Содержание
- 3. Order: Spirochaetales Family: Spirochaetaceae Genus: Treponema Borrelia Family: Leptospiraceae Genus: Leptospira Taxonomy
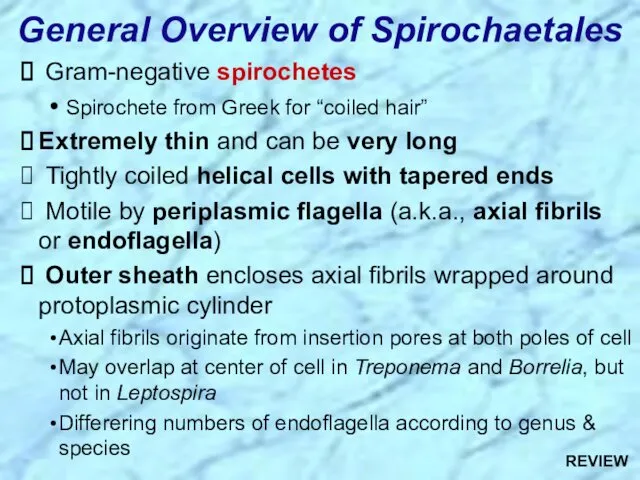
- 4. General Overview of Spirochaetales Gram-negative spirochetes Spirochete from Greek for “coiled hair” Extremely thin and can
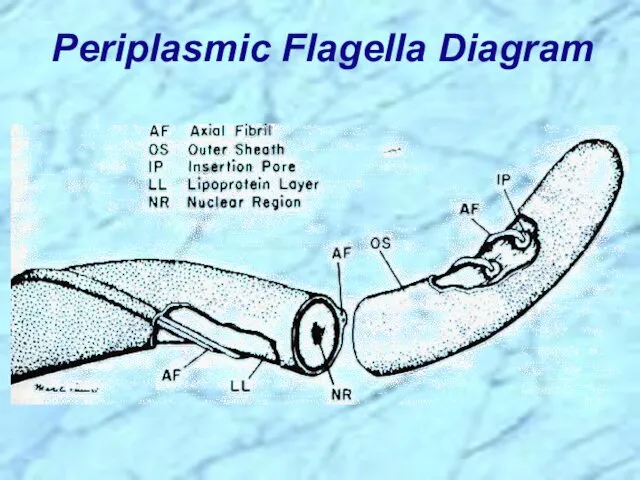
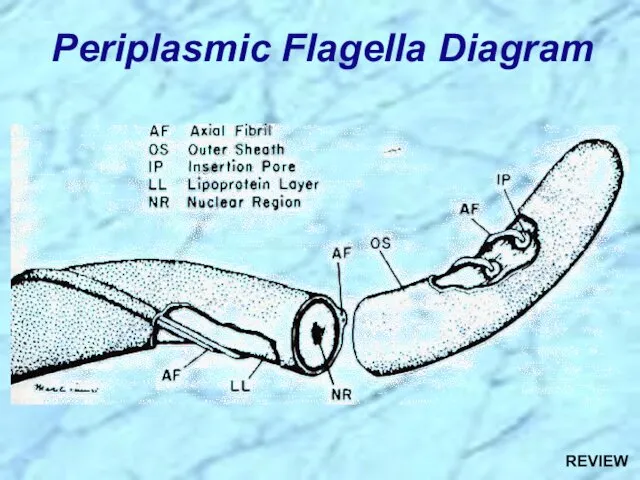
- 5. Periplasmic Flagella Diagram
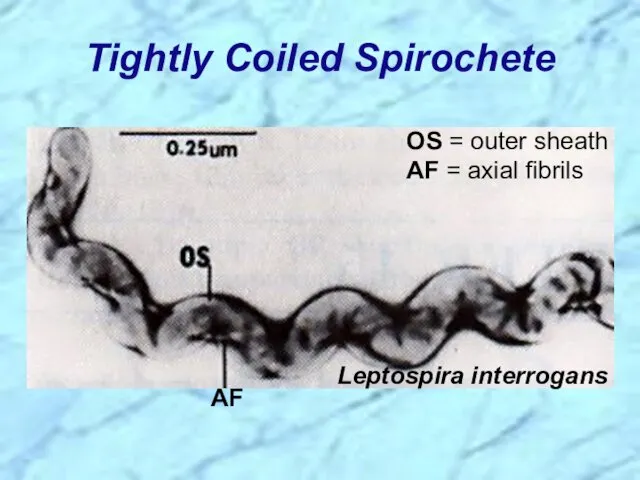
- 6. Tightly Coiled Spirochete
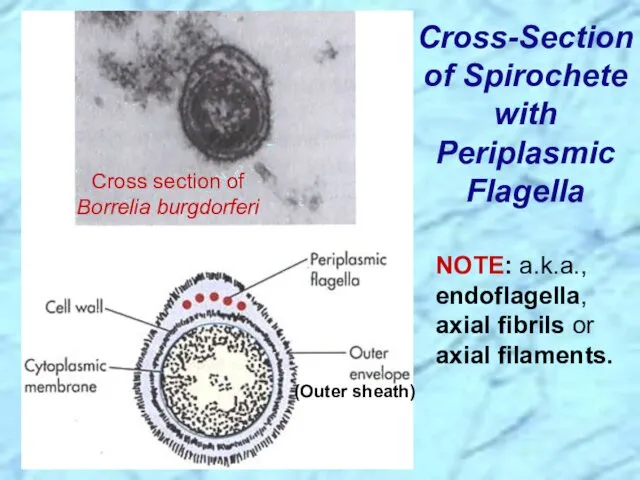
- 7. Cross-Section of Spirochete with Periplasmic Flagella NOTE: a.k.a., endoflagella, axial fibrils or axial filaments. (Outer sheath)
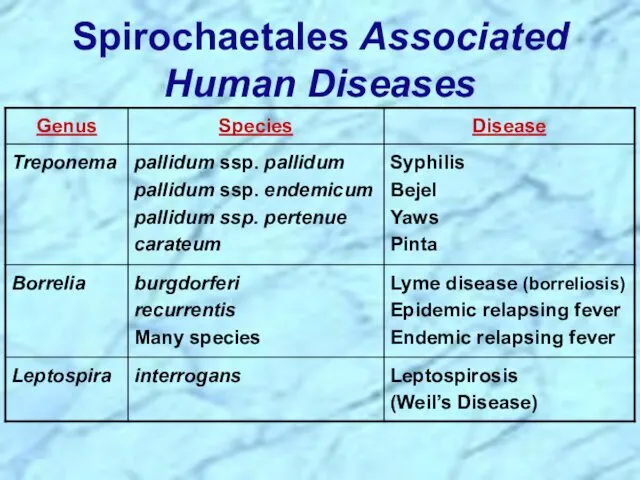
- 8. Spirochaetales Associated Human Diseases
- 10. Treponema spp.
