Содержание
- 2. 1H NMR SPECTROSCOPY
- 3. Метод я́дерного магни́тного резона́нса (ЯМР) основан на взаимодействии внешнего магнитного поля) основан на взаимодействии внешнего магнитного
- 4. Сабақтың мақсаты: Ядролық магнитті резонанс әдісімен танысу ЯМР қарапайым спектрлерімен танысу
- 5. Тілдік терминология Ядролық магнитті резонанс -magnetic nuclear resonance- ядерно магнитный резонанс
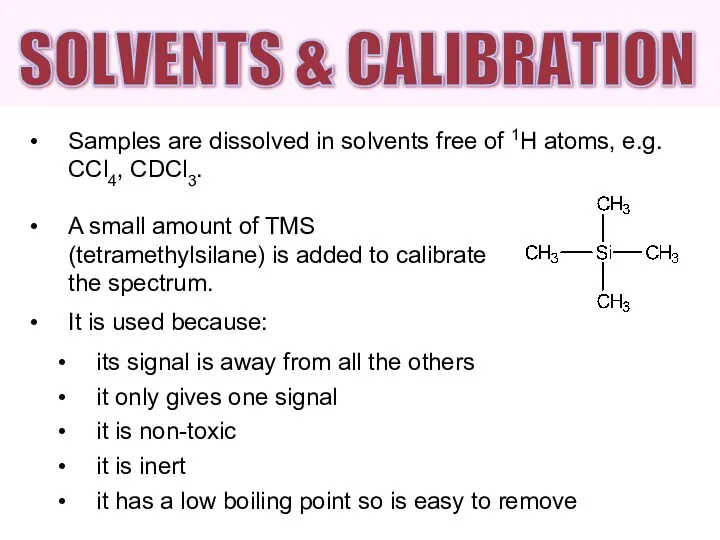
- 6. Samples are dissolved in solvents free of 1H atoms, e.g. CCl4, CDCl3. A small amount of
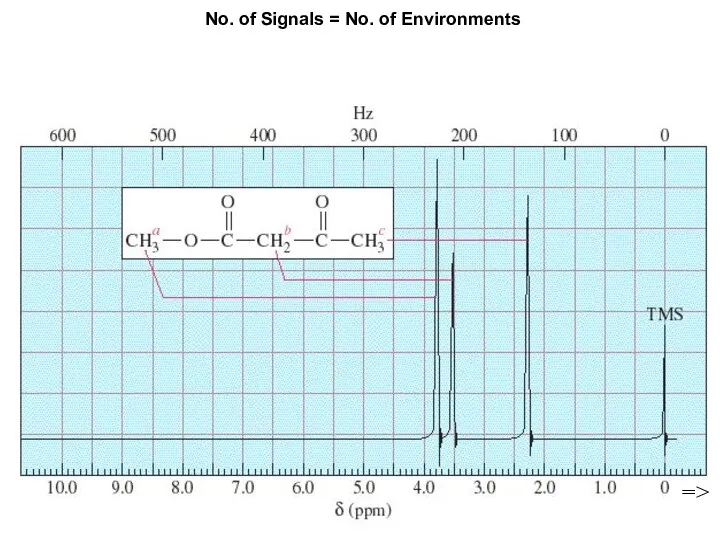
- 7. Chapter 13 No. of Signals = No. of Environments =>
