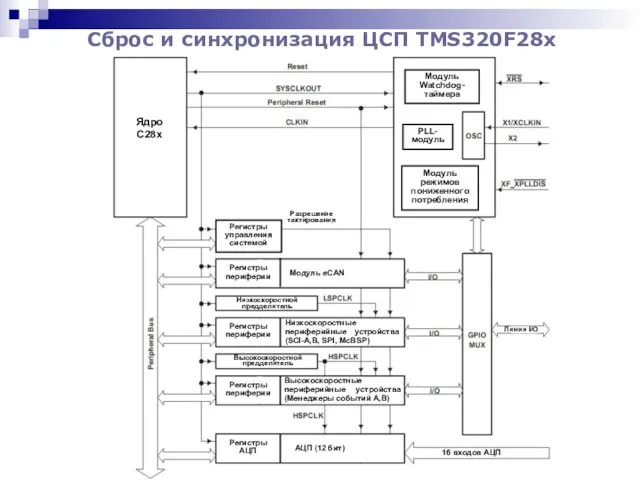 Сброс и синхронизация ЦСП TMS320F28x
Сброс и синхронизация ЦСП TMS320F28x