- 11. Nonvenereal Treponemal Diseases Bejel, Yaws & Pinta Primitive tropical and subtropical regions Primarily in impoverished children
- 12. Treponema pallidum ssp. endemicum Bejel (a.k.a. endemic syphilis) Initial lesions: nondescript oral lesions Secondary lesions: oral
- 13. Treponema pallidum ssp. pertenue (May also see T. pertenue) Papillomatous Lesions of Yaws: painless nodules widely
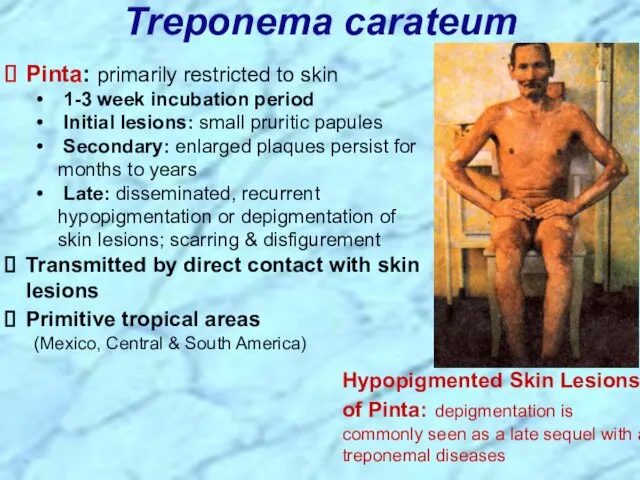
- 14. Treponema carateum Pinta: primarily restricted to skin 1-3 week incubation period Initial lesions: small pruritic papules
- 16. Treponema pallidum ssp. pallidum
- 17. Venereal Treponemal Disease Syphilis Primarily sexually transmitted disease (STD) May be transmitted congenitally
- 18. Darkfield Microscopy of Treponema pallidum
- 19. Too thin to be seen with light microscopy in specimens stained with Gram stain or Giemsa
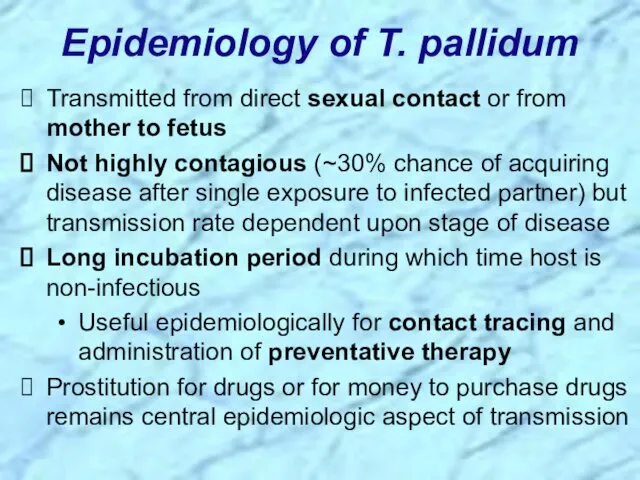
- 20. Epidemiology of T. pallidum Transmitted from direct sexual contact or from mother to fetus Not highly
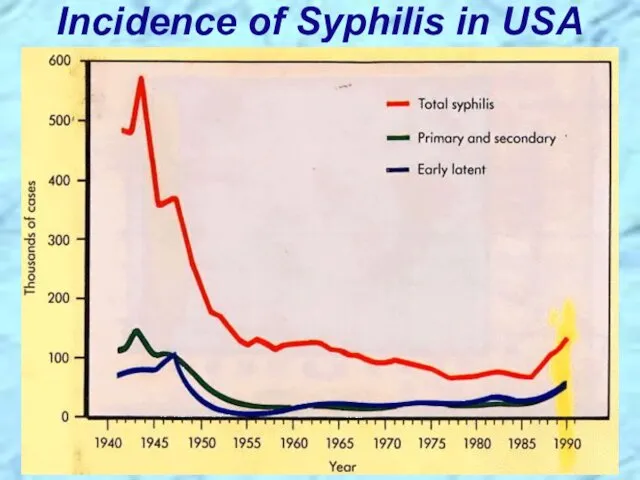