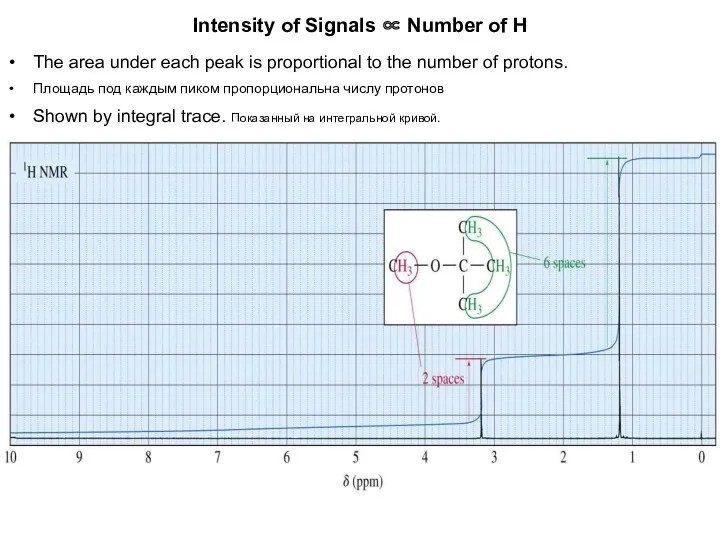
- 8. Intensity of Signals ∝ Number of H The area under each peak is proportional to the
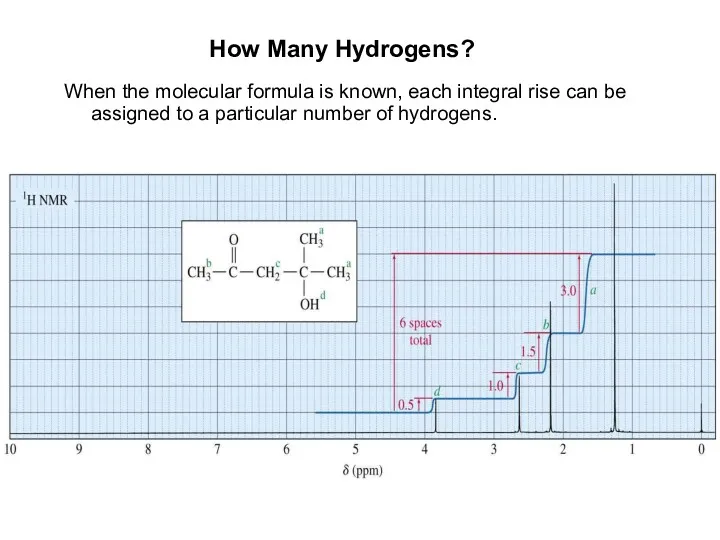
- 9. How Many Hydrogens? When the molecular formula is known, each integral rise can be assigned to
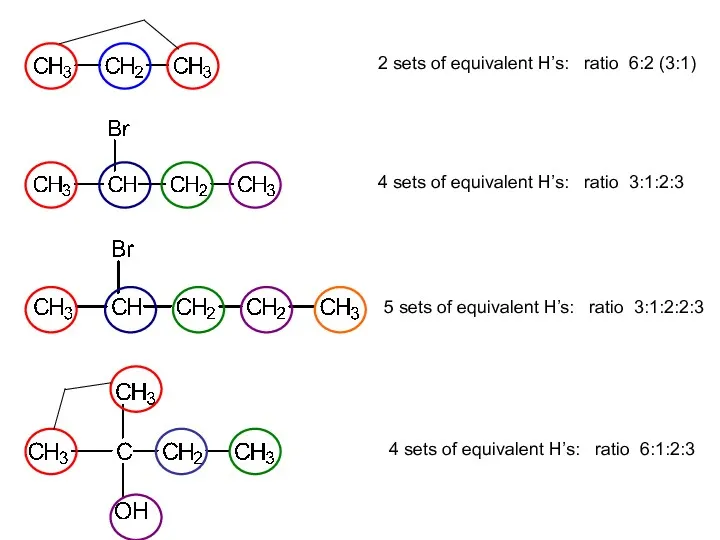
- 10. In a spectrum, there is one signal for each set of equivalent H atoms. В спектре
- 11. 2 sets of equivalent H’s: ratio 6:2 (3:1) 4 sets of equivalent H’s: ratio 3:1:2:3 5
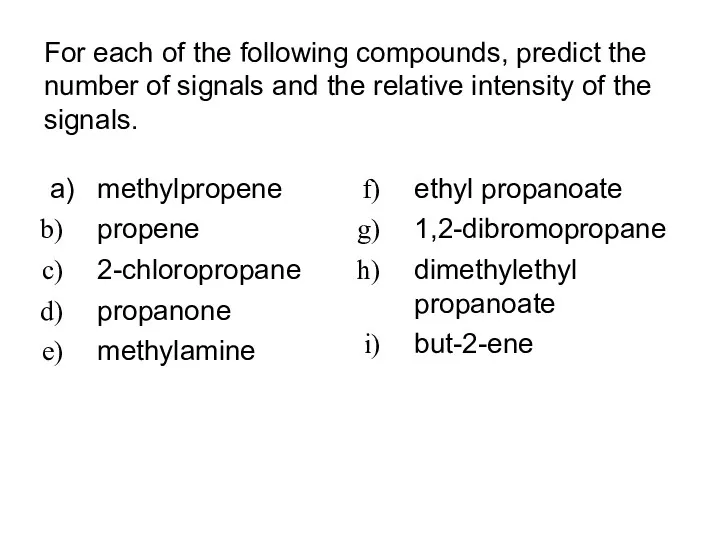
- 12. For each of the following compounds, predict the number of signals and the relative intensity of
