Содержание
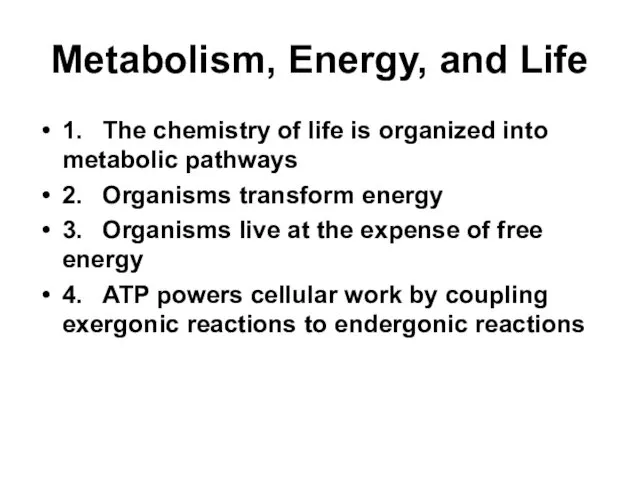
- 2. Metabolism, Energy, and Life 1. The chemistry of life is organized into metabolic pathways 2. Organisms
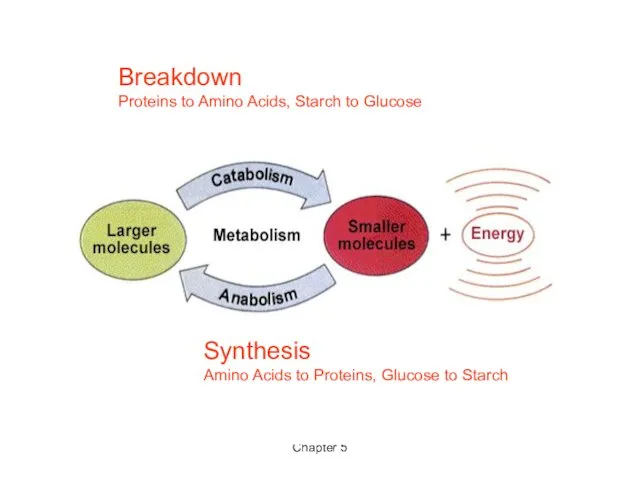
- 3. Chapter 5 Breakdown Proteins to Amino Acids, Starch to Glucose Synthesis Amino Acids to Proteins, Glucose
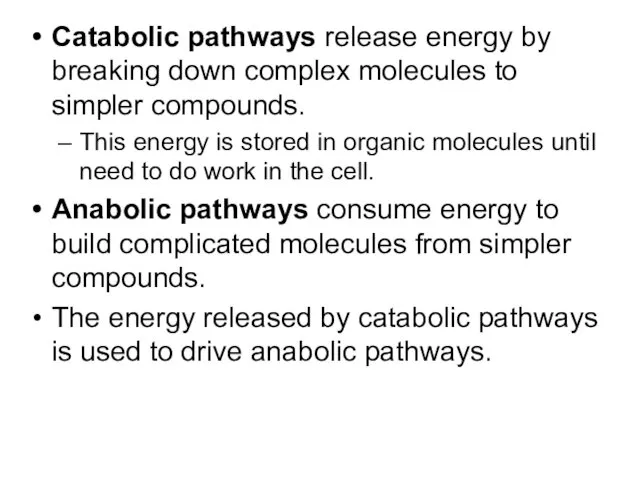
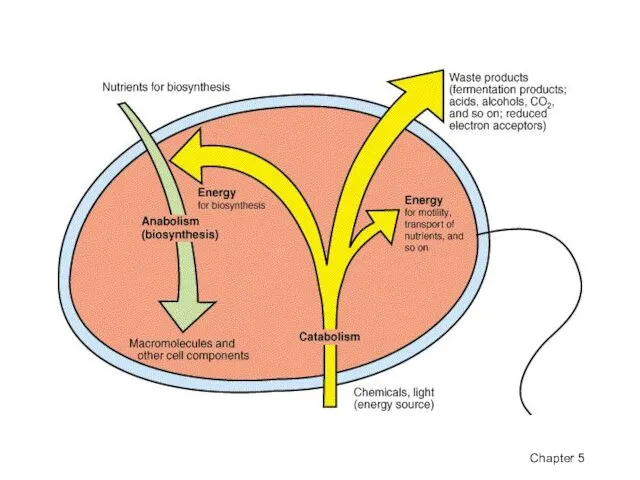
- 4. Catabolic pathways release energy by breaking down complex molecules to simpler compounds. This energy is stored
- 5. Chapter 5
- 6. Organisms transform energy Energy is the capacity to do work - to move matter against opposing
- 7. Organisms live at the expense of free energy Spontaneous processes can occur without outside help. The
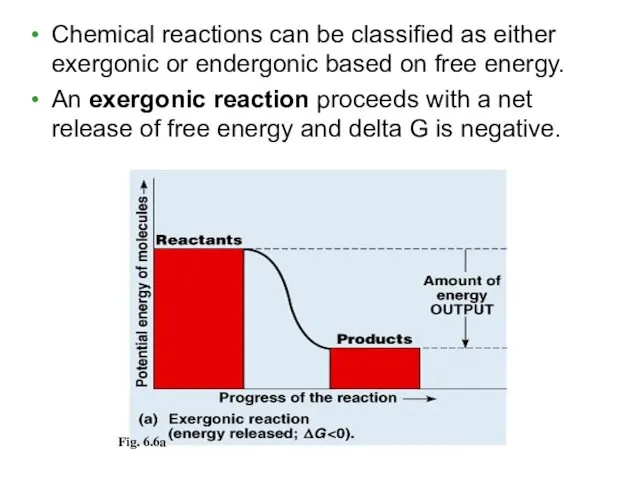
- 8. Chemical reactions can be classified as either exergonic or endergonic based on free energy. An exergonic
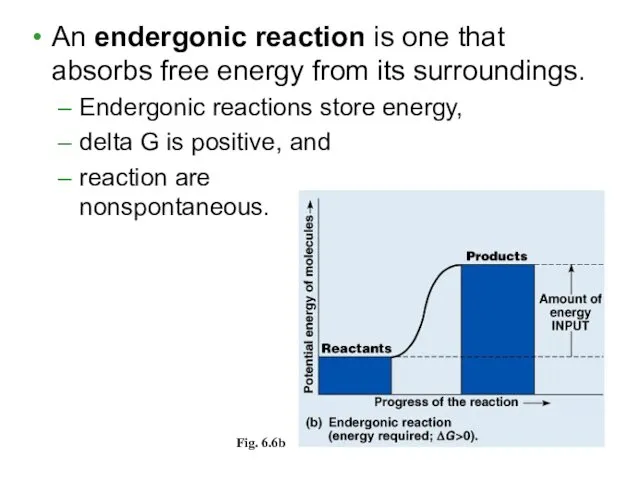
- 9. An endergonic reaction is one that absorbs free energy from its surroundings. Endergonic reactions store energy,
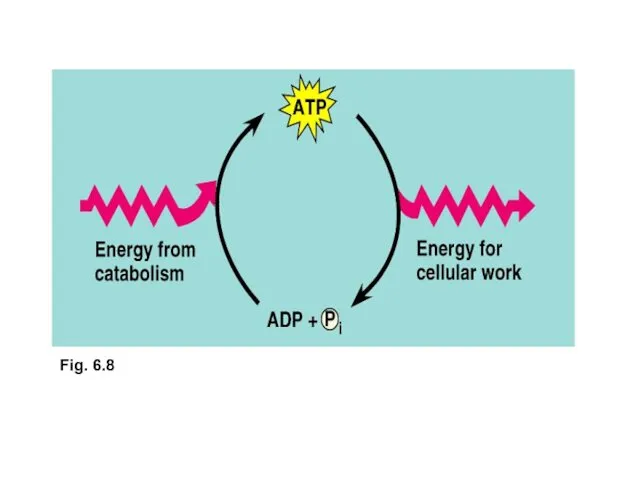
- 10. ATP ATP powers cellular work A cell does three main kinds of work: Mechanical work, contraction
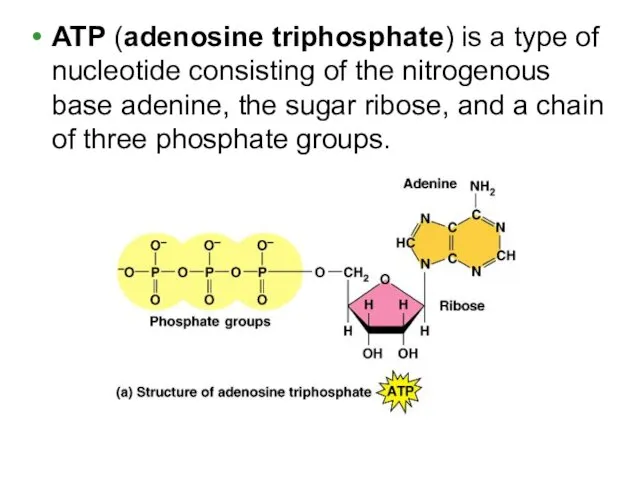
- 11. ATP (adenosine triphosphate) is a type of nucleotide consisting of the nitrogenous base adenine, the sugar
- 12. Fig. 6.8
- 14. Скачать презентацию


 Соединения костей
Соединения костей Мышцы шеи
Мышцы шеи Паразитические плоские черви
Паразитические плоские черви Разнообразие животных
Разнообразие животных Биотехнология в животноводстве
Биотехнология в животноводстве Виды комнатных растений