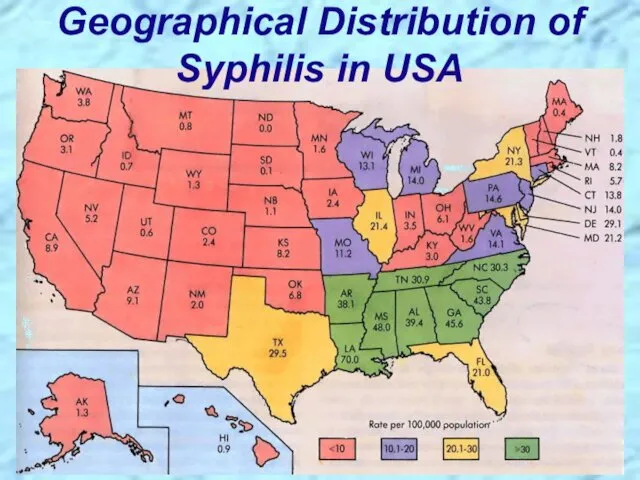
- 21. Incidence of Syphilis in USA
- 22. Geographical Distribution of Syphilis in USA
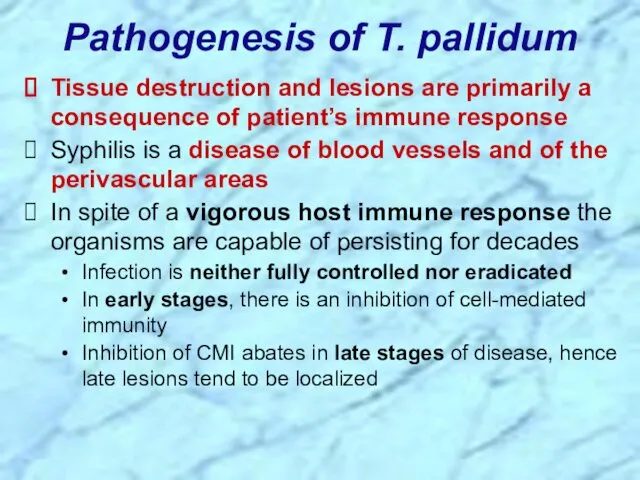
- 23. Pathogenesis of T. pallidum Tissue destruction and lesions are primarily a consequence of patient’s immune response
- 24. Virulence Factors of T. pallidum Outer membrane proteins promote adherence Hyaluronidase may facilitate perivascular infiltration Antiphagocytic
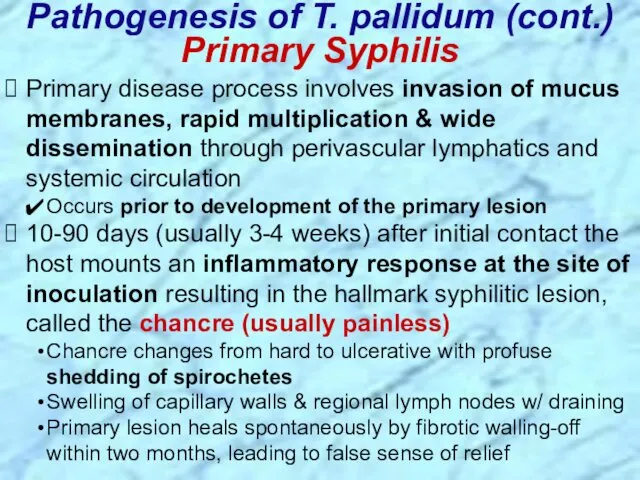
- 25. Primary disease process involves invasion of mucus membranes, rapid multiplication & wide dissemination through perivascular lymphatics
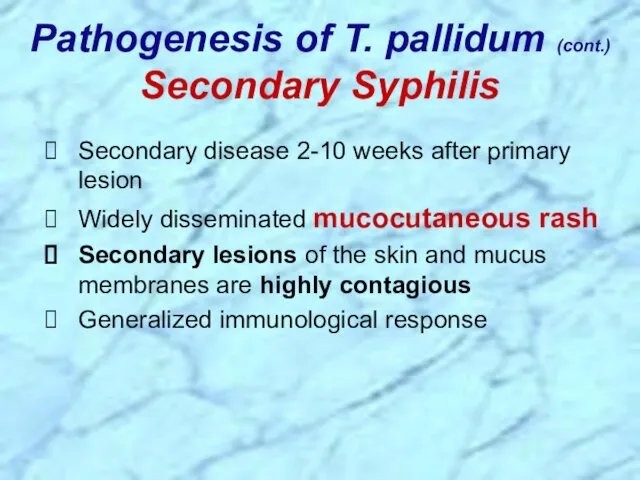
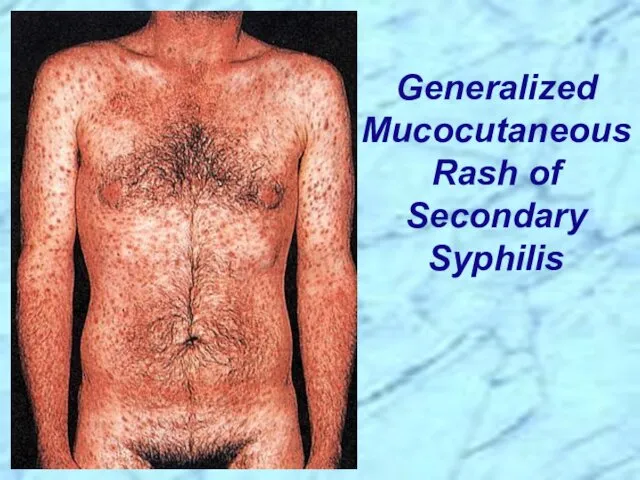
- 26. Secondary disease 2-10 weeks after primary lesion Widely disseminated mucocutaneous rash Secondary lesions of the skin
- 27. Generalized Mucocutaneous Rash of Secondary Syphilis
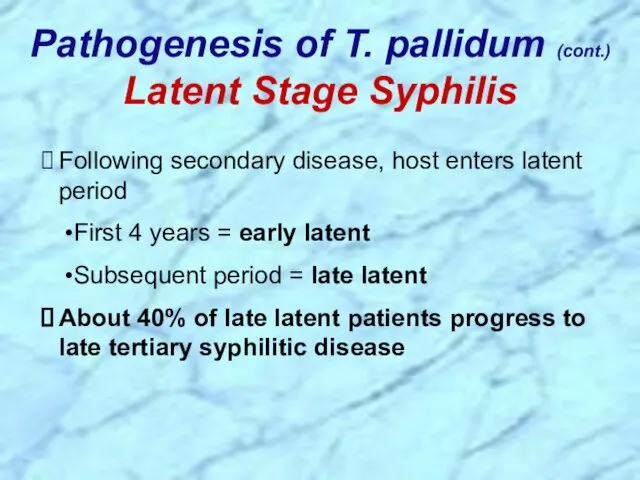
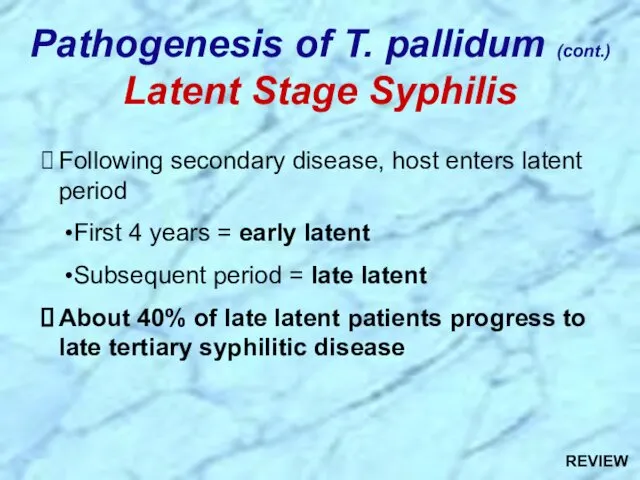
- 28. Following secondary disease, host enters latent period First 4 years = early latent Subsequent period =