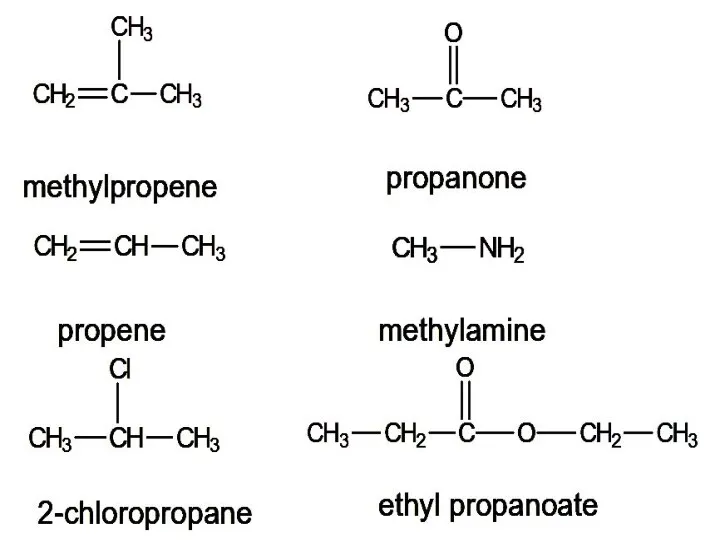
- 13. methylpropene propene 2-chloropropane propanone methylamine ethyl propanoate
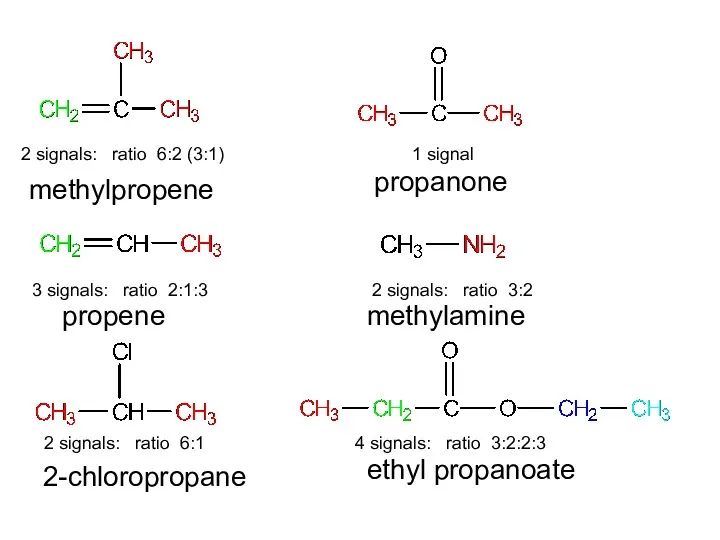
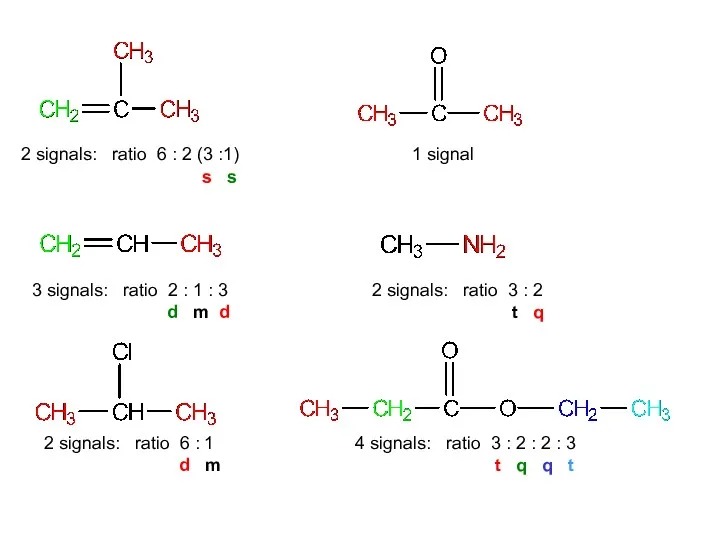
- 14. 2 signals: ratio 6:2 (3:1) 3 signals: ratio 2:1:3 2 signals: ratio 6:1 1 signal 2
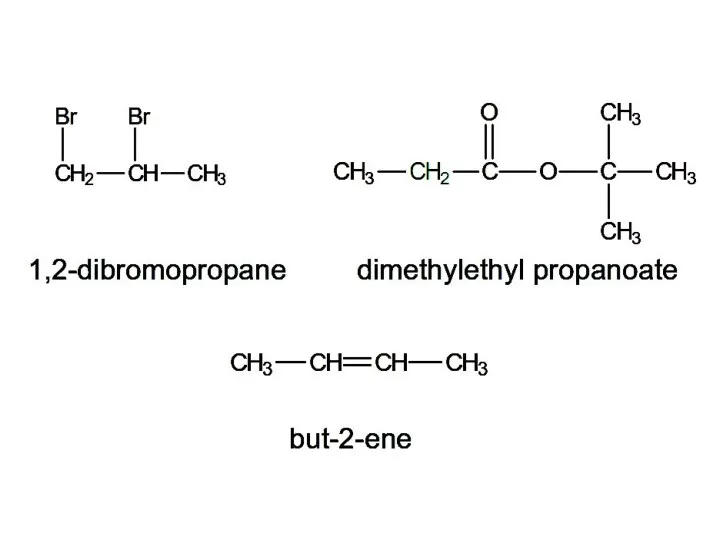
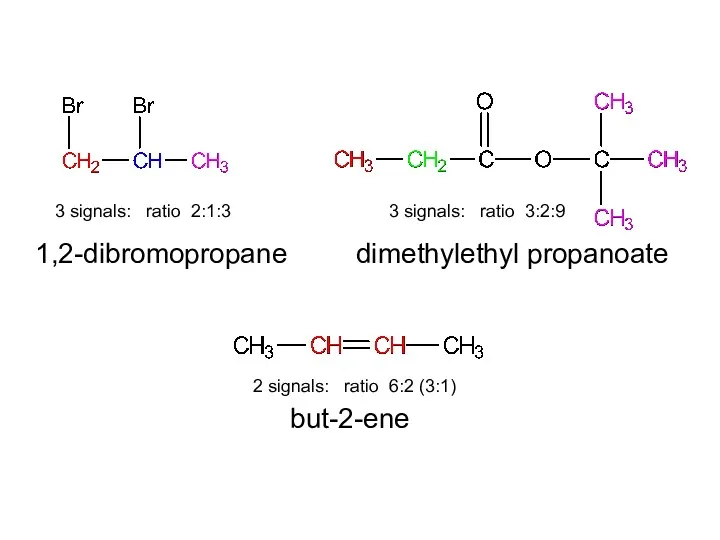
- 15. 1,2-dibromopropane dimethylethyl propanoate but-2-ene
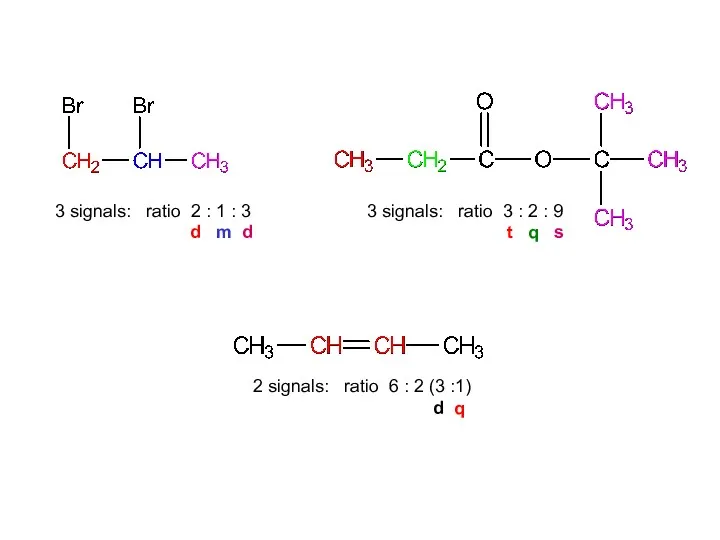
- 16. 3 signals: ratio 2:1:3 2 signals: ratio 6:2 (3:1) 3 signals: ratio 3:2:9 1,2-dibromopropane dimethylethyl propanoate
