Good Pharmacovigilance Practice (GVP) of the Eurasian Economic Union, approved by
the Decision of the Council of the Eurasian Economic Commission No. 87 of 03/11/2016 (entered into force on 06.05.2017)
Federal Law No. 61-FZ of April 12, 2010, About the Circulation of Medicinal Products (Chapter 13)
Law of the Ministry of Health of Russia No. 682n of 09/07/2016 “On approval of the form of the document containing the results of monitoring the effectiveness and safety of medicinal products for medical use, carried out by the holder or owner of the registration certificate of the medicinal products or an authorized legal entity”
Order of Roszdravnadzor No. 1071 of February 15, 2017 “On Approval of the Procedure for the Implementation of Pharmacovigilance”
Order of the Ministry of Health of Russia dated 07.09.2015 No. 5539 “On approval of the procedure for the implementation of selective quality control of drugs for medical use”
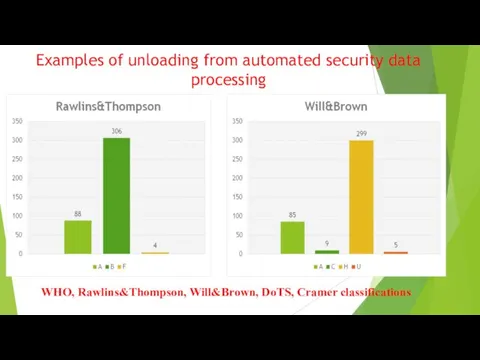
The Main Legal Acts in PV








 Декларация прав ребенка. Конвенция о правах ребенка
Декларация прав ребенка. Конвенция о правах ребенка Права потребителей
Права потребителей Организация и проведение экспертизы по делам об уголовной ответственности лиц медицинского персонала
Организация и проведение экспертизы по делам об уголовной ответственности лиц медицинского персонала Земельные правоотношения
Земельные правоотношения Преступления против половой неприкосновенности и половой свободы личности
Преступления против половой неприкосновенности и половой свободы личности Гражданин Российской Федерации
Гражданин Российской Федерации Учет поступления товаров. (Приход товаров)
Учет поступления товаров. (Приход товаров) Юридическая поддержка пациентов
Юридическая поддержка пациентов Правовой статус Президента РФ и его администрации в системе исполнительной власти
Правовой статус Президента РФ и его администрации в системе исполнительной власти Травматизм та професійні захворюваання в галузі. Розслідування нещасних випадків
Травматизм та професійні захворюваання в галузі. Розслідування нещасних випадків Правовые основы охраны труда
Правовые основы охраны труда Большие права маленького ребенка
Большие права маленького ребенка Конституция Российской Федерации 12 декабря 1993 года
Конституция Российской Федерации 12 декабря 1993 года Основные виды партийных систем в зарубежных странах
Основные виды партийных систем в зарубежных странах Правовые основы охраны труда в организации
Правовые основы охраны труда в организации Права, обязанности и ответственность военнослужащих. Размещение военнослужащих. Распределение времени. (Тема 2)
Права, обязанности и ответственность военнослужащих. Размещение военнослужащих. Распределение времени. (Тема 2) Навыки юриста. Тема 4
Навыки юриста. Тема 4 Понятие недвижимости. Регулирование рынка недвижимости
Понятие недвижимости. Регулирование рынка недвижимости Суб’єкт кримінального правопорушення
Суб’єкт кримінального правопорушення Основы конституционного права Соединенного Королевства Великобритании и Северной Ирландии
Основы конституционного права Соединенного Королевства Великобритании и Северной Ирландии Правовая система Новой Зеландии
Правовая система Новой Зеландии Парламент Республики Казахстан
Парламент Республики Казахстан Организационные формы предпринимательства в России
Организационные формы предпринимательства в России Теория государства и права. Происхождение государства и права. (Тема 2)
Теория государства и права. Происхождение государства и права. (Тема 2) Международная и региональная стандартизация
Международная и региональная стандартизация Нормативно-правовое обеспечение
Нормативно-правовое обеспечение Исполнение наказаний, не связанных с изоляцией осужденных от общества (НСИО). Тема № 6
Исполнение наказаний, не связанных с изоляцией осужденных от общества (НСИО). Тема № 6 Військовий облік військовозабов'язаних національної комісії, що здійснює державне регулювання у сфері ринків фінансових послуг
Військовий облік військовозабов'язаних національної комісії, що здійснює державне регулювання у сфері ринків фінансових послуг