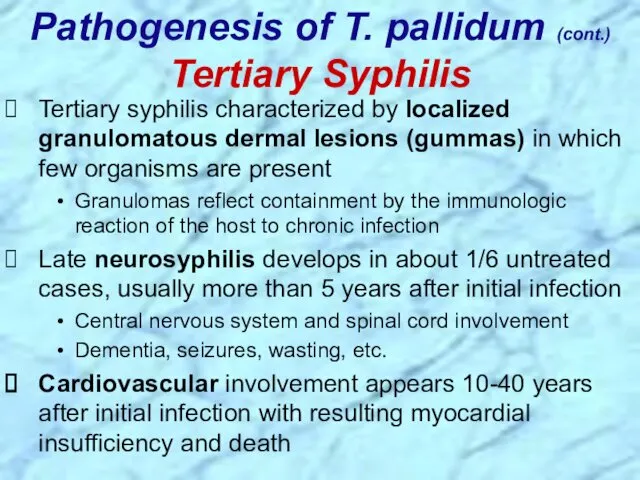
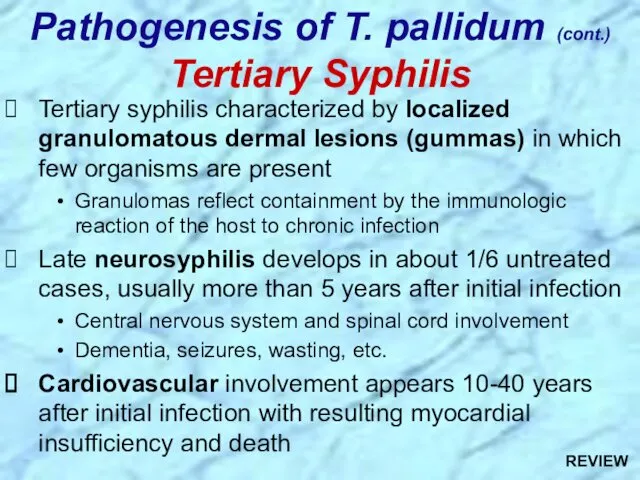
- 29. Tertiary syphilis characterized by localized granulomatous dermal lesions (gummas) in which few organisms are present Granulomas
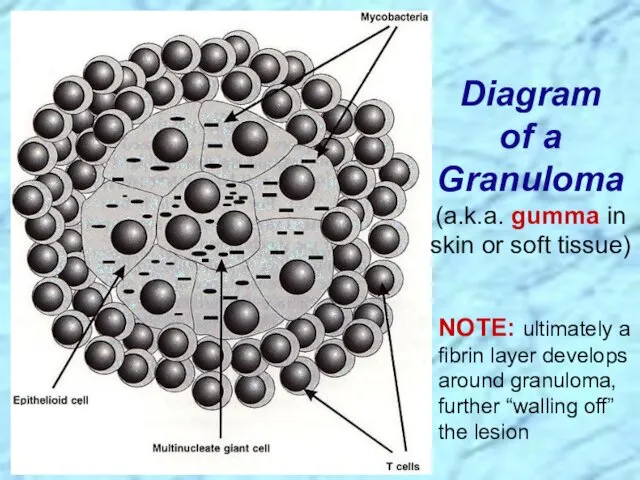
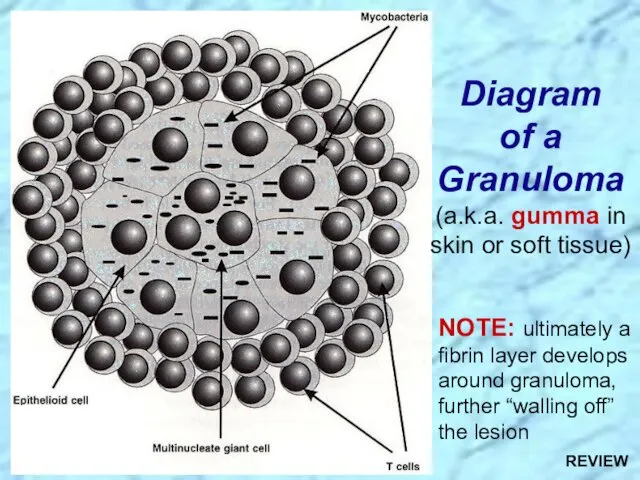
- 30. Diagram of a Granuloma (a.k.a. gumma in skin or soft tissue) NOTE: ultimately a fibrin layer
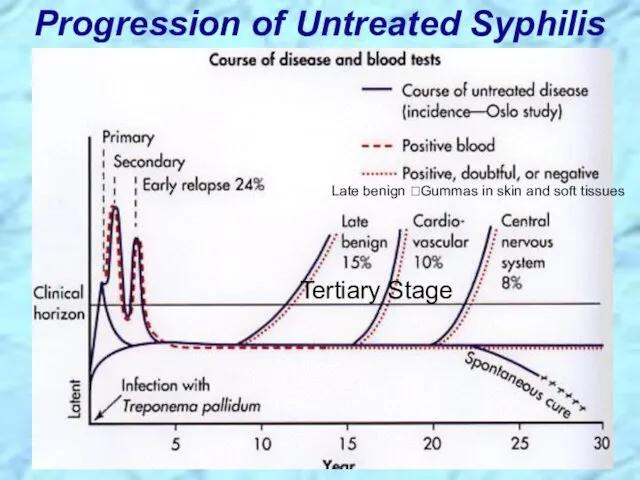
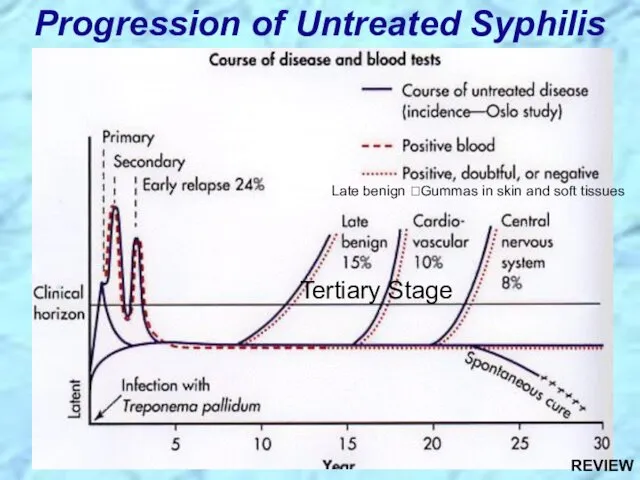
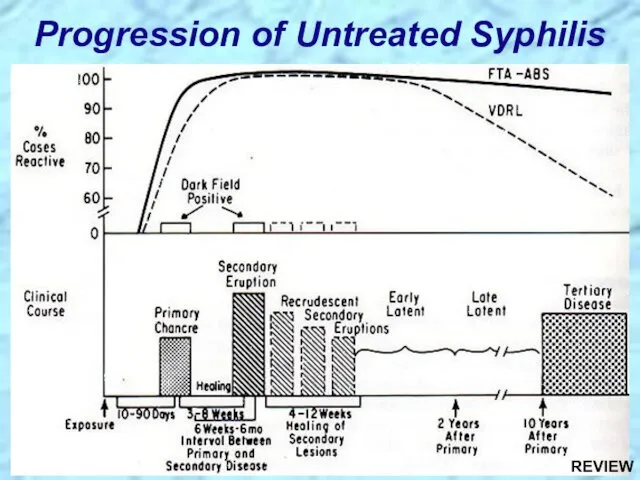
- 31. Progression of Untreated Syphilis Tertiary Stage Late benign ?Gummas in skin and soft tissues
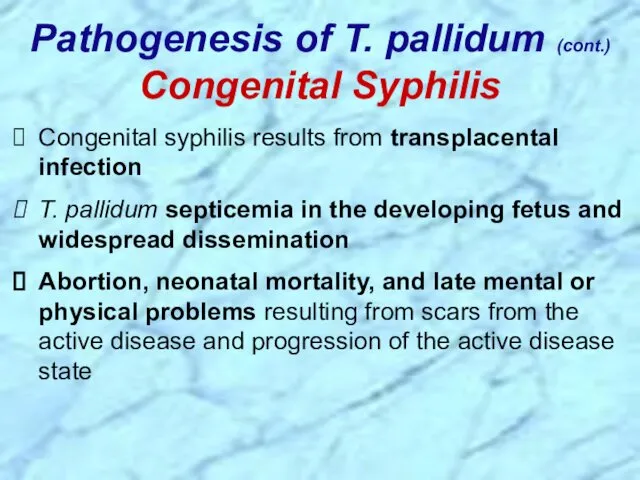
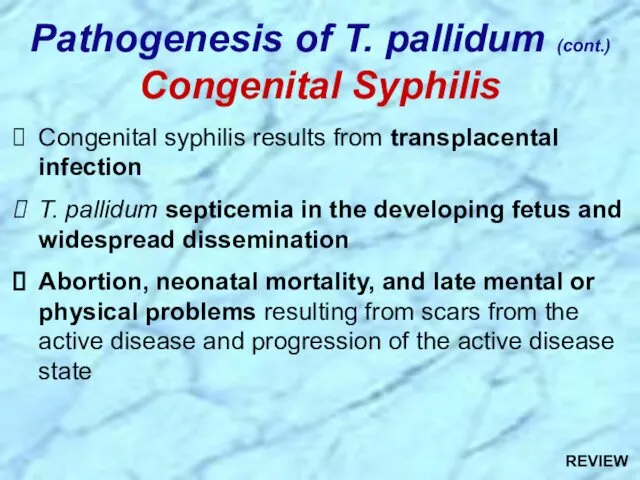
- 32. Congenital syphilis results from transplacental infection T. pallidum septicemia in the developing fetus and widespread dissemination
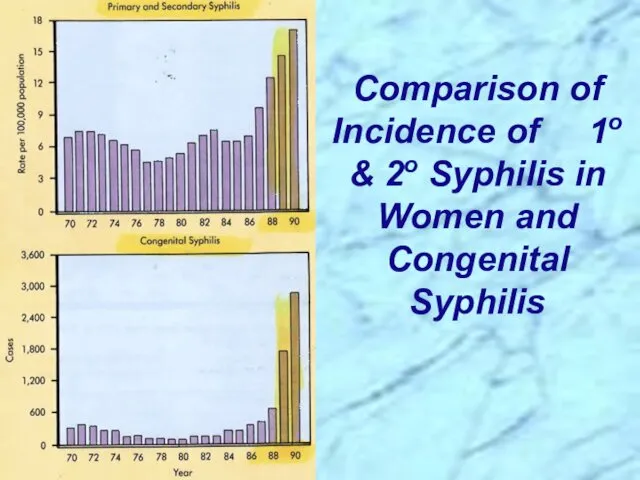
- 33. Comparison of Incidence of 1o & 2o Syphilis in Women and Congenital Syphilis
- 34. Prevention & Treatment of Syphilis Penicillin remains drug of choice WHO monitors treatment recommendations 7-10 days