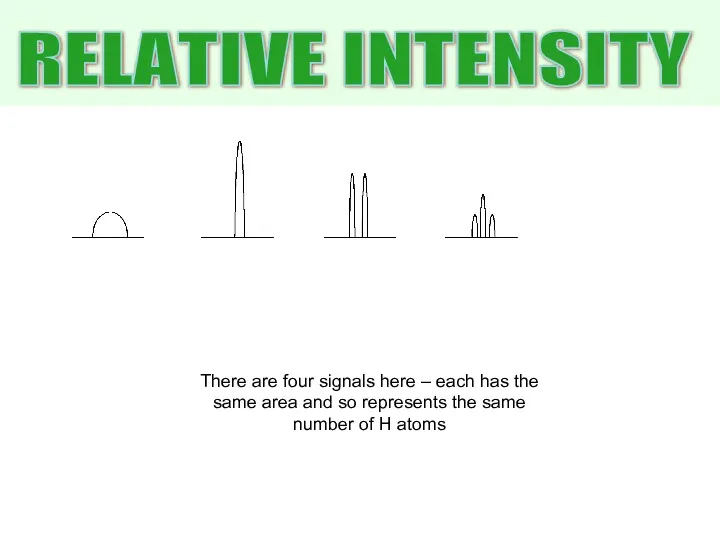
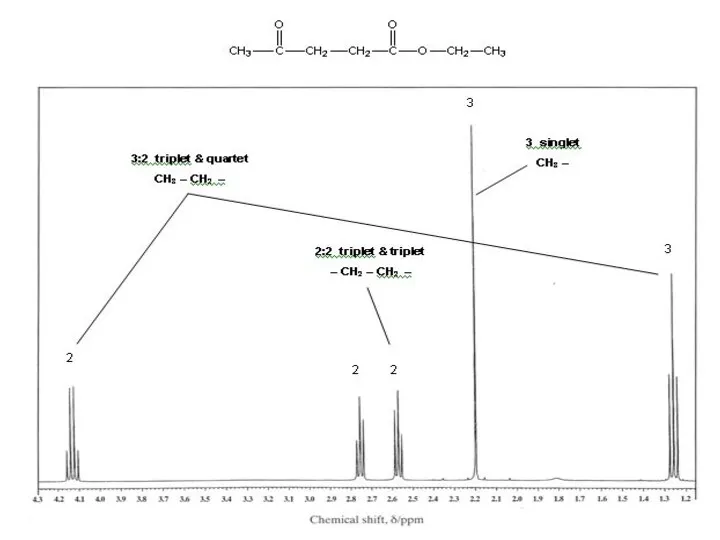
- 17. RELATIVE INTENSITY There are four signals here – each has the same area and so represents
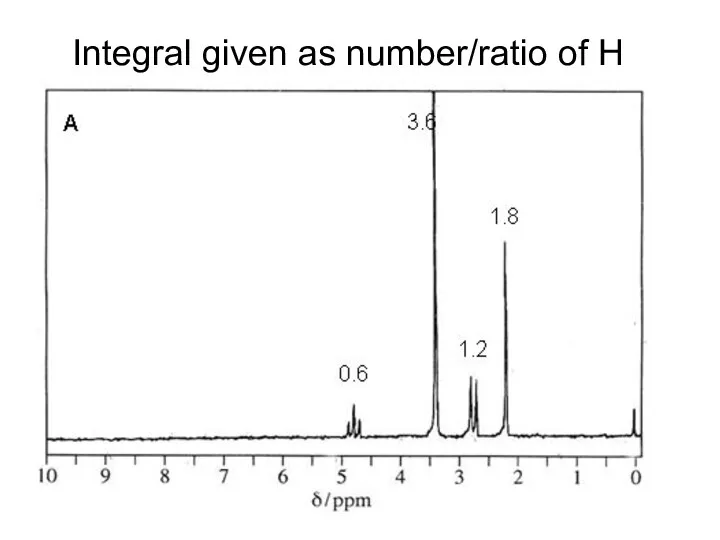
- 18. Integral given as number/ratio of H
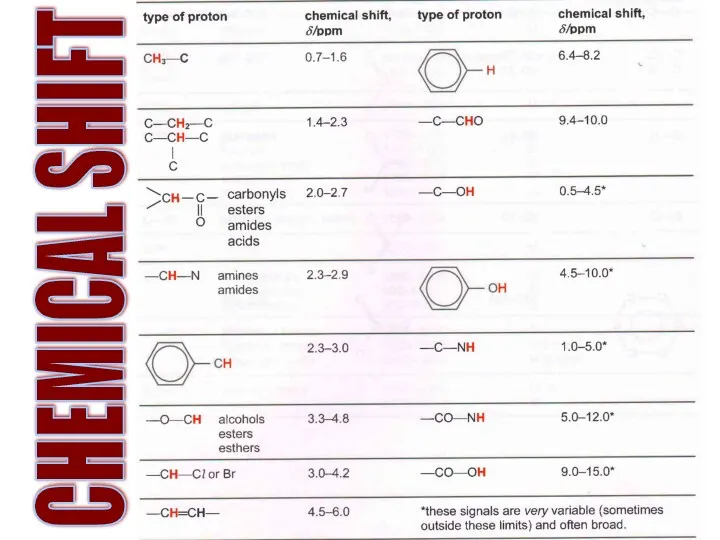
- 19. CHEMICAL SHIFT
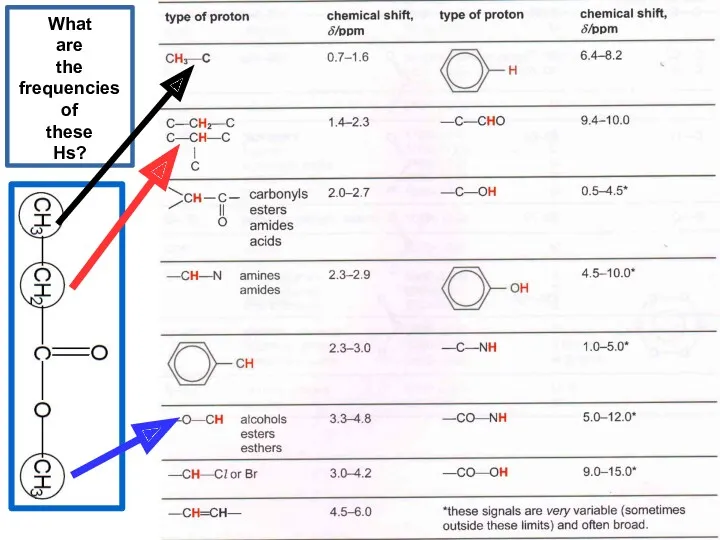
- 20. What are the frequencies of these Hs?
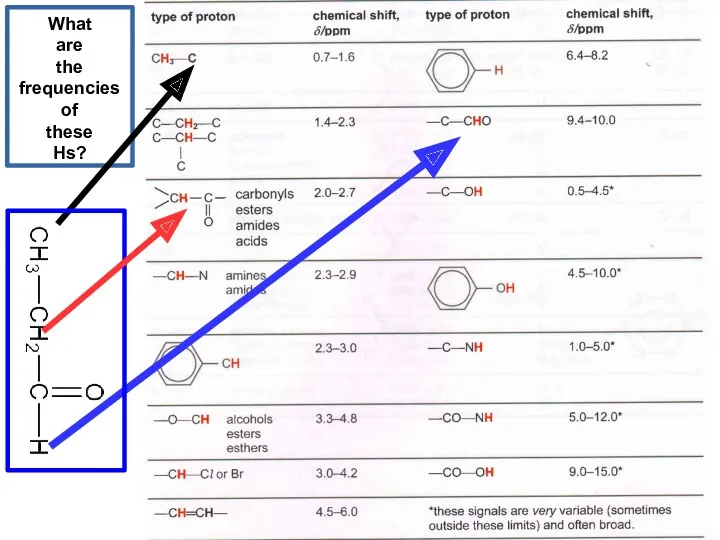
- 21. What are the frequencies of these Hs?
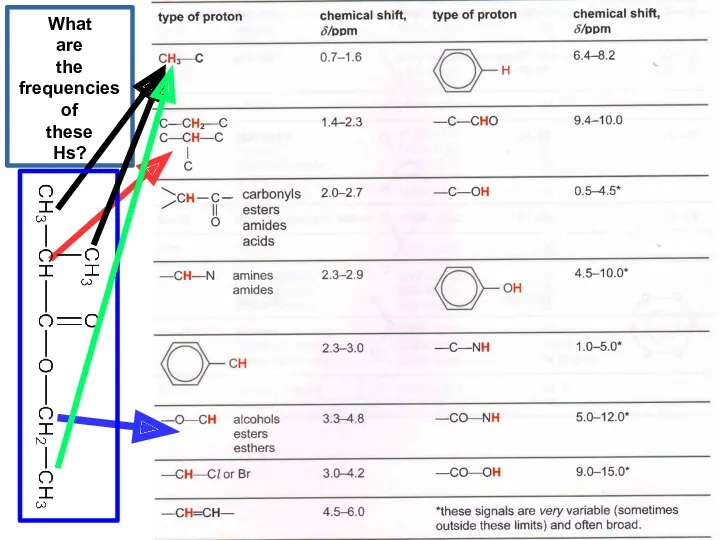
- 22. What are the frequencies of these Hs?
