Содержание
- 2. Systematic and trivial names, optical isomerism, formation of zwitterions of amino acids Lesson objectives - to
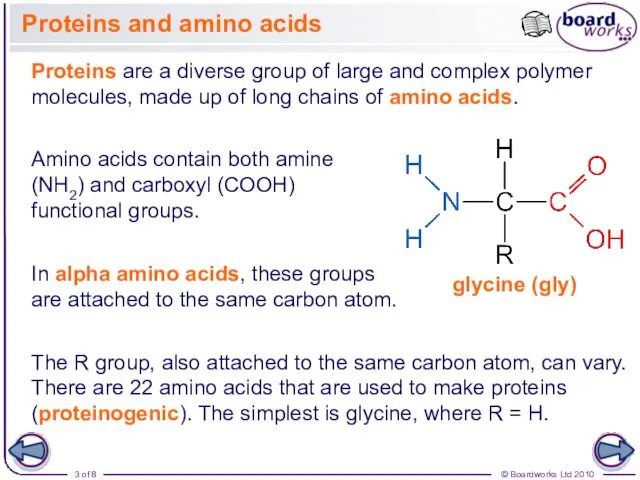
- 3. Proteins and amino acids Proteins are a diverse group of large and complex polymer molecules, made
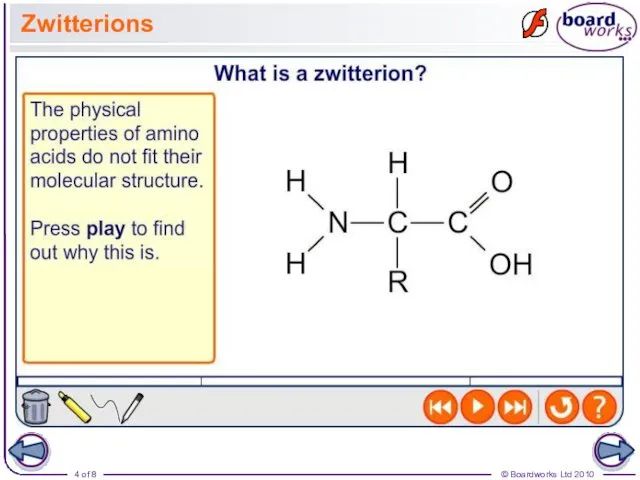
- 4. Zwitterions
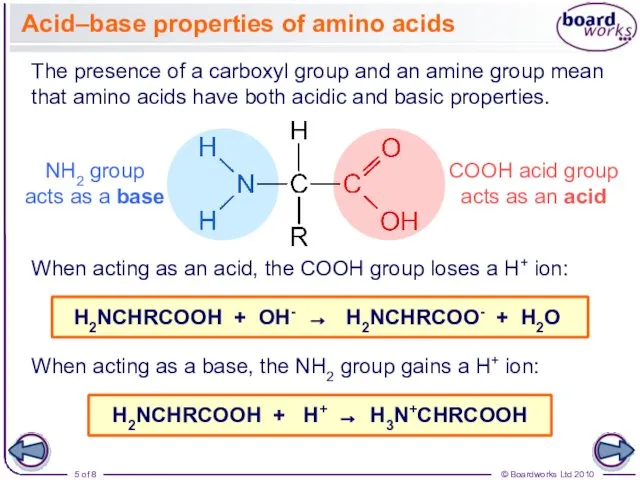
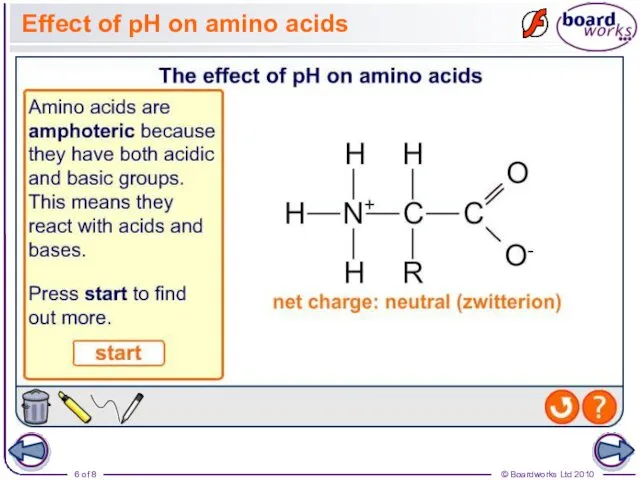
- 5. Acid–base properties of amino acids The presence of a carboxyl group and an amine group mean
- 6. Effect of pH on amino acids
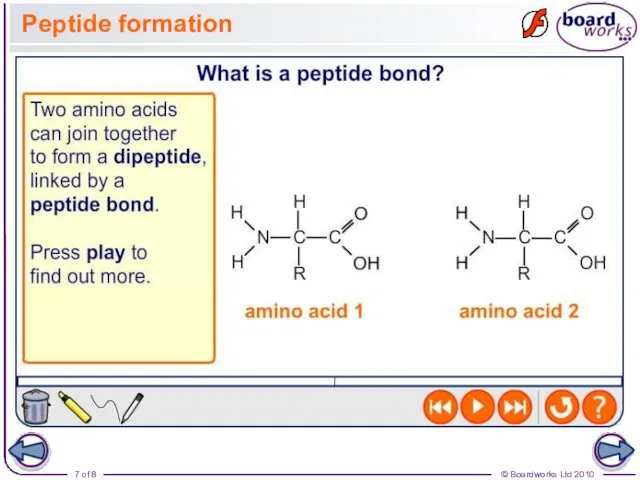
- 7. Peptide formation
- 9. Скачать презентацию
 Вирусы. Сходства вируса с живыми организмами. Размножение вирусов
Вирусы. Сходства вируса с живыми организмами. Размножение вирусов Вредители и болезни с /х культур
Вредители и болезни с /х культур Посттрансляционные модификации белков
Посттрансляционные модификации белков Поджелудочная железа
Поджелудочная железа Тип Членистоногие (Arthropoda). Многоножки
Тип Членистоногие (Arthropoda). Многоножки Черенкование комнатных растений
Черенкование комнатных растений Хай-тек отходы
Хай-тек отходы Хищные растения
Хищные растения Мәмлерің түрлері
Мәмлерің түрлері Бады - лечим или калечим
Бады - лечим или калечим Презентация Эмбриональное развитие
Презентация Эмбриональное развитие Классы и семейства цветковых растений