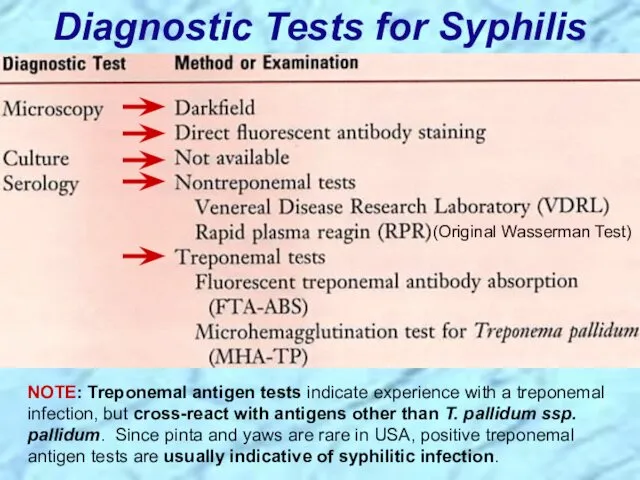
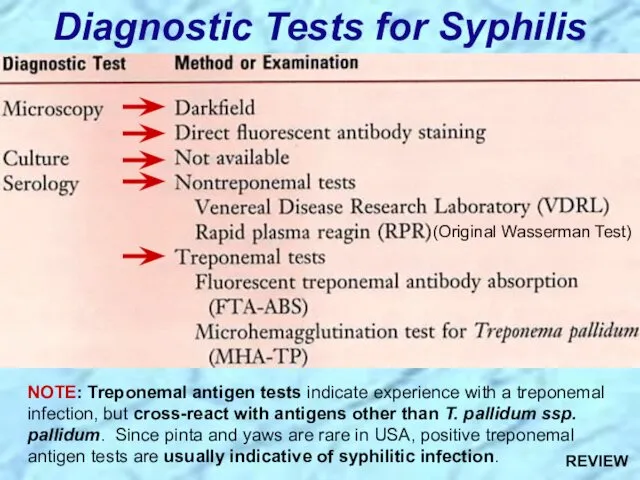
- 35. Diagnostic Tests for Syphilis NOTE: Treponemal antigen tests indicate experience with a treponemal infection, but cross-react
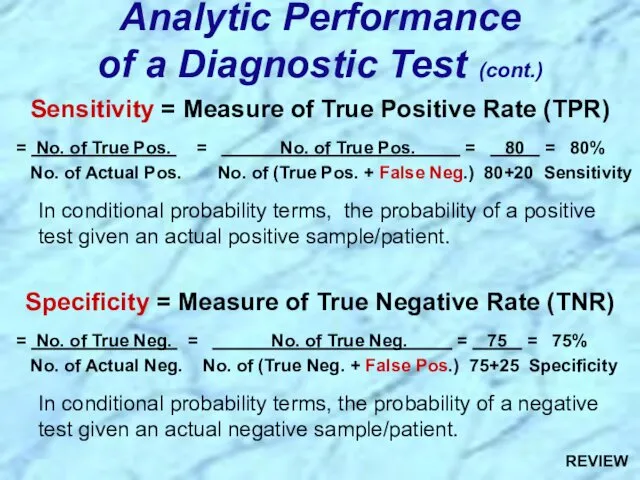
- 36. Sensitivity & Specificity of Serologic Tests for Syphillis
- 37. Review Handout on Sensitivity & Specificity of Diagnostic Tests
- 38. Conditions Associated with False Positive Serological Tests for Syphillis
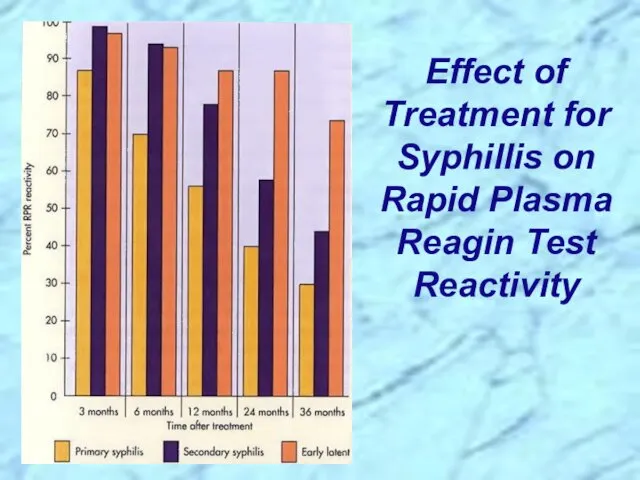
- 39. Effect of Treatment for Syphillis on Rapid Plasma Reagin Test Reactivity
- 41. Borrelia spp.
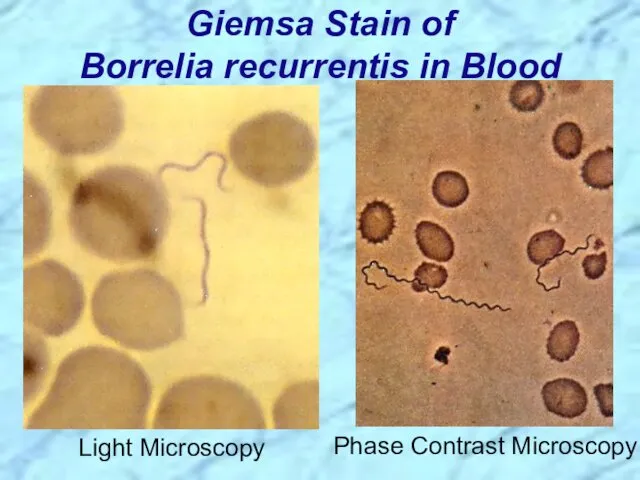
- 42. Giemsa Stain of Borrelia recurrentis in Blood Light Microscopy Phase Contrast Microscopy
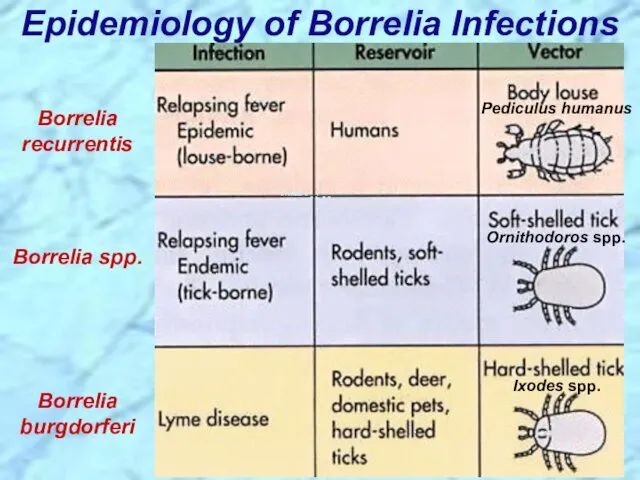
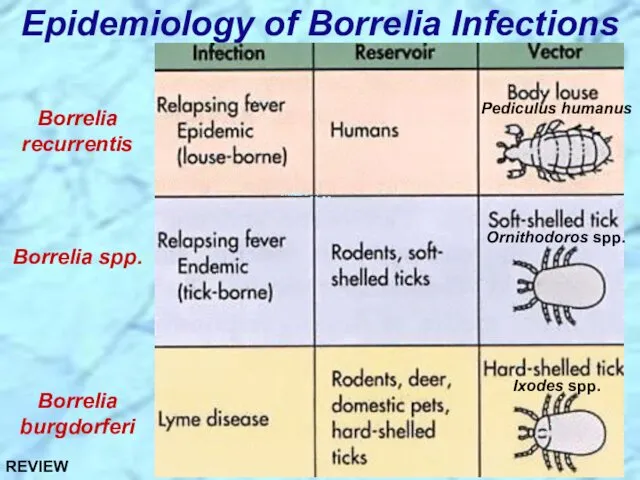
- 43. Epidemiology of Borrelia Infections Borrelia recurrentis Borrelia spp. Borrelia burgdorferi Ixodes spp. Ornithodoros spp. Pediculus humanus
- 44. Borrelia recurrentis & other Borrelia spp.