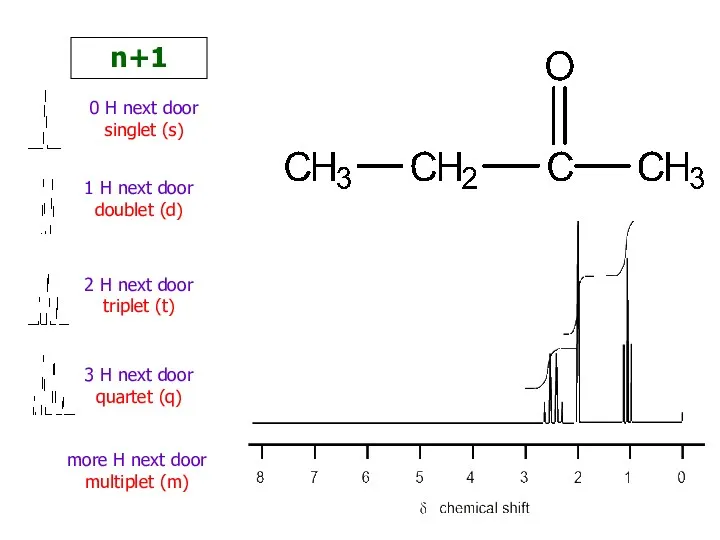
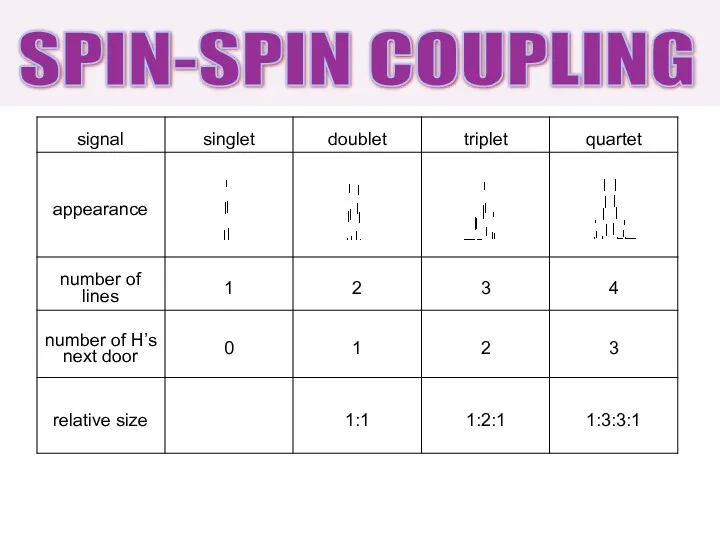
- 23. 0 H next door singlet (s) 1 H next door doublet (d) 2 H next door
- 24. SPIN-SPIN COUPLING
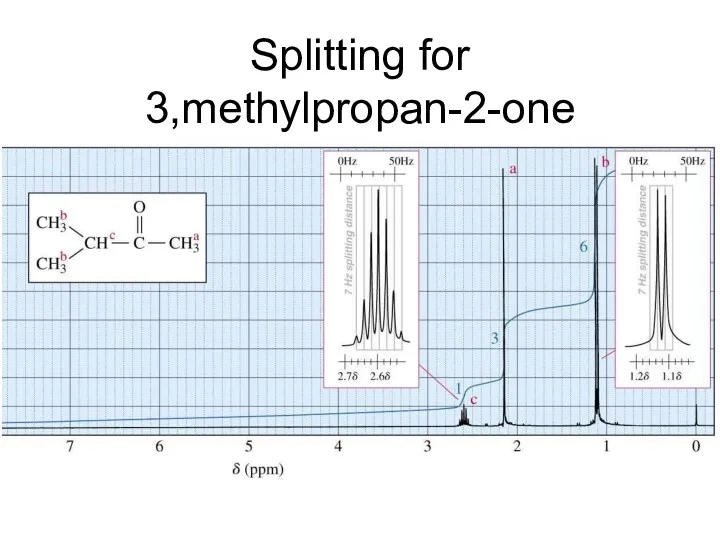
- 25. Splitting for 3,methylpropan-2-one
- 26. Number of H’s next door +1 But you don’t couple to H’s that are equivalent H’s
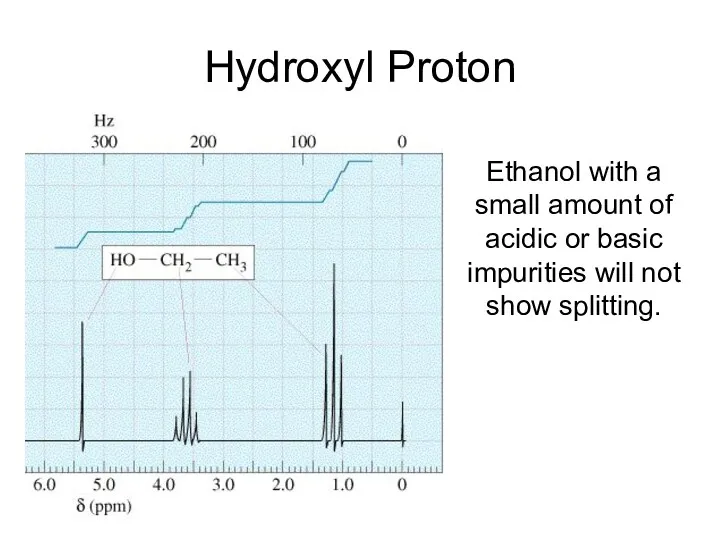
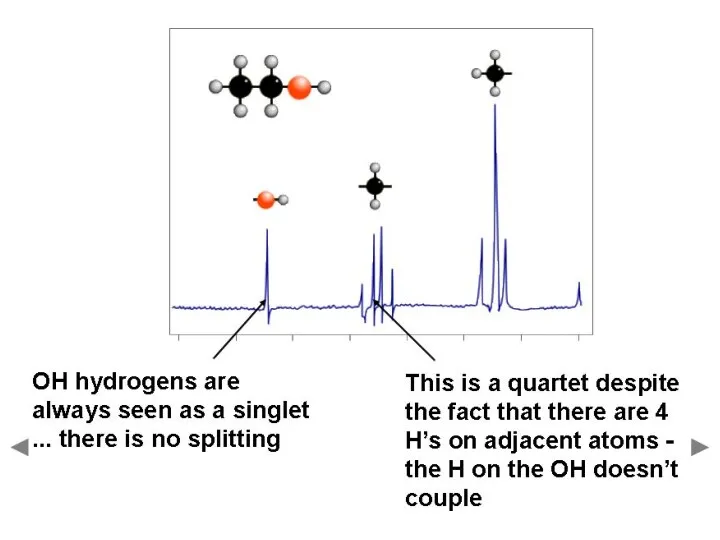
- 27. Hydroxyl Proton Ethanol with a small amount of acidic or basic impurities will not show splitting.