Классы и семейства цветковых растений Фотосинтез. Фазы фотосинтеза
Фотосинтез. Фазы фотосинтеза Строение клеток: органоиды. Клеточная теория (Шванн и Шлейден)
Строение клеток: органоиды. Клеточная теория (Шванн и Шлейден) Biologics in Rheumatology
Biologics in Rheumatology Презентация к уроку биологии в 11 классе Вид и его критерии
Презентация к уроку биологии в 11 классе Вид и его критерии Уникальность человека. Биологическое и социальное в человеке
Уникальность человека. Биологическое и социальное в человеке Устройство микроскопа и приёмы работы с ним. 5 класс
Устройство микроскопа и приёмы работы с ним. 5 класс Імунітет людини
Імунітет людини Простейшие. Корненожки. Радиолярии. Солнечники. Споровики
Простейшие. Корненожки. Радиолярии. Солнечники. Споровики Мир растений
Мир растений ЕГЭ Биология. Новый формат заданий. 2022
ЕГЭ Биология. Новый формат заданий. 2022 Породистые и непородистые кошки
Породистые и непородистые кошки Алгоритмы биоинформатики
Алгоритмы биоинформатики Этапы экспедиции Дарвина
Этапы экспедиции Дарвина Дикорастущие растения в кулинарии (часть 2)
Дикорастущие растения в кулинарии (часть 2) Дыхательная система
Дыхательная система evolyuciya_krovenosnoy_sistemy_7_klass_1427359430_105713
evolyuciya_krovenosnoy_sistemy_7_klass_1427359430_105713