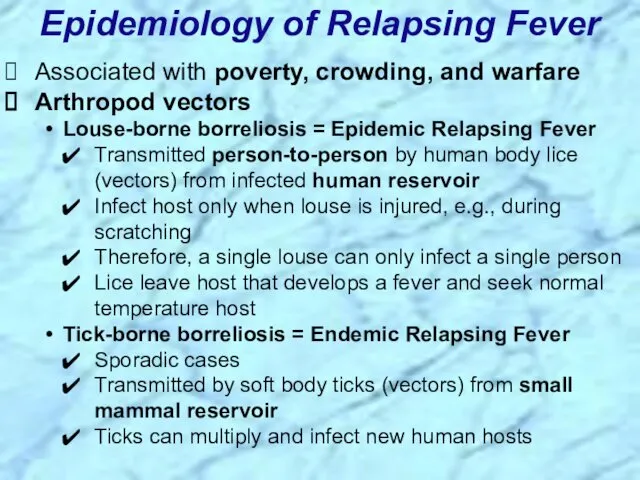
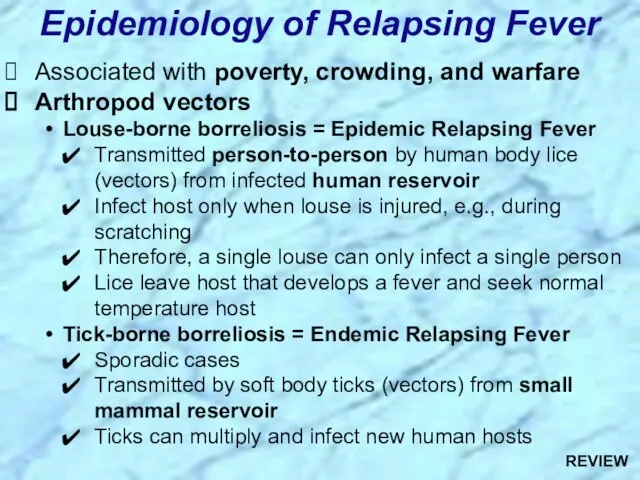
- 45. Associated with poverty, crowding, and warfare Arthropod vectors Louse-borne borreliosis = Epidemic Relapsing Fever Transmitted person-to-person
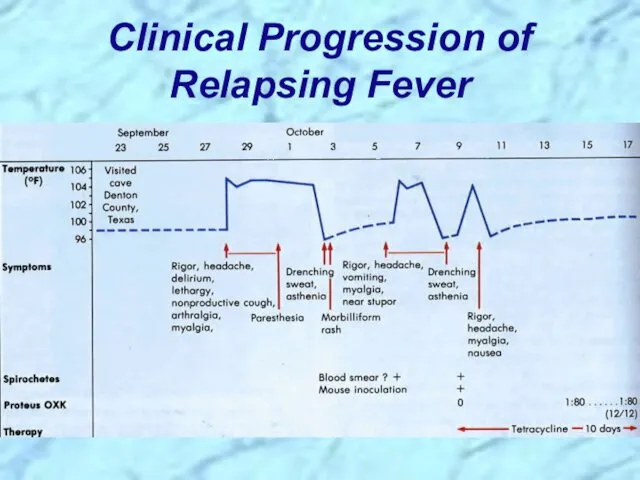
- 46. Pathogenesis of Relapsing Fever Relapsing fever (a.k.a., tick fever, borreliosis, famine fever) Acute infection with 2-14
- 47. Clinical Progression of Relapsing Fever
- 48. Borrelia burgdorferi
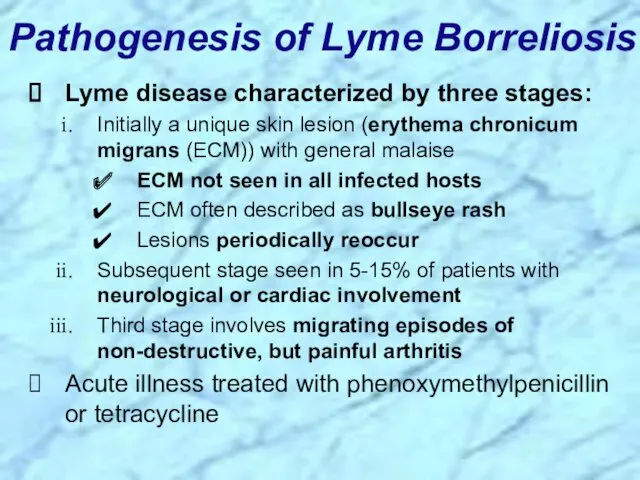
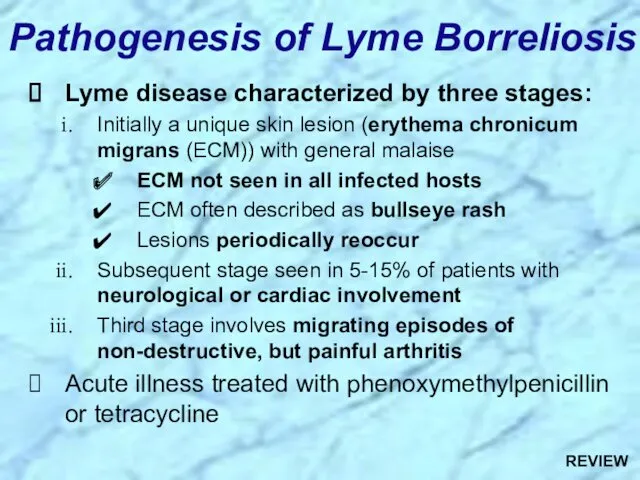
- 49. Pathogenesis of Lyme Borreliosis Lyme disease characterized by three stages: Initially a unique skin lesion (erythema
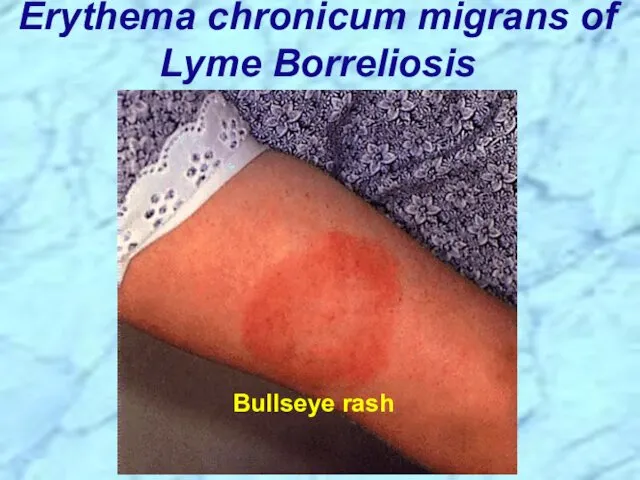
- 50. Erythema chronicum migrans of Lyme Borreliosis Bullseye rash
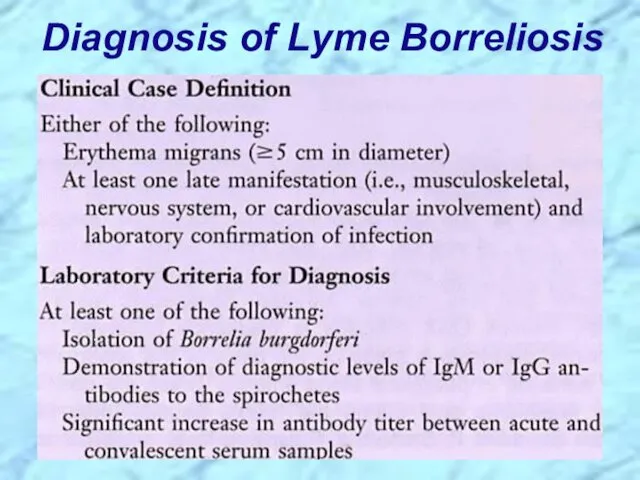
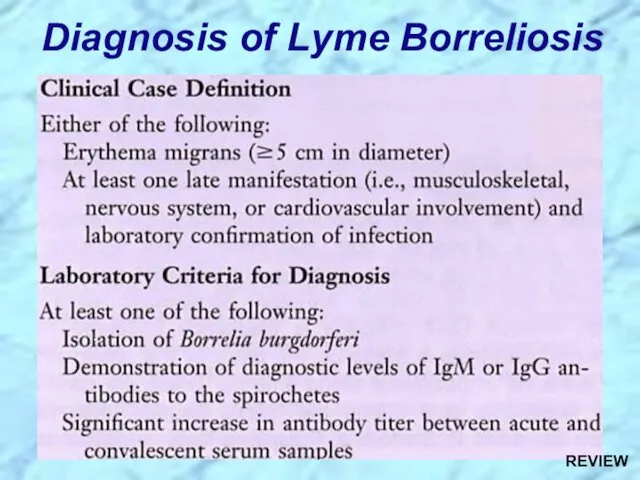
- 51. Diagnosis of Lyme Borreliosis
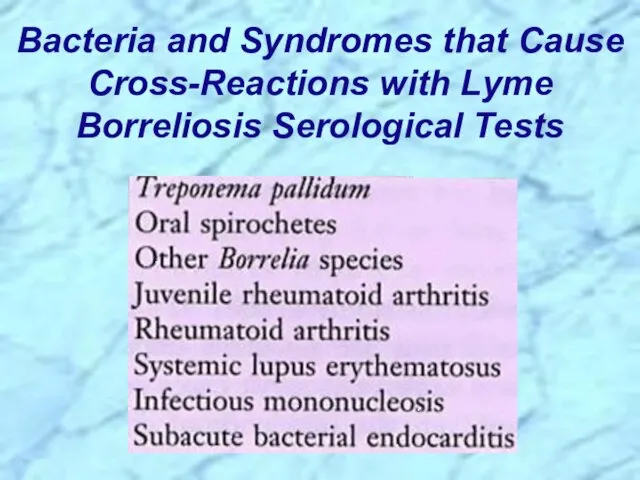
- 52. Bacteria and Syndromes that Cause Cross-Reactions with Lyme Borreliosis Serological Tests
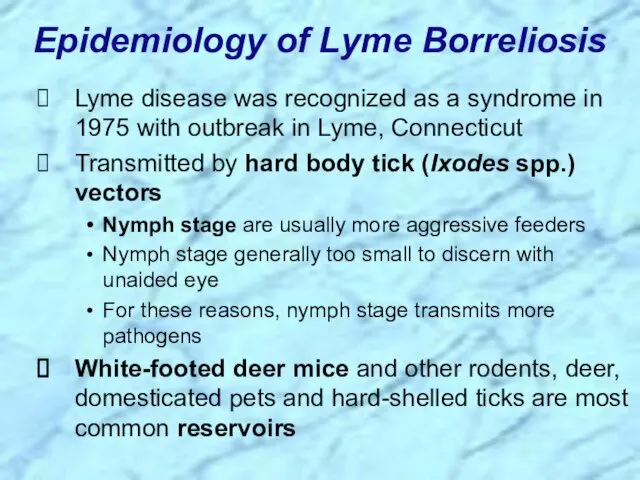
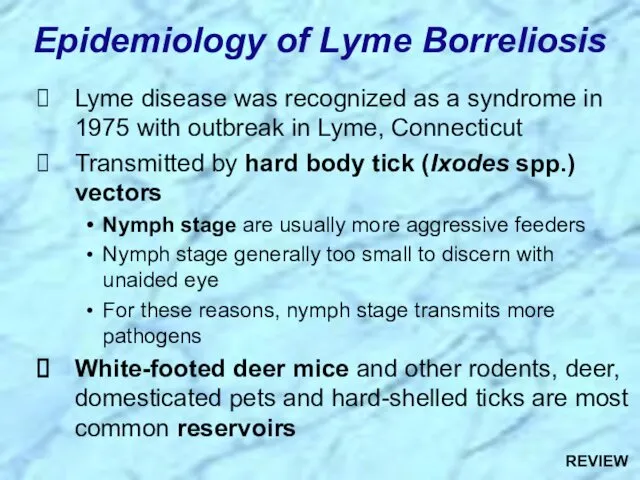
- 53. Lyme disease was recognized as a syndrome in 1975 with outbreak in Lyme, Connecticut Transmitted by
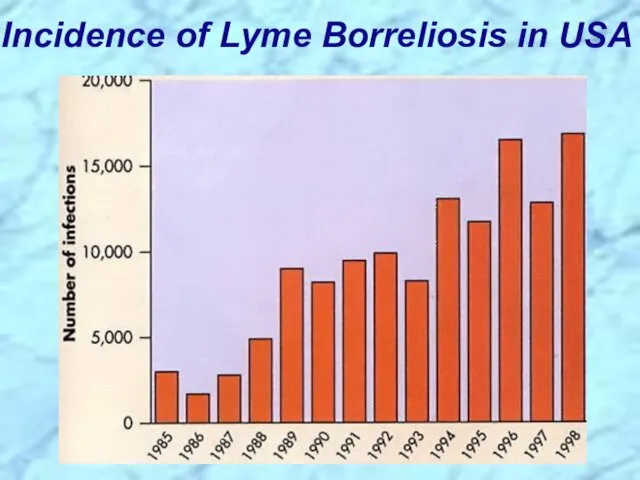
- 54. Incidence of Lyme Borreliosis in USA
- 56. Leptospira interrogans
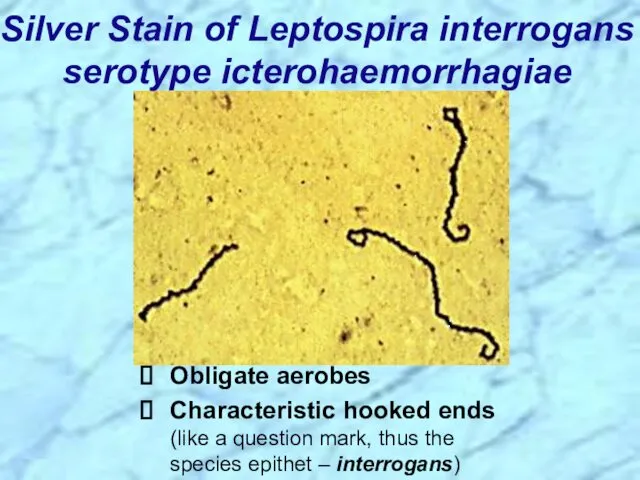
- 57. Silver Stain of Leptospira interrogans serotype icterohaemorrhagiae Obligate aerobes Characteristic hooked ends (like a question mark,
- 58. Leptospirosis Clinical Syndromes Mild virus-like syndrome (Anicteric leptospirosis) Systemic with aseptic meningitis (Icteric leptospirosis) Overwhelming disease
- 59. Leptospirosis, also called Weil’s disease in humans Direct invasion and replication in tissues Characterized by an
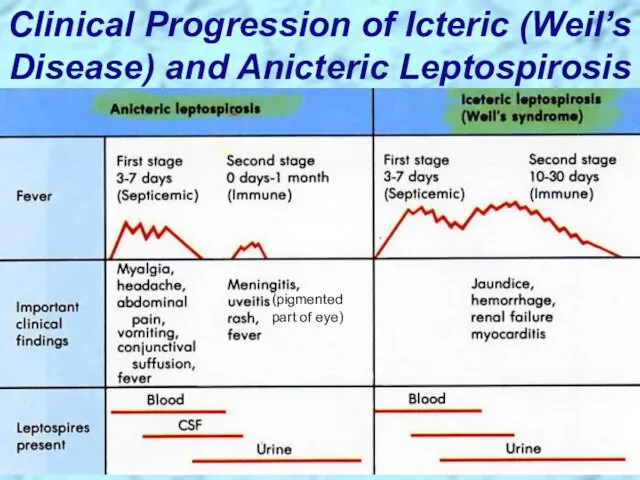
- 60. Clinical Progression of Icteric (Weil’s Disease) and Anicteric Leptospirosis (pigmented part of eye)
- 61. Epidemiology of Leptospirosis Mainly a zoonotic disease Transmitted to humans from a variety of wild and
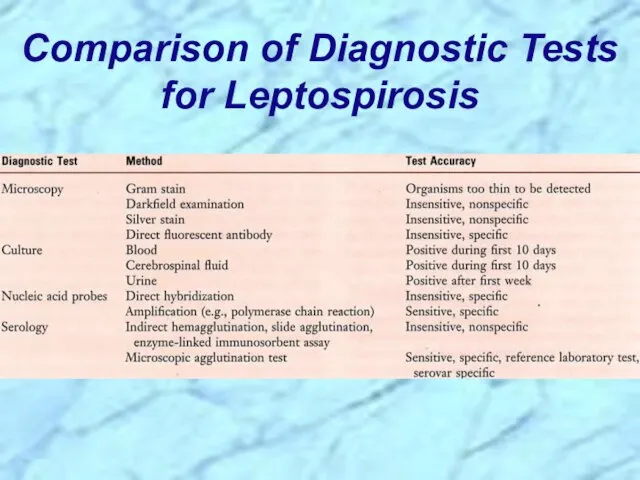
- 62. Comparison of Diagnostic Tests for Leptospirosis
- 64. REVIEW of Spirochaetales
- 65. General Overview of Spirochaetales Gram-negative spirochetes Spirochete from Greek for “coiled hair” Extremely thin and can
- 66. Periplasmic Flagella Diagram REVIEW
- 67. Spirochaetales Associated Human Diseases REVIEW
- 68. Review of Treponema
- 69. Summary of Treponema Infections REVIEW
- 70. Summary of Treponema Infections (cont.) REVIEW
- 71. Nonvenereal Treponemal Diseases Bejel, Yaws & Pinta Primitive tropical and subtropical regions Primarily in impoverished children
- 72. Review of Treponema pallidum ssp. pallidum