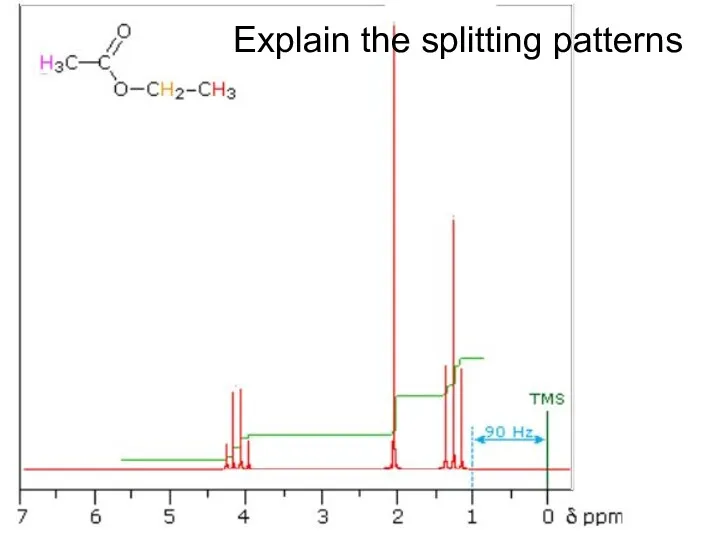
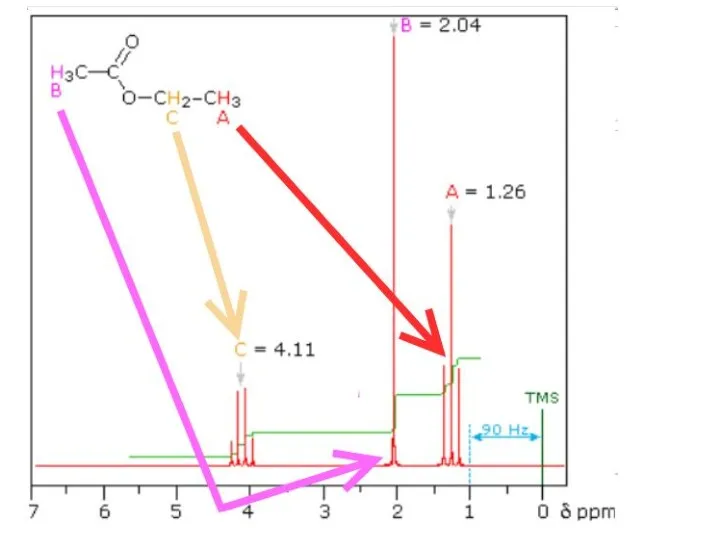
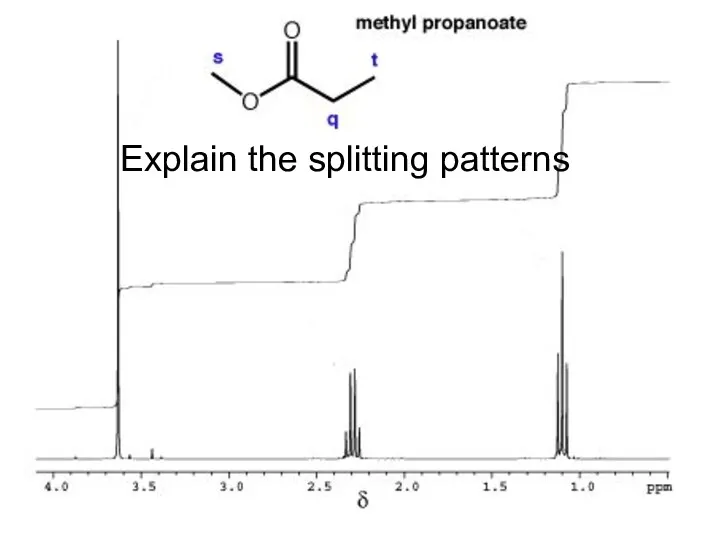
- 28. Explain the splitting patterns
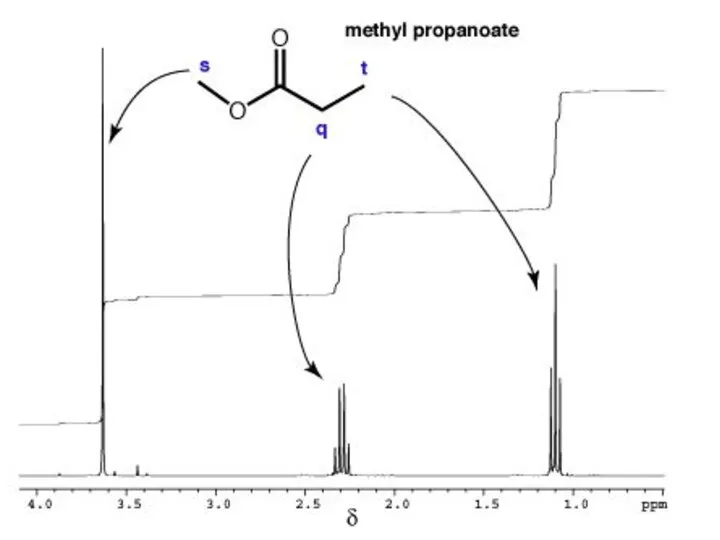
- 30. Explain the splitting patterns
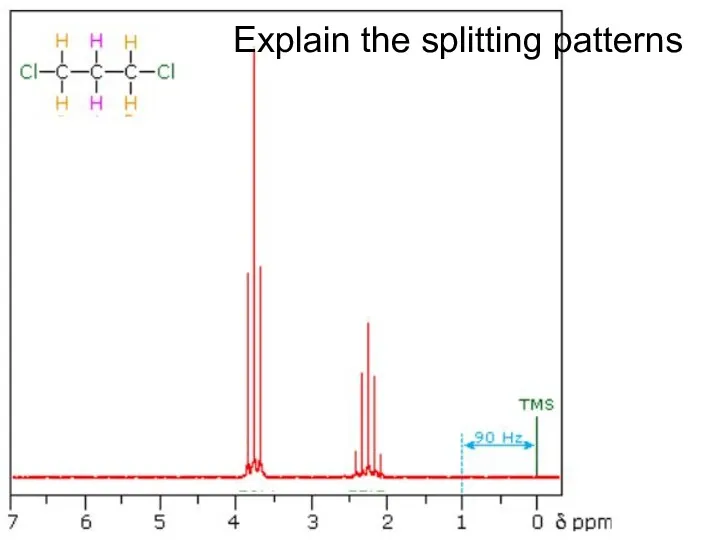
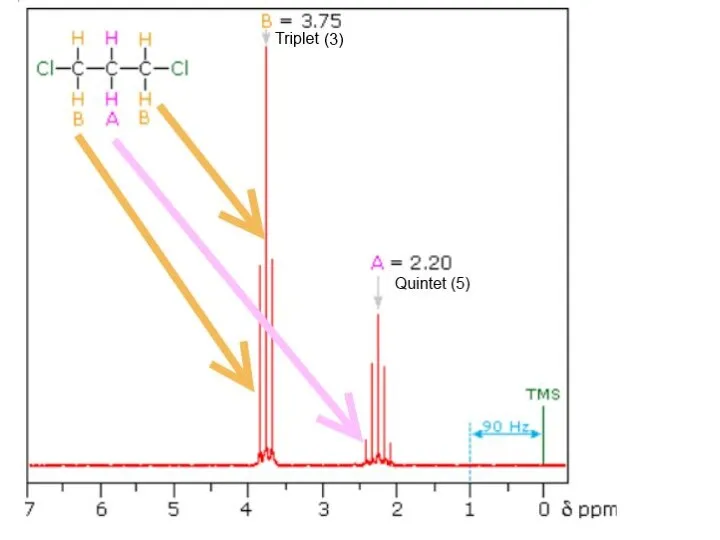
- 32. Explain the splitting patterns
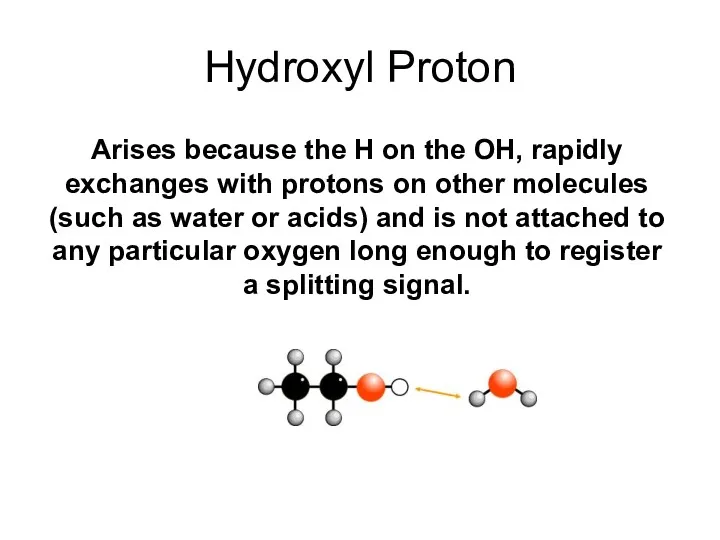
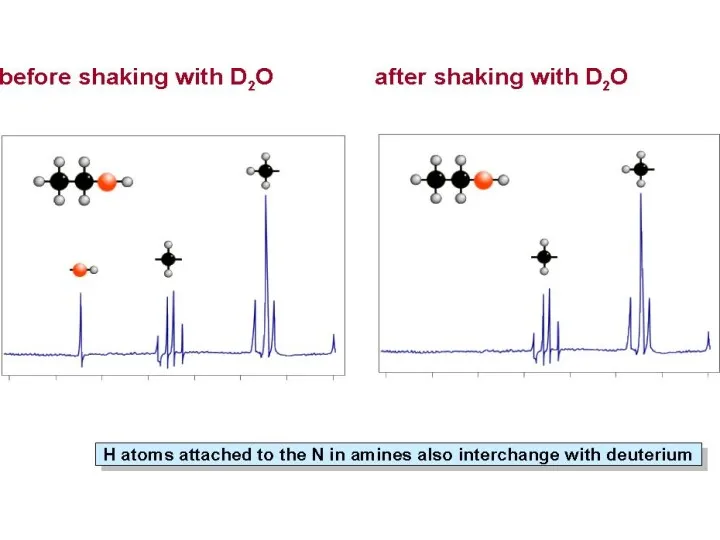
- 34. Hydroxyl Proton Arises because the H on the OH, rapidly exchanges with protons on other molecules
- 36. Identifying the O-H or N-H Peak Chemical shift will depend on concentration and solvent. To verify
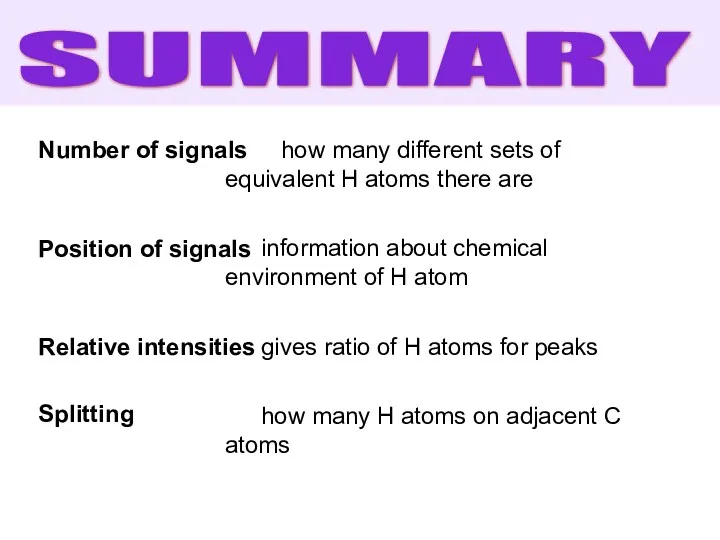
- 38. Number of signals Position of signals Relative intensities Splitting how many different sets of equivalent H
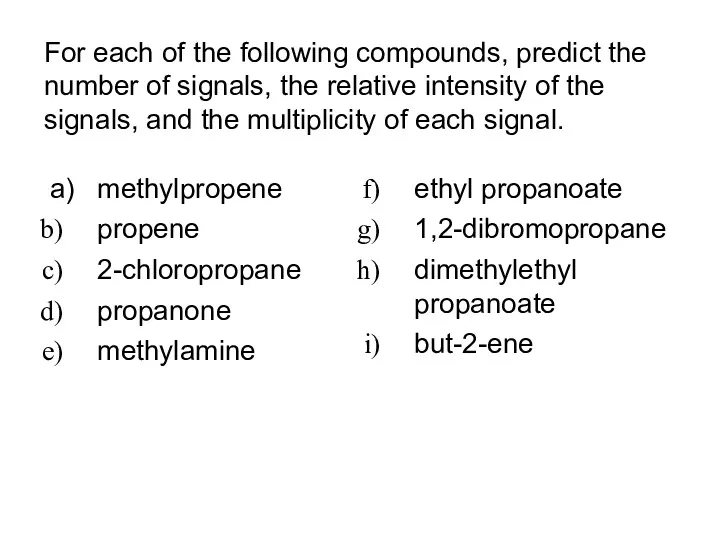
- 39. For each of the following compounds, predict the number of signals, the relative intensity of the
- 40. 2 signals: ratio 6 : 2 (3 :1) s s 3 signals: ratio 2 : 1
- 41. 3 signals: ratio 2 : 1 : 3 d m d 2 signals: ratio 6 :
- 43. 13C NMR SPECTROSCOPY
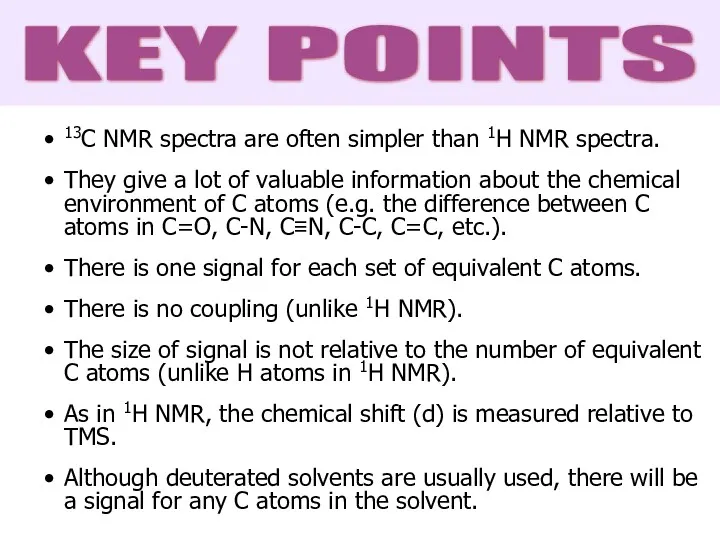
- 44. 13C NMR spectra are often simpler than 1H NMR spectra. They give a lot of valuable
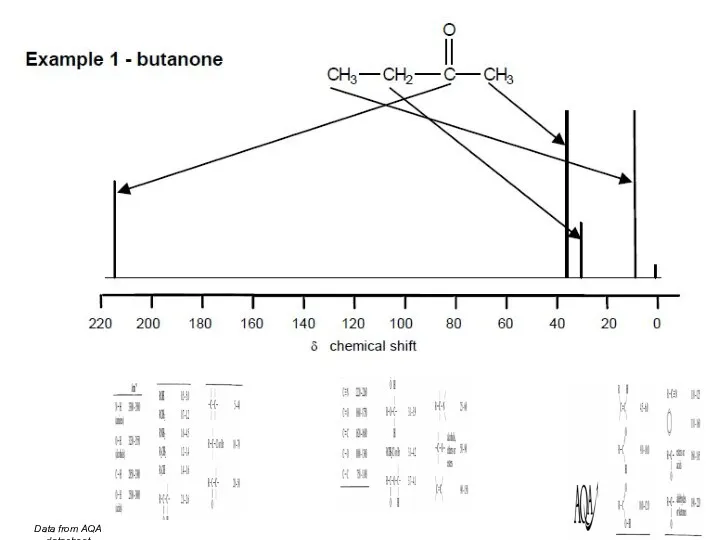