Виды комнатных растений Предмет ботаники. Растительная клетка. Протопласт и его производные
Предмет ботаники. Растительная клетка. Протопласт и его производные Міграція тварин. Метелики
Міграція тварин. Метелики Гистологическое строение печени
Гистологическое строение печени Ақуыз және Белок
Ақуыз және Белок Структура і функції ліпідів
Структура і функції ліпідів Черепные нервы: топографическая и клиническая анатомия
Черепные нервы: топографическая и клиническая анатомия Витамин A
Витамин A Методы генетики человека
Методы генетики человека В гости к весне. 4 класс
В гости к весне. 4 класс Атлас млекопитающих Москвы и Подмосковья
Атлас млекопитающих Москвы и Подмосковья Направления эволюции
Направления эволюции Анатомия и физиология больших пищеварительных желез
Анатомия и физиология больших пищеварительных желез Майстерність маскування
Майстерність маскування Функциональное состояние в структуре поведения
Функциональное состояние в структуре поведения Косточковые плоды
Косточковые плоды Основы медицинской паразитологии. Взаимодействия в системе паразит-хозяин на уровне особей. (часть 1)
Основы медицинской паразитологии. Взаимодействия в системе паразит-хозяин на уровне особей. (часть 1) Cell Membrane Fluid Mosaic
Cell Membrane Fluid Mosaic Прудовик обыкновенный
Прудовик обыкновенный Простейшие, царство одноклеточных или колониальных эукариот
Простейшие, царство одноклеточных или колониальных эукариот Внутреннее строение млекопитающих
Внутреннее строение млекопитающих Высшие растения мхи
Высшие растения мхи Приспособленность организмов к среде обитания. 9 класс
Приспособленность организмов к среде обитания. 9 класс