- 73. Too thin to be seen with light microscopy in specimens stained with Gram stain or Giemsa
- 74. Epidemiology of T. pallidum Transmitted from direct sexual contact or from mother to fetus Not highly
- 75. Pathogenesis of T. pallidum Tissue destruction and lesions are primarily a consequence of patient’s immune response
- 76. Virulence Factors of T. pallidum Outer membrane proteins promote adherence Hyaluronidase may facilitate perivascular infiltration Antiphagocytic
- 77. Primary disease process involves invasion of mucus membranes, rapid multiplication & wide dissemination through perivascular lymphatics
- 78. Secondary disease 2-10 weeks after primary lesion Widely disseminated mucocutaneous rash Secondary lesions of the skin
- 79. Following secondary disease, host enters latent period First 4 years = early latent Subsequent period =
- 80. Tertiary syphilis characterized by localized granulomatous dermal lesions (gummas) in which few organisms are present Granulomas
- 81. Diagram of a Granuloma (a.k.a. gumma in skin or soft tissue) NOTE: ultimately a fibrin layer
- 82. Progression of Untreated Syphilis Tertiary Stage Late benign ?Gummas in skin and soft tissues REVIEW
- 83. Progression of Untreated Syphilis REVIEW
- 84. Congenital syphilis results from transplacental infection T. pallidum septicemia in the developing fetus and widespread dissemination
- 85. Prevention & Treatment of Syphilis Penicillin remains drug of choice WHO monitors treatment recommendations 7-10 days
- 86. Diagnostic Tests for Syphilis NOTE: Treponemal antigen tests indicate experience with a treponemal infection, but cross-react
- 87. Review Handout on Sensitivity & Specificity of Diagnostic Tests
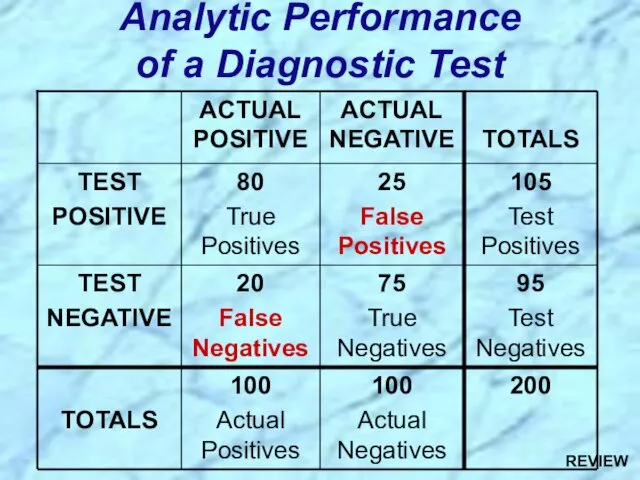
- 88. Analytic Performance of a Diagnostic Test REVIEW
- 89. Sensitivity = Measure of True Positive Rate (TPR) = No. of True Pos. = No. of
- 90. Review of Borrelia
- 91. Summary of Borellia Infections REVIEW
- 92. Summary of Borellia Infections (cont.) REVIEW
- 93. Epidemiology of Borrelia Infections Borrelia recurrentis Borrelia spp. Borrelia burgdorferi Ixodes spp. Ornithodoros spp. Pediculus humanus
- 94. Review of Borrelia recurrentis & other Borrelia spp.
- 95. Associated with poverty, crowding, and warfare Arthropod vectors Louse-borne borreliosis = Epidemic Relapsing Fever Transmitted person-to-person
- 96. Pathogenesis of Relapsing Fever Relapsing fever (a.k.a., tick fever, borreliosis, famine fever) Acute infection with 2-14
- 97. Review of Borrelia burgdorferi
- 98. Pathogenesis of Lyme Borreliosis Lyme disease characterized by three stages: Initially a unique skin lesion (erythema
- 99. Diagnosis of Lyme Borreliosis REVIEW