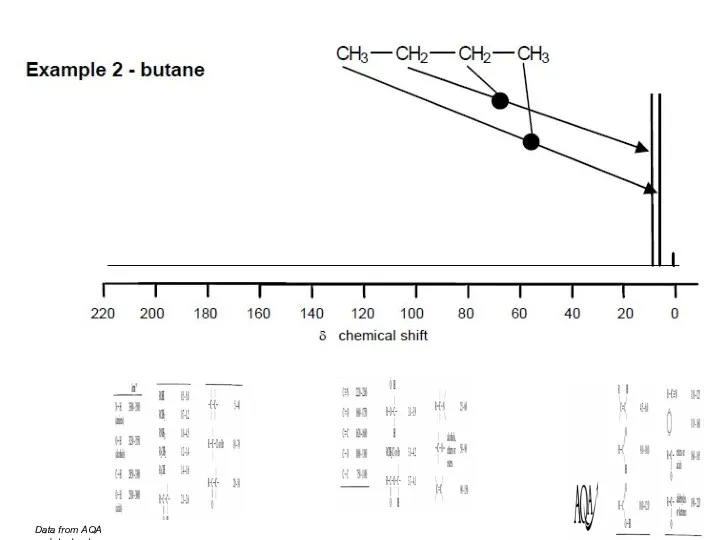
- 45. Data from AQA datasheet
- 46. Data from AQA datasheet
- 48. Скачать презентацию







 Методическая разработка урока Электроскоп. Электрометр. Проводники и диэлектрики
Методическая разработка урока Электроскоп. Электрометр. Проводники и диэлектрики Постоянные магниты. Магнитное поле Земли
Постоянные магниты. Магнитное поле Земли Елементи теорії поля. (Лекція 10)
Елементи теорії поля. (Лекція 10) Испарение. Поглощение энергии при испарении и выделение её при конденсации пара
Испарение. Поглощение энергии при испарении и выделение её при конденсации пара Реактивное движение. Ракеты
Реактивное движение. Ракеты Capacitance in an AC circuit
Capacitance in an AC circuit Нахождение механической силы через силу и перемещение
Нахождение механической силы через силу и перемещение конспект урока на тему: Атмосферное давление. Вес воздуха
конспект урока на тему: Атмосферное давление. Вес воздуха Первый закон Ньютона
Первый закон Ньютона Исследовательская работа в области естествознания. Анемометр в измерении силы ветра. Автор: Григорьев Никита, 11 лет, 6 Б класс
Исследовательская работа в области естествознания. Анемометр в измерении силы ветра. Автор: Григорьев Никита, 11 лет, 6 Б класс Научно-исследовательская работа по физике Электричество из овощей и фруктов (5 класс)
Научно-исследовательская работа по физике Электричество из овощей и фруктов (5 класс) Трансформатор. Қазіргі трансформаторлар
Трансформатор. Қазіргі трансформаторлар Почему внутри жидкостей существует давление?
Почему внутри жидкостей существует давление? Механічна енергія. Потенціальна і кінетична енергії тіла
Механічна енергія. Потенціальна і кінетична енергії тіла Рентгеновское излучение
Рентгеновское излучение Световые явления
Световые явления Изотопы
Изотопы Движение тела под действием силы трения
Движение тела под действием силы трения Атом құрылысы
Атом құрылысы Контур с током в магнитном поле
Контур с током в магнитном поле Учебный проект по теме Виды теплопередачи
Учебный проект по теме Виды теплопередачи Компенсация реактивной мощности
Компенсация реактивной мощности Элементы теории атомного ядра
Элементы теории атомного ядра Проезентация по теме Дисперсия света
Проезентация по теме Дисперсия света Введение в радиосвязь
Введение в радиосвязь 11 класс. Презентация по физике на тему Волновые явления.
11 класс. Презентация по физике на тему Волновые явления. Законы Кирхгофа
Законы Кирхгофа Устройство и назначение карданной передачи в автомобиле
Устройство и назначение карданной передачи в автомобиле