- 100. Lyme disease was recognized as a syndrome in 1975 with outbreak in Lyme, Connecticut Transmitted by
- 101. Review of Leptospira
- 102. Summary of Leptospira Infections REVIEW
- 103. Summary of Leptospira Infections (cont.) REVIEW
- 104. Leptospirosis Clinical Syndromes Mild virus-like syndrome (Anicteric leptospirosis) Systemic with aseptic meningitis (Icteric leptospirosis) Overwhelming disease
- 105. Leptospirosis, also called Weil’s disease in humans Direct invasion and replication in tissues Characterized by an
- 106. Epidemiology of Leptospirosis Mainly a zoonotic disease Transmitted to humans from a variety of wild and
- 108. Скачать презентацию



















































 General characteristics of 6 Kingdoms
General characteristics of 6 Kingdoms Биологически активные низкомолекулярные вещества
Биологически активные низкомолекулярные вещества Әр түрлі мөлшердегі минералды тыңайтқыштардың қант қызылшасының өнімділігіне әсерін анықтау барысындағы суреттер
Әр түрлі мөлшердегі минералды тыңайтқыштардың қант қызылшасының өнімділігіне әсерін анықтау барысындағы суреттер Растительная клетка. Лекция 1
Растительная клетка. Лекция 1 Актинидия. Особенности
Актинидия. Особенности Многообразие ракообразных
Многообразие ракообразных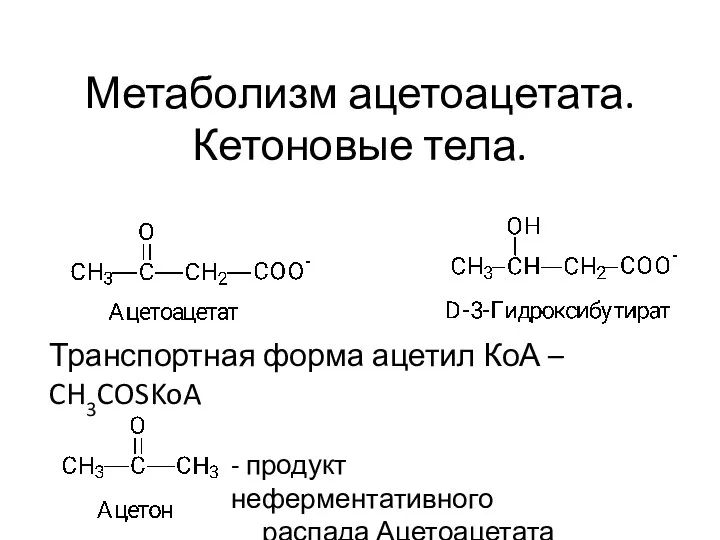 Кетоновые тела. Тема 10
Кетоновые тела. Тема 10 Лист. Морфология
Лист. Морфология Строение растительной клетки
Строение растительной клетки Генеративні органи рослини
Генеративні органи рослини Функциональная асимметрия мозга. Лекция 11
Функциональная асимметрия мозга. Лекция 11 Методы микроскопии
Методы микроскопии Самое редкое животное Африки - Окапи
Самое редкое животное Африки - Окапи Презентация, конспект урока, карточки - задания к уроку биологии для 6 класса на тему Фотосинтез.
Презентация, конспект урока, карточки - задания к уроку биологии для 6 класса на тему Фотосинтез. ПрезентацияТурнир по биологии в 8 классе.
ПрезентацияТурнир по биологии в 8 классе. Тема 12.Навоз, воздух, вода
Тема 12.Навоз, воздух, вода Лист — боковой орган побега
Лист — боковой орган побега Фотосинтез. История открытия
Фотосинтез. История открытия Миология. Мышцы тела человека
Миология. Мышцы тела человека Рослини ендеміки Австралії
Рослини ендеміки Австралії Деление клетки – митоз, мейоз
Деление клетки – митоз, мейоз Животные Республики Башкортостан
Животные Республики Башкортостан Физиология растений. Лекция 2
Физиология растений. Лекция 2 Красная книга Сызранского района
Красная книга Сызранского района В Буграх растут деревья и кустарники
В Буграх растут деревья и кустарники Сон и его значение
Сон и его значение Половое поведение животных
Половое поведение животных Ароматерапия. Химия эфирных масел
Ароматерапия. Химия эфирных масел